Published online Nov 15, 2023. doi: 10.4239/wjd.v14.i11.1603
Peer-review started: August 31, 2023
First decision: September 4, 2023
Revised: September 14, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 15, 2023
Over the past several decades, type 2 diabetes mellitus (T2DM) has been considered a global public health concern. Currently, various therapeutic modalities are available for T2DM management, including dietary modifications, moderate exercise, and use of hypoglycemic agents and lipid-lowering medications. Although the curative effect of most drugs on T2DM is significant, they also exert some adverse side effects. Biologically active substances found in natural medicines are important for T2DM treatment. Several recent studies have reported that active ingredients derived from traditional medicines or foods exert a therapeutic effect on T2DM. This review compiled important articles regarding the therapeutic effects of natural products and their active ingredients on islet β cell function, adipose tissue inflammation, and insulin resistance. Additionally, this review provided an in-depth understanding of the multiple regulatory effects on different targets and signaling pathways of natural medicines in the treatment of T2DM as well as a theoretical basis for clinical effective application.
Core Tip: This review compiled leading articles about the therapeutic effects of natural products and their active ingredients on islet β cell function, adipose tissue inflammation, and insulin resistance and provided an in-depth understanding of the multiple regulatory effects of different targets and signaling pathways of natural medicines in the treatment of type 2 diabetes mellitus.
- Citation: Wang T, Wang YY, Shi MY, Liu L. Mechanisms of action of natural products on type 2 diabetes. World J Diabetes 2023; 14(11): 1603-1620
- URL: https://www.wjgnet.com/1948-9358/full/v14/i11/1603.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i11.1603
According to the International Diabetes Federation, the number of patients with diabetes mellitus (DM) worldwide was 536 million in 2021, which is expected to reach 783 million by 2045[1]. Globally, China has the highest number of patients with DM, whose prevalence is increasing steadily. By 2045, the total number of patients with DM in China is expected to exceed 174 million[1]. Based on its etiology, mechanism, and clinical manifestations, DM can be classified into type 1 DM, type 2 DM (T2DM), specific types of DM due to other causes, and gestational DM[2]. In China, T2DM accounts for 90% of all DM cases[3]. T2DM is mainly caused by insulin resistance (IR) associated with obesity, deficiencies in insulin secretion (INS), and reduction in islet cell numbers due to apoptosis[3]. DM and its complications are serious health and economic problems that affect individuals worldwide and require urgent prevention and early intervention.
Through diet management, lifestyle changes, and oral use of biguanides and sulfonylureas, blood sugar levels can be effectively controlled to treat T2DM. Although these treatment modalities can relieve symptoms and improve patients’ conditions to a certain extent, they cannot completely prevent the occurrence and progression of complications; moreover they exert toxic side effects[4]. Natural medicines have become a hotspot in the exploration of alternative treatments for DM owing to their minimal side effects. Natural products mainly refer to small or macromolecular active substances with pharmacological properties and are extracted from plants, animals, or microorganisms. They can be used to treat DM and its complications through multiple targets and pathways. The antidiabetic ingredients of natural products include monomeric compounds such as flavonoids, alkaloids, terpenes, polyphenols, saponins, and quinines[5].
The current literature on T2DM treatment with natural products is mostly based on their different active ingredients; however, reviews on their regulation mechanisms are lacking. This review aimed to summarize the mechanism of natural products and/or their monomers on T2DM treatment (Table 1). It also provided a theoretical basis for comprehensively understanding the mechanism and clinical application of natural medicines in the treatment of T2DM by summarizing the signal pathways involved in the regulation.
| Classification, extracts/monomers | Model | Signaling pathway | Related genes/proteins | Improvement effect | Ref. | |
| In vivo | In vitro | |||||
| Flavonoids | ||||||
| Quercetin | STZ-induced Wistar rats | - | IKK/NF-κB/TNF-α | Serum SOD and GSH ↑, TNF-α ↓ | Lowered blood glucose, cholesterol, and triglyceride levels and restores the number of islet β cells | Abdelkader et al[13] |
| Fructose-treated Wistar rats | INS-1 β cells | Akt/FoxO1 | p-Akt, JAK2, and STAT3 ↑, Akt/FoxO1 and Socs3 ↓ | Protected β cell mass and function | Li et al[35] | |
| STZ-induced Sprague–Dawley rats | - | - | Islet β cell number ↑, total cholesterol ↓ | Caused regeneration of islets and increased insulin release | Vessal et al[39] | |
| Balb/c mouse | - | - | HO-1 and Bcl-2 ↑, NO, iNOS, and Bax ↓ | Enhanced islet viability, reduced apoptosis | Kim et al[67] | |
| db/db mice | INS-1 cells | SIRT3-FoxO3a | SOD2, CAT, and Sirt3 ↑, cleaved-caspase-3 and Bax/Bcl-2 ratio ↓ | Protected islet β cells against apoptosis | Wang et al[68] | |
| HFD-induced C57BL/6 mice | - | AMPKα1/SIRT1 | GLUT4, AMPK, and SIRT1 ↑, TNF-α, IL-6, and MSP-1 ↓ | Suppressed ATM infiltration and inflammation, increased insulin sensitivity, and decreased adipose tissue weight | Dong et al[103] | |
| Hesperidin | STZ-induced Wistar rats | - | PI3K/Akt | FFA, p-IRS-1, Akt, IL-6, and TNF-α ↓ | Enhanced the antioxidant defense system while inhibiting the production of proinflammatory cytokines | Mahmoud et al[16] |
| STZ-induced Wistar rats | - | - | Antioxidative enzyme activities ↑, MDA, NO, and lipid peroxidation ↓ | Decreased oxidative stress while preserving the integrity of β cells | Coskun et al[36] | |
| db/db mice | Palmitic acid-induced MIN-6 cells | ERK1/2 | Bcl-2/Bax ratio↑, caspase-3, caspase-9, and caspase-12 ↓ | Inhibited cell apoptosis, improved fat metabolism disorders, and reduced blood sugar levels | Zhuang et al[38] | |
| Puerarin | HFD-induced C57BL/6J mice | High glucose-induced MIN-6 cells | - | GLP-1R ↑, PDX-1, caspase-3, and Foxo1 ↓ | Improved glucose homeostasis and protected β cell survival | Yang et al[42] |
| STZ-induced C57BL/6 mice | CoCl2-induced MIN-6 cells | PI3K/Akt/mTOR | Bcl-2/BAX ratio and SOD and GPX1 activity↑, caspase-3 ↓ | Protected pancreatic β cell function and survival | Li et al[43] | |
| 3Cyanidin-3-glucoside | - | High glucose-induced MIN-6 cell | NF-κB/MAPK/caspase | β cell viability ↑, ROS, ERK, p-ERK, JNK, p-JNK, caspase-3, and Bax ↓ | Decreased the generation of intracellular reactive oxygen species, DNA fragmentation, and apoptosis rate; prevented pancreatic β cell apoptosis | Lee et al[69] |
| Kaempferol | - | PA induced INS-1E cells | PDX-1/cAMP/PKA/CREB | β cell activity and Bcl-2 ↑, caspase-3 and Bax ↓ | Promoted pancreatic β cell survival and function | Zhang et al[73] |
| - | High glucose-induced INS-1E β cells | cAMP/Akt/CREB | Bcl-2 ↑ | Improved insulin secretory function and synthesis in β cells | Zhang and Liu[74] | |
| Butein | - | 3T3-L1 cells | NF-κB/AMPK | iNOS, NO, ERK, JNK, and p38MAPK ↓ | Prevented adipose tissue inflammation and obesity-linked IR | Wang et al[96] |
| Naringin | - | 3T3-L1 cells | NF-κB/ERK/TNF-α | TNF-α and IL-6 ↓ | Repressed FFA secretion to alleviate IR induced by FFA | Yoshida et al[98] |
| HFD-induced C57BL/6 mice | 3T3-L1 cells | IκB-α/JNK/TNF-α | TNF-α, TLR2, and MCP-1 ↓ | Decreased blood glucose levels | Yoshida et al[99] | |
| Baicalin | HFD-induced C57BL/6 mice | - | - | β-cell activity ↑, HOMA-IR ↓ | Improved IR by inhibiting macrophage-mediated inflammation | Na et al[115] |
| HFD-induced C57BL/6 mice | - | - | MCP-1 ↓ | Suppressed macrophage infiltration into the adipose tissue | Yoshida et al[100] | |
| HFD-induced C57BL/6J mice, C57BL/6 mice | - | IRS1/PI3K/Akt, AMPKα | MAPK, NF-κB, and p85 ↑, FFA, IRS1, and Akt ↓ | Exerted an anti-inflammatory effect, inhibited IR | Pu et al[125] | |
| Icariin | High-sugar HFD and STZ-induced SD rats | - | AMPK/GLUT-4 | p-AMPK, and GLUT4 ↑, islets cell number ↓ | Reduced hyperglycemia | Li et al[76] |
| Cyanidin-3-glucoside | - | H2O2-induced MIN-6 cells | - | Islet cell apoptosis, ERK, p38, and caspase-3 ↓ | Prevented diabetes by inhibiting oxidative stress-induced β cell apoptosis | Lee et al[70] |
| Anthocyanins | STZ-induced SD rats | - | - | Caspase-3 ↓ | Reduced IR and β cell apoptosis | Nizamutdinova et al[71] |
| Polyphenols | ||||||
| Curcumin | STZ-induced SD rats | High- fructose-induced U937 monocytes | IKK/NF-κB/TNF-α | TNF-α, IL-6, and MCP-1 ↓ | Reduced inflammation and oxidative stress levels | Jain et al[18] |
| High fructose fed Wistar rats | - | IKK/NF-κB /COX-2 | Proliferation of β cells and SOD ↑, TNF-α and COX-2 ↓ | Reduced glucose intolerance and IR | Maithilikarpagaselvi et al[19] | |
| STZ-induced SD rats | PA and high fructose-induced INS-1 cells | - | Caspase-3 and Bax ↑ | Inhibited apoptosis | Li et al[83] | |
| - | 3T3-L1 and BV-2 cells | IKK/NF-κB/TNF-α | TNF-α, IL-1β, IL-6, and COX-2 ↓ | Inhibited chronic inflammation | Gonzales et al[111] | |
| Gallic acid and p-coumaric acid | STZ-induced Albino rats | - | IKK/NF-κB/iNOS | PPARγ mRNA and adiponectin ↑, TNF-α, IL-1, and IL-6 ↓ | Decreased glucose and glycosylated hemoglobin levels, increased insulin level and body weight | Abdel-Moneim et al[23] |
| Resveratrol | HFD-induced SD rats | - | IKK/NF-κB/TNF-α | ICAM-1, MCP-1, IL-1, and TNF-α ↓ | Improved IR and vascular permeability and attenuated inflammatory injury | Zheng et al[28] |
| HFD + STZ-induced SD rats | PA-induced INS-1E cells | SIRT1/NF-κB/TNF-α | PPAR-γ, SIRT1, FOXO-3a, and TNF-α ↑ | Decreased blood glucose and insulin levels | Cao et al[29] | |
| - | UA-induced MIN-6 cells | PI3K/Akt | miR-126 ↑, Bax, cleaved-caspase-3, and iNOS ↓ | Enhanced cell viability, reduced cell apoptosis, and increased insulin secretion | Xin et al[77] | |
| Human islet cells | - | - | VEGF, insulin, and C-peptide secretion ↑, ROS and HIF-1α ↓ | Diminished apoptosis and enhanced islet survival and function | Keshtkar et al[79] | |
| Sargassum oligocystum | STZ-induced Wistar rats | - | - | - | Enhanced the number of insulin-positive β cells, facilitated the survival of islet β cells, and conserved islet mass | Akbarzadeh et al[46] |
| HSHFD-induced SD rats | - | - | - | Decreased blood glucose levels, alleviated pancreas, liver, and kidney damage | Motshakeri et al[45] | |
| Genistein | HF + STZ-induced C57BL/6 mice | - | - | - | Improved glycemic control, glucose tolerance, and insulin levels while enhancing islet β cell survival | Fu et al[47] |
| HFD + STZ-induced Wistar rats | - | ERK1/2 /Akt | Bcl-2 and caspase-3 ↓ | Regulated pancreatic β cell function, enhanced the morphology of pancreatic β cells, and mitigated cellular apoptosis | Yousefi et al[49] | |
| Mangiferin | PPX C57BL/6J mice | - | - | Cyclins D1 and D2 and cyclin-dependent kinase 4 ↑, p27Kip1 and p16INK4a ↓ | Stimulated β cell proliferation and suppressed β cell apoptosis | Wang et al[53] |
| Cranberries | - | 3T3-L1 cells | - | AP2, FAS, LPL, HSL, and PLIN1 mRNA ↓ | Inhibited mass production of the adipose tissue | Kowalska et al[105] |
| - | 3T3-L1 cells | - | IL-6, PAI-1, McP-1, and leptin ↓ | Exerted an anti-inflammatory effect | Kowalska and Olejnik[106] | |
| Peanut skin extract | HFD-induced mice | - | - | TNF-α, IL-1β, IL-6, and PAI-1 ↓ | Maintained the gut microbiota, inhibited inflammation, and reduced fasting blood glucose levels, body weight, and food intake | Xiang et al[109] |
| Luteolin | - | 3T3-L1 cells | AMPK/SIRT1 | p-p65 ↑, TNF-α, IL-6, and MCP-1 ↓ | Inhibited inflammation and promoted glucose disposal | Xiao et al[112] |
| HFD-induced C57BL/6N mice | - | - | IL-1β and PAI-1 ↓ | Enhanced dyslipidemia, ameliorated hepatic steatosis, improved IR, and reduced inflammation | Kwon et al[131] | |
| HDF-induced C57BL/6J mice | - | - | PPARγ, SREBP1, SREBP2, ACC G6PD, Fas, ME, PAP, HMCGR, and ACAT ↓ | Attenuated hepatic lipotoxicity and improved circulating fatty acid levels as well as hepatic insulin sensitivity | Kwon et al[130] | |
| Mulberry anthocyanin extract | db/db mice | Palmitic acid and high-fructose-induced HepG2 cells | PI3K/Akt | Proliferation of islet β cells, AKT, GSK-3β, and GYS-2 levels ↑, TC, TG, FOXO-1, and PGC-1α ↓ | Decreased fasting blood glucose, serum insulin, leptin, triglyceride, IR, and cholesterol levels and increased adiponectin levels | Yan et al[123] |
| Terpenoids | ||||||
| Geniposide | HFD-induced C57BL/6J mice | MIN-6 cells | β-catenin/TCF7L2 | TCF7L2 and GLP-1R ↑, GSK3 ↓ | Promoted β cell survival by inducing proliferation and inhibiting apoptosis | Yao et al[56] |
| Paeoniflorin | - | INS-1 cells | MAPK/caspase | Bax, p38, JNK, caspase-3 activity ↓ | Enhanced insulin secretion and inhibited β cell apoptosis | Liu et al[90] |
| - | High-fructose-induced INS-1 cells | - | HO-1 and Bcl-2 ↑, caspase-3 and Bax ↓ | Protected β cells and reduced apoptosis | Liu et al[88] | |
| Alpha-mangostin | - | STZ-induced INS-1 cells | PI3K/Akt and ERK | Bax, p38, JNK, and caspase-3 activity ↓ | Improved insulin secretion in pancreatic β cells and prevented apoptosis | Lee et al[87] |
| Ethanolic extracts of Pluchea indica | STZ-induced BALB/C mice | - | - | IFN-γ, TNF-α, IL-1β, caspase-3, caspase-8, and caspase-9 ↓ | Maintained body weight, reduced hyperglycemia, restored islet function, and inhibited β cell apoptosis | Nopparat et al[89] |
| Dioscorea batatas extract | HDF-induced C57BL/6 mice | - | PI3K/Akt | p-Akt ↑, p-ERK, and p-S6K1 ↓ | Reduced glucose and insulin levels and improved IR | Kim et al[132] |
| Alkaloids | ||||||
| Rhizoma coptidis | HFD/STZ-induced Wistar rats | - | PI3K/p-Akt | PPAR-γ ↑, TNF-α, GLUT4, HOMA-IR, TC, TG, and p-Akt ↓ | Enhanced insulin sensitivity of the adipose tissue, regulated adipogenesis, elevated glucose uptake in adipocytes, and preserved β cell function | Gandhi et al[127] |
| Berberine | db/db mice | PA-induced MIN6 cells | iPLA2β/OL/OPA1 | TNF-α, IL-1, NO, PEG2, and CRP ↑ | Prevented apoptosis of β cells and enhanced islet β cell function | Li et al[84] |
| Brucea javanica, luteolin, protocatechuic acid | NA/STZ-induced SD rats | - | - | TG, TC, IL-6, INF-γ, TNF-α, ROS, and MDA ↓ | Improved hepatic glucose and carbohydrate metabolism, suppressed oxidative stress, and prevented inflammation | Li et al[83] |
| Coffee | STZ-induced C57BL/6J | - | - | Caspase-3 and Bax ↓ | Reduced glucose levels and maintained pancreatic insulin contents | Kobayashi et al[85] |
| Caffeic acid, naringenin, and quercetin | - | INS-1 cells | PI3K/Akt | HSP90 mRNA ↑, caspase-3 and Bax ↓ | Enhanced glucose-induced insulin secretion and sensitivity and improved β cell survival and function | Kobayashi et al[86] |
| Quinones | ||||||
| Thymoquinone | STZ-induced Wistar rats | - | - | Survivin CD31 and IL-10 ↑, caspase-3, IL-1β, and TBARSS ↓ | Promoted β cell regeneration, mitigating inflammation and oxidative stress, suppressed apoptosis of β cells, and enhanced revascularization of islets | El-Shemi et al[60] |
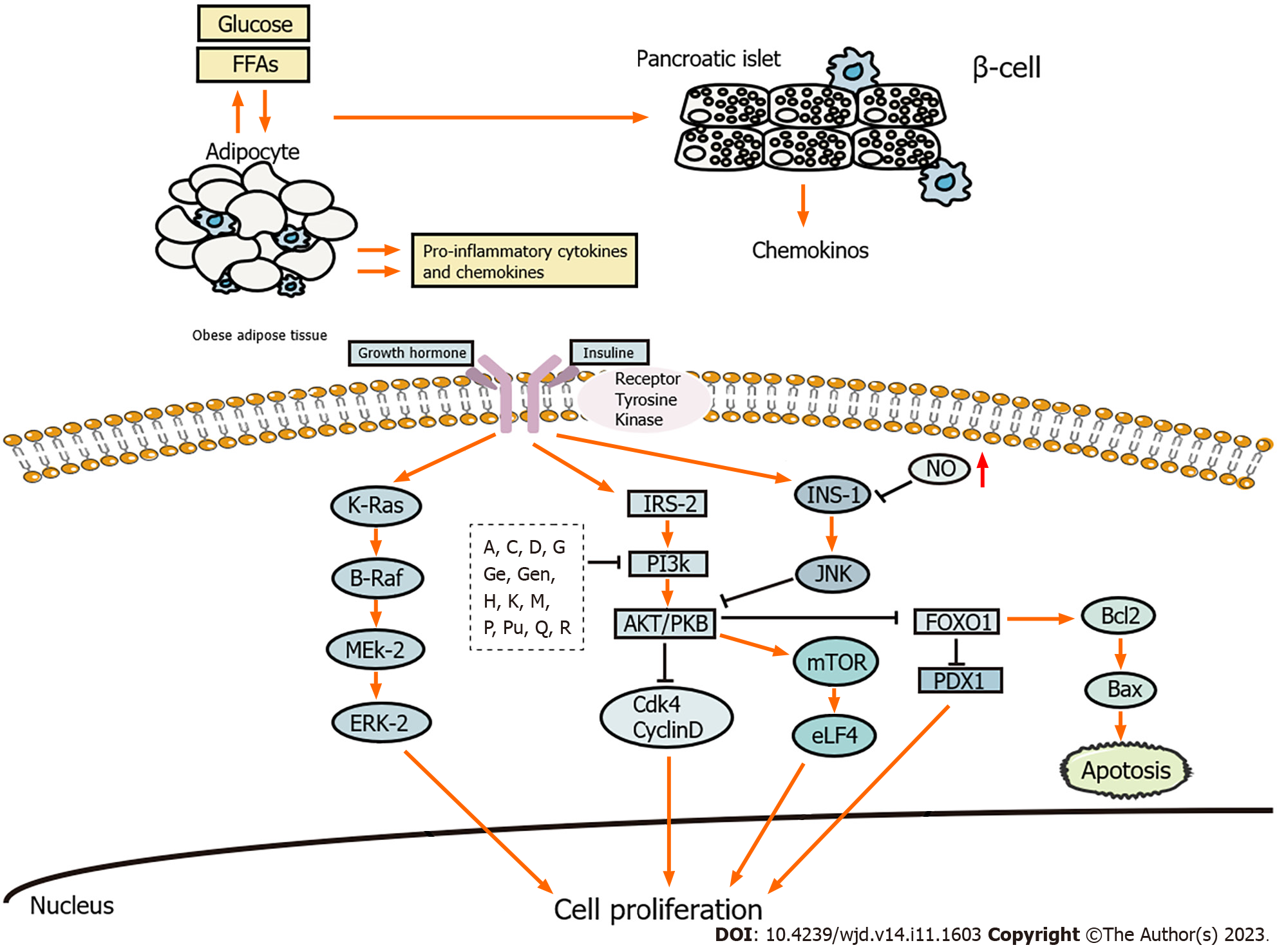
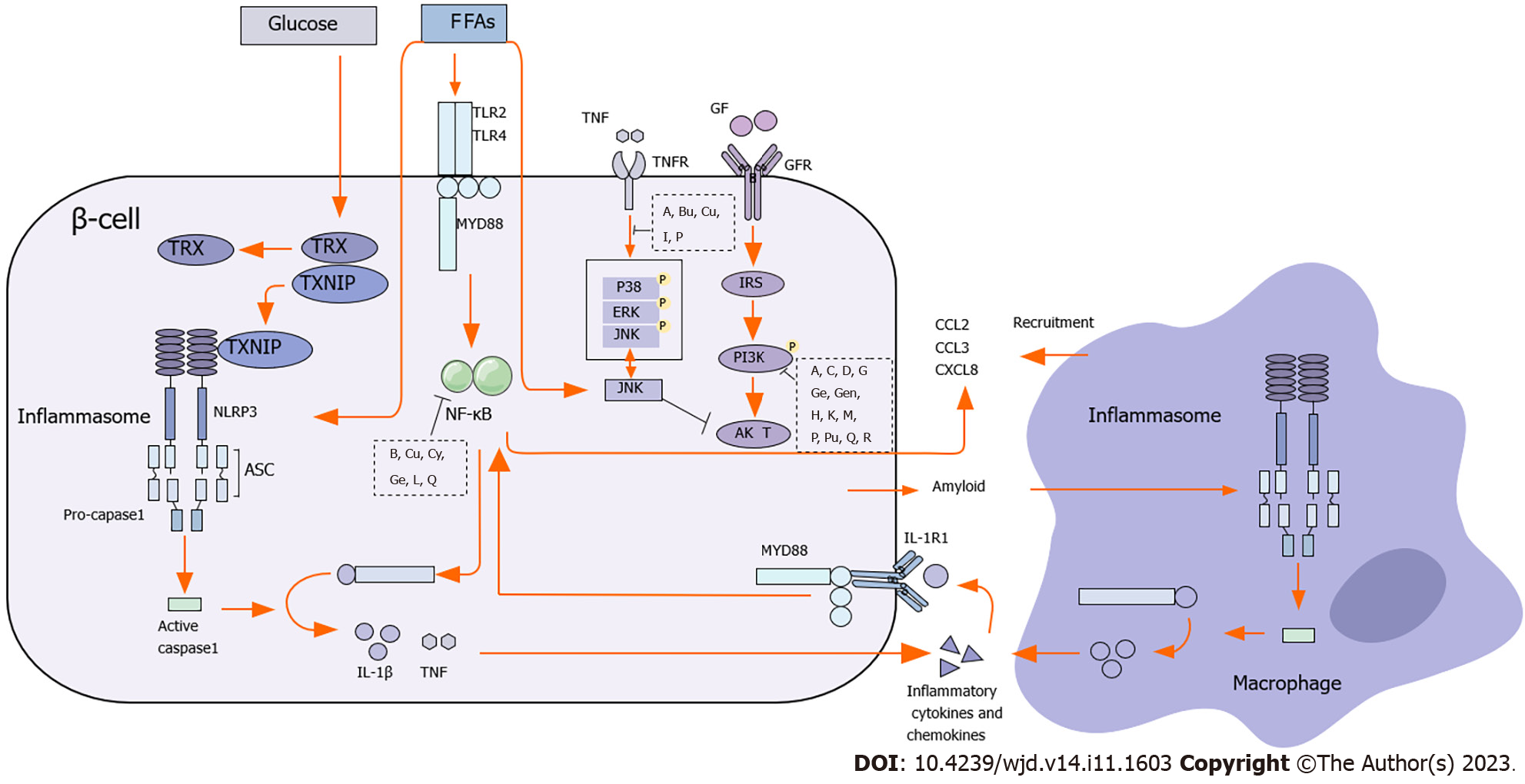
Inhibition of islet β cell function is a prerequisite for T2DM occurrence. β cell impairment and IR are crucial in the development and pathogenesis of T2DM[6]. During the course of the illness, islet β cell function failure is observed along with frequent episodes of exacerbation[7,8]. Natural products exhibit notable effectiveness in reducing the inflammation, promoting the regeneration, and inhibiting the apoptosis of islet β cells (Figure 1).
The accumulation of intra-islet macrophages is observed in T2DM, which represents the primary source of proinflammatory cytokines within the islets[9]. Activated monocytes and macrophages release proinflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1)[10], which activate inflammatory signaling pathways, such as the inhibitor of kappa B kinase and c-Jun N-terminal kinase (JNK), and impair the insulin signaling pathway by regulating the levels of phosphoinositide 3-kinase (PI3K)and protein kinase B(Akt).
Flavonoids: Quercetin is one of the most important bioflavonoids found in vegetables, cereals, fruits, and other plants. It is widely detected in green tea, onions, and apples and exerts antioxidative, anti-inflammatory, and antifibrotic effects[11]. A previous study reported that the anti-inflammatory effect of quercetin is mediated by the upregulation of peroxisome proliferator-activated γ (PPAR-γ), which interferes with proinflammatory transcriptional factors, such as signal transducer and activator of transcription (STAT) and nuclear factor-kappa B (NF-κB), and reduces the expression of IL-1β, IL-6, and TNF-α[12]. Abdelkader et al[13] also demonstrated that the anti-inflammatory effect of quercetin decreased the expression of IκB-α by inhibiting the expression of IKK-α and IKK-β in islets β cells, thereby inhibiting NF-κB activation and decreasing TNF-α levels.
Naringin and hesperidin are abundant in citrus fruits and exert antioxidative, antidiabetic, lipid-lowering, anti-atherosclerotic, and anti-inflammatory effects[14,15] They can reduce the expression of TNF-α and IL-6, regulate the level of nitric oxide (NO), activate the JNK pathway, inhibit the PI3K/Akt pathway, inactivate the lipid peroxide reaction, and reduce the levels of free radicals in high-fat diet/streptozocin (HFD/STZ)-induced rats with diabetes[16].
Polyphenols: Curcumin is a bioactive molecule found in the rhizome of turmeric plants; it exhibits extensive pharmacological and biological activities, such as exerting anti-inflammatory and hypoglycemic effects, improving β cell function, preventing β cell death, and improving IR[17]. It has been reported that curcumin indirectly inhibits the NF-κB pathway to prevent inflammation by inhibiting IκB-α degradation as well as reduces the levels of IL-6, MCP-1, and TNF-α in the serum of rats with diabetes[18]. Another study reported that curcumin decreased the expression of JNK, cyclooxygenase-2 (COX-2), protein kinase C, extracellular signal-regulated kinase (ERK), and p38, reduced the level of malondialdehyde (MDA), and prevented inflammation[19].
Gallic and p-coumaric acids are found in plants such as tea, mango, and cocoa; they exert anti-inflammatory, antioxidative, and antiobesity effects[20,21]. IL-1β reportedly induces NO production, increases NF-κB DNA binding, activates inducible NO synthase (iNOS) in islet β cells, and aggravates the inflammatory injury in islet β cells. Oral administration of gallic and p-coumaric acids also increases the expression of PPAR-γ[22], suppresses the expression of NF-κB, decreases the levels of proinflammatory cytokines (IL-1, IL-6, and TNF-α), iNOS expression, and nitrite production, and increases insulin sensitivity[23].
Luteolin is widely found in vegetables, fruits, and natural herbs, such as parsley, thyme and celery. It exerts various antitumor and anti-inflammatory effects by inducing cell apoptosis and inhibiting NF-κB activation, respectively[24]. Luteolin reportedly inhibits the NF-κB pathway and increases IL-10 levels in lipopolysaccharide-activated macrophage-like cell lines, thus exerting its anti-inflammatory effect[25].
Resveratrol is detected in cereals, fruits, and plant derived-beverages. It exerts antidiabetic, anti-inflammatory, and antioxidative effects[26]. Resveratrol also inhibits the production of inflammatory factors by activating sirtuin 1 (SIRT1) and inhibiting p65/RelA acetylation, which results in decreased mRNA expression of ICAM-1, MCP-1, and TNF-α[27-29].
Genistein is an isoflavone found in legumes and herbs. It is a natural estrogen and tyrosine kinase inhibitor with potential hypolipidemic, antioxidative, and antiapoptotic effects[30]. Genistein reportedly inhibits p65 acetylation by activating SIRT1 to reduce the levels of IL-1β, IL-6, and TNF-α in ovariectomized rats with diabetes as well as the expression of NF-κB[31].
Alkaloids: Brucea javanica belongs to the bitter wood family, which is generally used for the treatment of DM[32]. A previous study showed that it effectively reduced the levels of TNF-α and IL-6 in rats, inhibited the NF-κB pathway, enhanced the expression of insulin receptor substrate-1 (IRS-1), and GLUT4 and played an anti-inflammatory role[33].
β cells are key to maintaining balance in glucose metabolism. A decrease in the number of β cells leads to insufficient insulin production, which is one of the key factors in the pathogenesis of T2DM. β cell regeneration can be considered a new approach for treating T2DM[34].
Flavonoids: Quercetin promotes the differentiation and regeneration of β cells[13]. Previous studies have revealed that quercetin decreases the phosphorylation of Akt and FoxO1 in fructose-fed rat islets and increases the expression of nuclear FoxO1 in fructose-treated INS-1 cells[35]. Quercetin significantly decreases MDA and NO levels, increases antioxidative enzyme activities, and enhances insulin staining and β-cells preservation[36]. Oyedemi et al[37] reported that quercetin increased the number of pancreatic islets and β cells and can normalize the weight ratio of rat pancreas, suggesting that quercetin has the potential to regenerate pancreatic β cells. Furthermore, Zhuang et al[38] reported that quercetin improved the vacuolation of β cells and increased the number of pancreatic islets in db/db mice, consistent with the regeneration of pancreatic islets in STZ-induced rats with diabetes after 7 d of treatment with quercetin[39].
Puerarin, the dry root of pueraria, exerts neuroprotective, antioxidative, anti-inflammatory, and antiapoptotic effects[40]. It reportedly increases the mass and proliferation of mouse β cells, leading to the activation of glucagon-like peptide 1 receptor signaling[41]. Another study confirmed that puerarin protects pancreatic β cell function and promotes survival by mediating the PI3K/Akt pathway, thereby exhibiting resistance to the toxicity of cobalt chloride[42,43].
Polyphenols:Sargassum is a brown macroalgae found in shallow sea meadows. It exerts anti-inflammatory, antioxidative, and immune regulatory effects[44]. Pathological analysis of the islets revealed that the water extract of Sargassum can restore the damaged islet structure. Previous studies have revealed that the islet area and regeneration percentage increased and the regeneration function of pancreatic β cells improved after 30 d of supplementation with hydroalcoholic extract of Sargassum[45,46].
Genistein intake can improve hyperglycemia, increase insulin levels, and enhance glucose tolerance in mice with diabetes[47]. Akt and ERK1/2 are markers of cell proliferation and growth[48]. Genistein reportedly increases the expression of p-ERK1/2, p-Akt, and Bcl-2 and suppresses the expression of caspase-3, concomitant with improved morphology and mass of islet β cells[49].
Mangiferin is a polyphenolic compound isolated from Anemarrhena. C-glycoside, which is isolated from mango leaves, is a type of mangiferin exhibiting biological activities. It reduces blood glucose levels and contributes to the regeneration of pancreas and islet cells in rats with diabetes[50]. Neurogenin-3 (Ngn3) is a marker of new endocrine progenitor β cells[51]. A previous study reported that mangiferin increased the expression of Ngn3, FoxO-1, and PDX after partial pancreatectomy in mice and contributed to the proliferation of β cells. Mangiferin can also regulate the cell cycle through the activation of p16INK4a and promote islet regeneration in rats[52,53].
Terpenoids: Geniposide is widely found in herbs. It exhibits anti-inflammatory, antioxidative, and antidiabetic effects[54]. T cell transcription factor 7-like 2 (TCF7L2) is a key factor involved in the Wnt/β-catenin pathway, which is an important regulator of β cell survival and regeneration. Geniposide reportedly increases the expression of TCF7L2 by activating Wnt signaling[55]. Furthermore, it inhibits GSK3β activity as well as promotes the nuclear translocation of β-catenin and regeneration of β cells. Geniposide can also induce ductal cell differentiation by upregulating TCF7L2 expression and activating the JAK2/STAT3 pathway. Thus, it can promote β cell survival and regeneration by activating β-catenin/TCF7L2 signaling[56].
Astragalus belongs to the legume family and possesses many pharmacological properties, including antidiabetic, antioxidative, anti-inflammatory, and antiapoptotic effects[57]. A previous study reported that Astragalus strengthens the structure of pancreatic islet cells; the researchers also reported the appearance of new pancreatic islet cells and abundant capillaries around the islets, which promote β cell regeneration in HDF/STZ-induced Wistar rats with diabetes[58].
Quinones: Thymoquinone is the most abundant constituent in the volatile oil of Nigella sativa seeds. It exerts antioxidative, anti-inflammatory, and immunomodulatory effects[59]. In rats with diabetes, treatment with thymoquinone can efficiently ameliorate the histomorphological deteriorations of pancreatic islets, replenish the mass of β cells; and restore the function of β cells[60]. It has also been shown that thymoquinone inhibits COX-2 activity, relieves lipid peroxidation, and enhances antioxidative enzyme activity, thereby protecting pancreatic β-cells[61].
β cell apoptosis is a common pathological feature of T2DM. Mass production of superoxide ions and endoplasmic reticulum stress caused by high concentrations of free fatty acids lead to β cell apoptosis and dysfunction. Furthermore, the impaired balance between oxidation and antioxidation promotes β cell apoptosis and dysfunction[62,63]. Excessive production of reactive oxygen species (ROS) and reactive nitrogen species induces IR and chronic inflammation through abnormal changes in intracellular signaling pathways[64]. Inflammation also promotes β cell apoptosis and dysfunction[64,65].
Flavonoids: The decrease in mitochondrial membrane potential is an early indicator of apoptosis[66]. Previous studies have reported that quercetin reverses the decrease in mitochondrial membrane potential, inhibits the activation of caspase-3, caspase-9, and caspase-12, and increases the Bcl-2/Bax ratio, thereby suppressing apoptosis[38,67]. Quercetin also protects islet β cells from oxidation-induced apoptosis via SIRT3. After treating INS-1 cells and mice with diabetes were treated with quercetin, superoxide dismutase 2 and SIRT3 proteins levels increased, whereas the cleaved caspase-3 levels and Bax/Bcl-2 ratio decreased, along with reduced blood glucose levels and elevated insulin levels[68].
According to a previous study, cyanidin-3-glucoside decreased the apoptotic rate, intracellular ROS generation, and caspase-3 activity as well as reduced MAPK phosphorylation in MIN-6 cells treated with high levels of glucose[69]. The same results were observed in MIN-6 cells treated with H2O2[70]. A previous study revealed that anthocyanins protected the pancreatic tissue from STZ-induced apoptosis by regulating the levels of caspase-3, Bax, and Bcl-2 proteins in rats with diabetes[71].
Kaempferol is a flavanol compound found in various Chinese medicinal herbs[72]. It has been reported that kaempferol protects β cells and human islets from palmitate-induced apoptosis via the upregulation of the PDX-1/cAMP/PKA/CREB signaling cascade[73], increases the expression of Bcl-2 via CREB to activate the PI3K/Akt pathway, maintaining β-cell survival under high-glucose conditions, and reduces the expression of caspase-3[74].
Icariin is the main active ingredient of the natural medicine epimedium. It is considered a potential therapeutic agent for various diseases and is known to exert antioxidative, antineuroinflammatory, and antiapoptotic effects[75]. Icariin reportedly increases GLUT4 mRNA expression and promotes AMP-activated protein kinase (AMPK) phosphorylation to reduce the loss of islets in the pancreatic tissue[76].
Puerarin promotes the proliferation and reduces the apoptosis of pancreatic β-cells. It also reverses the effect of impaired glucose tolerance[41,42]. Isoflavone glycosides (the main component of puerarin) inhibit apoptosis and protect β cells via Akt phosphorylation[43].
Polyphenols: Resveratrol reportedly alleviates uric acid-induced apoptosis, reduces the expression of Bax, cleaved-caspase-3, and iNOS, and activates the PI3K/Akt pathway by upregulating the expression of miR-126[77]. A previous study demonstrated that ROS overproduction affected cell apoptosis by destroying the mitochondrial membranes, releasing cytochrome C, and stabilizing HIF-1 and p53[78]. Previous research has also revealed that resveratrol inhibited the production of ROS and HIF-1α[79]. Another study showed that the PI3K/Akt pathway reduced ROS production and inhibited p53 expression and pancreatic islet cell apoptosis[80].
Curcumin possesses antiapoptotic activity and improves the function of pancreatic islets. On the one hand, it interferes with the interaction among Beclin1, Bcl-2, and Bim through the signal pathway mediated by JNK-1 and AMPK, thereby regulating the transition between apoptosis and autophagy[81,82]. On the other hand, it decreases palmitate-induced oxidative stress in pancreatic islet cells by regulating the NADPH pathway, increases insulin levels, reduces the expression of cleaved caspase-3 and Bax, and protects cells from apoptosis[83].
Alkaloids: In a previous study, overexpression of independent phospholipase A2β and treatment with berberine significantly attenuated palmitate-induced apoptosis. Furthermore, silencing independent phospholipase A2β partially abolished the antiapoptotic effect of berberine and inhibited cardiolipin/Opa1 signaling in MIN6 cells[84]. In another study, coffee ingestion protected β cells from STZ cytotoxicity, suppressed hyperglycemia, inhibited β cells apoptosis, and maintained the pancreatic insulin content by inhibiting the activity of poly ADP ribose polymerase[85]. Based on a previous research, caffeic acid, naringin and quercetin increased the expression of GLUT2, Ins1, β2, Pdx1, Akt1, Bcl2 and Hsp70/90, reduced the expression of caspase-3 and Bax, and inhibited apoptosis of INS-1E cells[86].
Terpenoids: Mangostin reduces ROS, p38, and JNK phosphorylation, restores the impaired secretory function of pancreatic β cells, and exerts its antiapoptotic effect on STZ-induced INS-1 cells[87]. Geniposide inhibits the apoptosis of INS-1 cells induced by high levels of glucose, thereby preventing caspase-3 cleavage. Further research demonstrated that AMPK siRNA attenuated the effects of geniposide on apoptosis-associated proteins and cell viability, suggesting that AMPK plays a key role in protecting β cells from high-glucose-induced apoptosis[88]. According to a previous study, pretreatment with licorice extract inhibited the expression of caspase-3, caspase-8, caspase-9, and other apoptotic factors as well as the expression of p-STAT1, thereby hindering STZ-induced β cell apoptosis[89].
Paeoniflorin is a glycoside extracted from the root of Paeonia lactiflora Pall. It inhibits the activation of the p38MAPK and JNK signaling pathway and reduces the phosphorylation of p38MAPK and ERK1/2 by increasing the expression of Bcl-2 and inhibiting the expression of Bax and caspase-3. It also increases the survival rate of STZ-induced INS-1 cells[90].
Adipose tissue is an important endocrine organ that regulates insulin sensitivity and energy homeostasis throughout the body. It can secrete various hormones such as adiponectin, leptin, resistin, and visfatin as well as typical cytokines such as TNF-α and IL-6. It can also activate the MAPK and NF-κβ pathways[10,91]. Adipose tissue inflammation is a mechanistic pathogenesis of T2DM. Fat-infiltrated macrophages, basophils, and regulatory T cells cooperate with adipocytes to mediate adipose tissue inflammation by secreting proinflammatory factors[92]. Activation of monocytes and release of MCP-1 cause the transformation of white fat cells into the proinflammatory phenotype[93]. MCP-1 recruits macrophages into adipose tissue, which in turn produce inflammatory cytokines. PPARα/γ agonists also reduce the expression of IL-6, CXC-L10, and MCP-1 in human adipocytes[94].
Butein is isolated from the bark of the sumac tree. It exerts antioxidative, anti-inflammatory, antidiabetic, and neuroprotective effects[95]. It has been reported that pretreatment with butea results in the complete blockade of TNF-α-induced IκB-α degradation, prevents p65 phosphorylation at Ser311 and Ser536, and inhibits ERK, JNK, and p38MAPK phosphorylation in 3T3-L1 adipocytes[96]. These results are consistent with the previous findings, indicating that butein suppresses the expression of IL-6, TNF-α, and MCP-1, increases the expression of HO-1, and activates the p38MAPK/Nrf2/HO-1 pathway in the epididymal white adipose tissue of HFD-fed mice[97]. These findings suggest that butein plays an anti-inflammatory role in adipocytes in vitro and in vivo.
Naringin possesses strong antioxidative activity. Previous studies have demonstrated that naringin suppresses TNF-α–induced activation of NF-κB and ERK pathways in 3T3-L1 adipocytes[98]. Naringenin presumably exerts an anti-inflammatory effect by inhibiting IκB-α degradation and p-JNK expression, thereby inhibiting the expression of TLR2 in TNF-α induced adipocytes[99]. It was found to suppress macrophage infiltration into the adipose tissue by inhibiting MCP-1 production[100]. A recent study demonstrated that naringenin suppresses neutrophil infiltration into the adipose tissue by regulating MCP-3 expression and macrophage infiltration[101].
SIRT1 activators suppress inflammatory responses by promoting p65 deacetylation and inhibiting NF-κB activity in adipocytes[102]. Quercetin increases antioxidative activity as well as p-AMPK and SIRT1 expression in the adipose tissue of HFD-fed mice. Moreover, it reduces proinflammatory enzymatic activity and cytokine levels[103].
Cranberry contains various types of bioactive components with high antioxidative and anti-inflammatory potentials. It also exerts beneficial effects on adipogenesis and lipid metabolism in vitro[104]. Cranberries reportedly reduce lipid accumulation during adipocyte differentiation by decreasing the levels of acid-binding protein, lipoprotein lipase, fatty acid synthase, and perilipin 1[105]. In addition, they reduce H2O2-induced inflammation in 3T3-L1 cells by decreasing the expression of IL-6, PAI-1, MCP-1, and leptin in adipose tissue[106].
Peanut skin extract is a rich source of polyphenols[107]. It is effective in the treatment of various diseases, such as DM, obesity, and inflammation[108-110]. A previous study reported that peanut skin extracts significantly alleviate adipose tissue inflammation by reducing the expression of TNF-α, IL-1β, IL-6, and PAI-1[109].
According to another study, the combined use of curcumin and resveratrol inhibited the activation of NF-κB, decreased the expression of IL-1β, TNF-α, IL-6, and COX2, and reduced the damage induced by chronic inflammation in adipocytes[111]. Based on a previous study, luteolin increases the expression of p-AMPK and SIRT1, suppresses the expression of p-p65, and decreases the mRNA expression of TNF-α, IL-6, and MCP-1 in 3T3-L1 cells[112]. Studies have shown that SIRT1 inhibits NF-κB activation[113], and AMPK antagonizes inflammation through SIRT1[114].
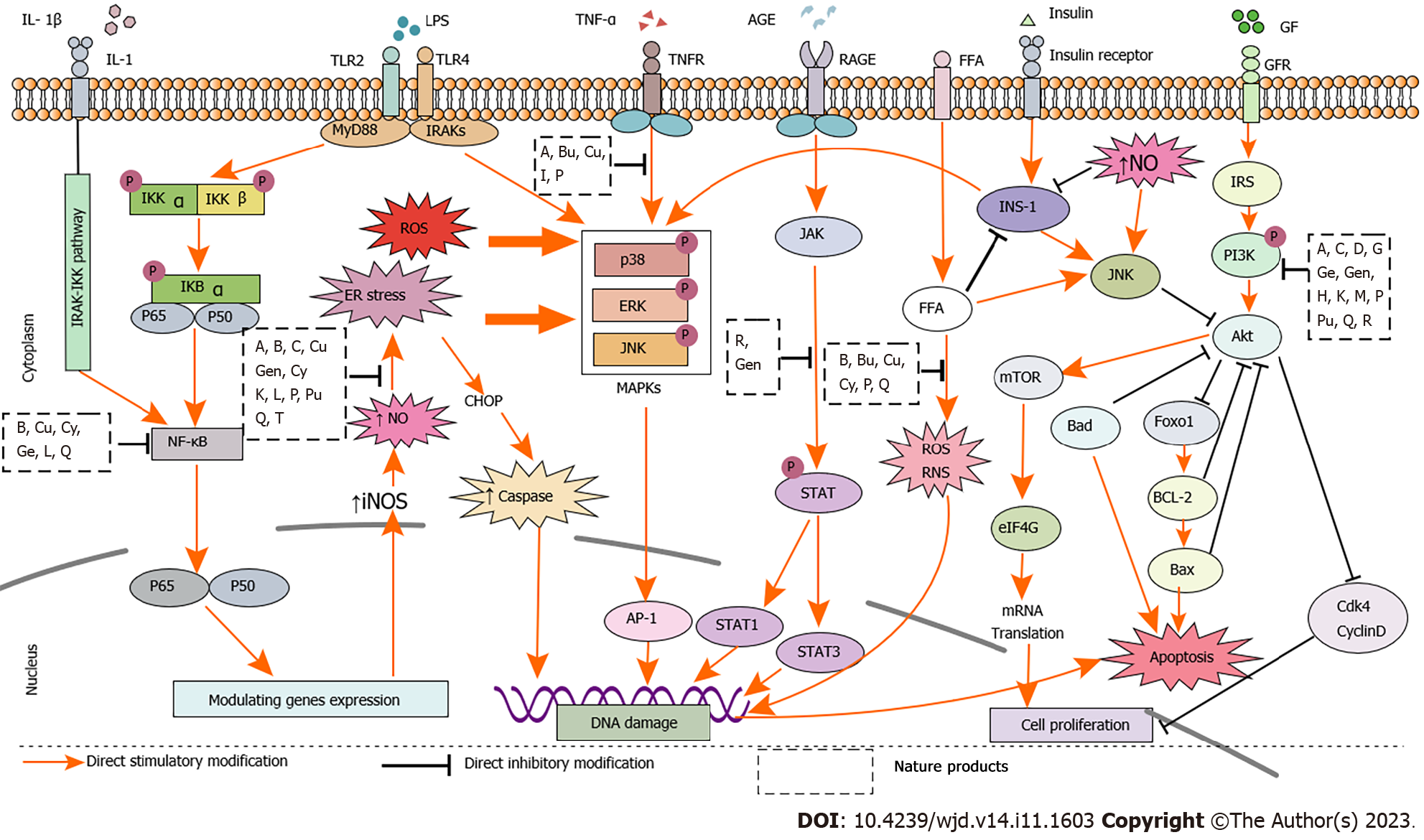
IR usually refers to the reduction in insulin-induced glucose uptake and utilization efficiency in the muscle, body fat, and liver, leading to compensatory INS, which ultimately results in a series of clinical manifestations such as hyperglycemia, hyperinsulinemia, and dyslipidemia[115,116]. A previous study reported that lipid accumulation in the liver and adipose tissue accelerated IR in patients with T2DM[117]. Inflammatory factors such as TNF-α and IL-6 activate the NF-κB pathway and inhibit the expression of IRS-1 and GLUT4, thereby promoting IR[118,119]. IL-1β also inhibits the IRS-1 pathway and promotes IR[120]. In general, IR is related to the NF-κB, JNK, p38MAPK, and PI3K/Akt pathways. When the energy intake is high, the activation of the PI3K/Akt pathway can alleviate obesity and IR[121](Figure 2).
Anthocyanins reportedly improve INS and IR[122]. A previous study showed that mulberry anthocyanin extract activates the PI3K/Akt pathway, increases the phosphorylation of its downstream target GSK3β, activates GYS2, and alleviates IR in HepG2 cells induced by high levels of glucose and palmitic acid. According to in vivo experiments, mulberry anthocyanin extract reduces the secretion of leptin and insulin and increases the levels of adiponectin in the serum, thereby improving IR[123].
According to a previous study, baicalein reduced the expression of TNF-α and F4/80, activated AMPK, p-AKT, and IRS-1, and induced dephosphorylation of ERK, NF-κB and JNK, thereby reducing IR[124]. A study by Pu et al[125] confirmed that the inhibitory effect of baicalein on IR was mediated by the inhibition of the MAPK pathway and activation of the IRS1/PI3K/Akt pathway.
Naringin possesses strong antioxidative activity. It reportedly increases the expression of GLUT4, adiponectin, and Ch-REBPβ in white adipocytes, promotes energy consumption and insulin sensitivity, and inhibits the proliferation of fat cells[126].
Gallic acid increases the expression of PPAR-γ in the adipose tissue, liver, and skeletal muscle, enhances tyrosine kinas activity, promotes IRS phosphorylation, and improves insulin-dependent glucose transport through GLUT4 in the PI3K/p-Akt dependent pathway in the adipose tissue, thereby improving IR in rats[127]. Adiponectin plays an important role in regulating insulin function as well as the occurrence and development of T2DM[128]. Gallic acid reduces the levels of serum total cholesterol and triglycerides by inhibiting adipogenesis and increasing adiponectin activity. The combined use of gallic acid and p-coumaric acid increases the levels and mRNA expression of PPAR-γ and reduces the levels of serum adiponectin in STZ-induced rats with diabetes[23].
Luteolin reportedly reduces blood lipid and glucose and improves hyperinsulinemia and IR through PPAR-γ[129]. It increases the absorption of circulating free fatty acids and reduces liver fat toxicity by increasing the protein expression of PPARγ in the adipose tissue[130]. In HFD-fed mice, luteolin reduces lipid formation, increases fatty acid oxidation, and significantly reduces the levels of IL-1, IL-6, and PAI-1, thereby improving obesity and metabolic disorders[131].
HFD-induced IR in mouse visceral adipose tissue is characterized by increased p-ERK and decreased p-Akt expression. The therapeutic effect of the Dioscorea batatas extract decreased the protein expression of p-ERK and p-S6K1 and enhanced the translocation of GLUT4 to the plasma membrane of the visceral adipose tissue in mice. It has been speculated that the Dioscorea batatas extract attenuates IR by upregulating the expression of GLUT4 in the plasma membrane of the visceral adipose tissue in HFD-fed mice[132]. The discoloration mixture of Astragalus membranaceus and Potentilla anserina reportedly increases the mRNA expression of PPARγ and PI3K in the liver, reduces FPG levels, and improves IR in mice[133].
To date, only a few clinical studies have been reported on natural medicines for treating DM. Most previous studies have focused on the addition of natural medicines to the diet to examine their effects on blood glucose levels, blood lipid levels, and body mass index in patients with T2DM. The addition of soluble fibers from psyllium to the normal diet of patients with T2DM significantly improved the levels of fasting blood sugar, hemoglobin A1c, C-peptide, Homeostasis Model Assessment-IR, and Homeostasis Model Assessment-B after 8 wk of administration[134]. A study by Noureddin et al[135] also showed that psyllium supplementation decreased the body weight, blood glucose levels, and cholesterol levels and increased the high-density lipoprotein cholesterol levels in patients with T2DM. Similar results were reported in other clinical trials[136,137].
A previous study showed that dietary raspberries significantly reduced serum glucose levels at 2 h and 4 h after intake and decreased the serum levels of IL-6 and TNF-α[138]. These results indicated that propolis increased the serum activity of superoxide dismutase and GPx, decreased the levels of fasting blood sugar, 2-h postprandial glucose and insulin, and alleviated IR[139]. In a previous study, based on the results of the area under the curve, the consumption of bitter melon for 3 mo increased INS and decreased the body weight, body mass index, and glucose in patients with T2DM, possibly by increasing uncoupling protein expression or inhibiting PPAR-γ[140].
These results indicated that quercetin intake was inversely correlated with T2DM prevalence in the Chinese population. Moreover, quercetin intake reduced pancreatic β-cell inflammation, thus successfully treating T2DM[141].
Accumulating studies including clinical trials and animal experiments have confirmed the effectiveness of natural products. In vivo and in vitro studies have demonstrated that the active ingredients of monomeric compounds, such as flavonoids, polyphenols, alkaloids, terpenes, and quinones in natural medicines can inhibit the release of inflammatory mediators and reduce oxidative stress. Thus, reduction in IR and lipid accumulation can protect islet cells and treat T2DM. The mechanisms by which natural medicines treat T2DM include the following: (1) β cell inflammation was mainly inhibited by IKK/IκB/NF-κB, PI3K/Akt, and SIRT1/NF-κB pathways; (2) β cell regeneration was mainly promoted via ERK1/2/MDA, PI3K/Akt/mTOR, Wnt/β-catenin, and JAK2/STAT3/Ngn3 pathways; (3) β cell apoptosis was inhibited through MAPK/caspase-3, PI3K/Akt/caspase-3, and SIRT1/HIF-1/P53 pathways; (4) Adipose tissue inflammation was attenuated by PPAR-γ/SREBP, TGF-β/STAT3/Smad2/3, P38MAPK/Nrf2/HO-1, JNK/MCP-1, and AMPK/SIRT1 pathways; and (5) IR was alleviated mainly through IRS1/PI3K/Akt, TGF-β/Smad, LKB1/AMPK/PGC1α, and mTOR/S6K1 pathways (Figure 3).
Commonly used drugs for treating T2DM, such as α-glutaminase inhibitors, sulfonylureas, biguanides, and glitalactone, can be used alone or in combination to regulate blood glucose levels. However, the multiple side effects and high cost of these drugs have led to the urgent need to explore natural medicines to treat T2DM. In recent years, an increasing number of studies have explored various effective active ingredients of natural medicines for treating T2DM to discover a new alternative medicine. The plants and their main components reported in this review can alleviate the effects of T2DM on the body to a certain extent and provide a theoretical basis for the development of new drugs. Further studies in the following areas are still warranted: (1) The potential toxicity of natural medicines and the interactions between drug compatibilities remain unclear. Common adverse effects associated with the intake of natural medicines include gastrointestinal disturbances such as abdominal pain, diarrhea, constipation, nausea, and vomiting[142-145]. However, more severe toxicities may occur and affect patients’ cardiovascular systems, auditory functions, or reproductive health[146,147]. Furthermore, the concomitant use of natural medicines and established antidiabetic drugs may increase the risk of hypoglycemia in patients with T2DM[148]. Thus, further studies are warranted on the specific mechanism of action and long-term toxic side effects of these natural products; and (2) Although some natural products have shown positive effects in cell and animal models, their activities have not yet been verified. Thus, further clinical studies are warranted to confirm the efficacy of natural medicines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balbaa ME, Egypt; Papazafiropoulou A, Greece; Lu Cai, United States S-Editor: Lin C L-Editor: Filipodia P-Editor: Yu HG
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1958] [Cited by in F6Publishing: 2289] [Article Influence: 1144.5] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Tao M, Chen C, Zhao X, Feng Q, Chen G, Fu Y. BAFF Blockade Attenuates DSS-Induced Chronic Colitis via Inhibiting NLRP3 Inflammasome and NF-κB Activation. Front Immunol. 2022;13:783254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2249] [Cited by in F6Publishing: 2633] [Article Influence: 438.8] [Reference Citation Analysis (0)] |
| 4. | Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 289] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 5. | Xu L, Li Y, Dai Y, Peng J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol Res. 2018;130:451-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 6. | Aguayo-Mazzucato C, van Haaren M, Mruk M, Lee TB Jr, Crawford C, Hollister-Lock J, Sullivan BA, Johnson JW, Ebrahimi A, Dreyfuss JM, Van Deursen J, Weir GC, Bonner-Weir S. β Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance. Cell Metab. 2017;25:898-910.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Gerber PA, Rutter GA. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid Redox Signal. 2017;26:501-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 8. | Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab. 2012;23:477-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Wu J, Shi S, Wang H, Wang S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr Polym. 2016;144:474-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 2020;16:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 11. | Sato S, Mukai Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J Inflamm Res. 2020;13:421-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Chen S, Jiang H, Wu X, Fang J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflamm. 2016;2016:9340637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Abdelkader NF, Eitah HE, Maklad YA, Gamaleldin AA, Badawi MA, Kenawy SA. New combination therapy of gliclazide and quercetin for protection against STZ-induced diabetic rats. Life Sci. 2020;247:117458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24:1777-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 311] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 15. | Elshazly SM, Abd El Motteleb DM, Ibrahim IAAE. Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats. Chem Biol Interact. 2018;291:153-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 17. | Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 18. | Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Zachariah B. Curcumin prevents inflammatory response, oxidative stress and insulin resistance in high fructose fed male Wistar rats: Potential role of serine kinases. Chem Biol Interact. 2016;244:187-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Choubey S, Goyal S, Varughese LR, Kumar V, Sharma AK, Beniwal V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini Rev Med Chem. 2018;18:1283-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Pei K, Ou J, Huang J, Ou S. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 2016;96:2952-2962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 314] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 22. | Cieślak M, Wojtczak A, Cieślak M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim Pol. 2015;62:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Abdel-Moneim A, El-Twab SMA, Yousef AI, Reheim ESA, Ashour MB. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and p-coumaric acid: The role of adipocytokines and PPARγ. Biomed Pharmacother. 2018;105:1091-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 25. | Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181-187. [PubMed] [Cited in This Article: ] |
| 26. | Malaguarnera L. Influence of Resveratrol on the Immune Response. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 27. | Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front Endocrinol (Lausanne). 2019;10:187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 28. | Zheng X, Zhu S, Chang S, Cao Y, Dong J, Li J, Long R, Zhou Y. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur J Pharmacol. 2013;147-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Cao MM, Lu X, Liu GD, Su Y, Li YB, Zhou J. Resveratrol attenuates type 2 diabetes mellitus by mediating mitochondrial biogenesis and lipid metabolism via Sirtuin type 1. Exp Ther Med. 2018;15:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Ahmed QU, Ali AHM, Mukhtar S, Alsharif MA, Parveen H, Sabere ASM, Nawi MSM, Khatib A, Siddiqui MJ, Umar A, Alhassan AM. Medicinal Potential of Isoflavonoids: Polyphenols That May Cure Diabetes. Molecules. 2020;25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Yousefi H, Alihemmati A, Karimi P, Alipour MR, Habibi P, Ahmadiasl N. Effect of genistein on expression of pancreatic SIRT1, inflammatory cytokines and histological changes in ovariectomized diabetic rat. Iran J Basic Med Sci. 2017;20:423-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 32. | Zhao L, Li C, Zhang Y, Wen Q, Ren D. Phytochemical and biological activities of an anticancer plant medicine: Brucea javanica. Anticancer Agents Med Chem. 2014;14:440-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Ablat A, Halabi MF, Mohamad J, Hasnan MH, Hazni H, Teh SH, Shilpi JA, Mohamed Z, Awang K. Antidiabetic effects of Brucea javanica seeds in type 2 diabetic rats. BMC Complement Altern Med. 2017;17:94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018;27:57-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 35. | Li JM, Wang W, Fan CY, Wang MX, Zhang X, Hu QH, Kong LD. Quercetin Preserves β -Cell Mass and Function in Fructose-Induced Hyperinsulinemia through Modulating Pancreatic Akt/FoxO1 Activation. Evid Based Complement Alternat Med. 2013;2013:303902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005;51:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 488] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 37. | Oyedemi SO, Nwaogu G, Chukwuma CI, Adeyemi OT, Matsabisa MG, Swain SS, Aiyegoro OA. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: In silico studies of molecular interaction of quercetin with hexokinase and catalase. J Food Biochem. 2020;44:e13127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Zhuang M, Qiu H, Li P, Hu L, Wang Y, Rao L. Islet protection and amelioration of type 2 diabetes mellitus by treatment with quercetin from the flowers of Edgeworthia gardneri. Drug Des Devel Ther. 2018;12:955-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 388] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 41. | Chen X, Yu J, Shi J. Management of Diabetes Mellitus with Puerarin, a Natural Isoflavone From Pueraria lobata. Am J Chin Med. 2018;46:1771-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 42. | Yang L, Yao D, Yang H, Wei Y, Peng Y, Ding Y, Shu L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol Endocrinol. 2016;30:361-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Li Z, Shangguan Z, Liu Y, Wang J, Li X, Yang S, Liu S. Puerarin protects pancreatic β-cell survival via PI3K/Akt signaling pathway. J Mol Endocrinol. 2014;53:71-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Yende SR, Harle UN, Chaugule BB. Therapeutic potential and health benefits of Sargassum species. Pharmacogn Rev. 2014;8:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Motshakeri M, Ebrahimi M, Goh YM, Othman HH, Hair-Bejo M, Mohamed S. Effects of Brown Seaweed (Sargassum polycystum) Extracts on Kidney, Liver, and Pancreas of Type 2 Diabetic Rat Model. Evid Based Complement Alternat Med. 2014;2014:379407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Akbarzadeh S, Gholampour H, Farzadinia P, Daneshi A, Ramavandi B, Moazzeni A, Keshavarz M, Bargahi A. Anti-diabetic effects of Sargassum oligocystum on Streptozotocin-induced diabetic rat. Iran J Basic Med Sci. 2018;21:342-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 47. | Fu Z, Gilbert ER, Pfeiffer L, Zhang Y, Fu Y, Liu D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl Physiol Nutr Metab. 2012;37:480-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes. 2002;51:966-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Yousefi H, Karimi P, Alihemmati A, Alipour MR, Habibi P, Ahmadiasl N. Therapeutic potential of genistein in ovariectomy-induced pancreatic injury in diabetic rats: The regulation of MAPK pathway and apoptosis. Iran J Basic Med Sci. 2017;20:1009-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 50. | Aswal S, Kumar A, Chauhan A, Semwal RB, Semwal DK. A Molecular Approach on the Protective Effects of Mangiferin Against Diabetes and Diabetes-related Complications. Curr Diabetes Rev. 2020;16:690-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Cheng CW, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, Cohen P, Sneddon JB, Perin L, Longo VD. Fasting-Mimicking Diet Promotes Ngn3-Driven β-Cell Regeneration to Reverse Diabetes. Cell. 2017;168:775-788.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 52. | Wang HL, Li CY, Zhang B, Liu YD, Lu BM, Shi Z, An N, Zhao LK, Zhang JJ, Bao JK, Wang Y. Mangiferin facilitates islet regeneration and β-cell proliferation through upregulation of cell cycle and β-cell regeneration regulators. Int J Mol Sci. 2014;15:9016-9035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Wang H, He X, Lei T, Liu Y, Huai G, Sun M, Deng S, Yang H, Tong R, Wang Y. Mangiferin induces islet regeneration in aged mice through regulating p16INK4a. Int J Mol Med. 2018;41:3231-3242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Zhou YX, Zhang RQ, Rahman K, Cao ZX, Zhang H, Peng C. Diverse Pharmacological Activities and Potential Medicinal Benefits of Geniposide. Evid Based Complement Alternat Med. 2019;2019:4925682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci U S A. 2007;104:6247-6252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 56. | Yao DD, Yang L, Wang Y, Liu C, Wei YJ, Jia XB, Yin W, Shu L. Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway. Cell Death Dis. 2015;6:e1746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol. 2020;87:89-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 153] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 58. | Li J, Huang Y, Zhao S, Guo Q, Zhou J, Han W, Xu Y. Based on network pharmacology to explore the molecular mechanisms of astragalus membranaceus for treating T2 diabetes mellitus. Ann Transl Med. 2019;7:633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Darakhshan S, Bidmeshki Pour A, Hosseinzadeh Colagar A, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95-96:138-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 60. | El-Shemi AG, Kensara OA, Alsaegh A, Mukhtar MH. Pharmacotherapy with Thymoquinone Improved Pancreatic β-Cell Integrity and Functional Activity, Enhanced Islets Revascularization, and Alleviated Metabolic and Hepato-Renal Disturbances in Streptozotocin-Induced Diabetes in Rats. Pharmacology. 2018;101:9-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Al Wafai RJ. Nigella sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas. 2013;42:841-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Boyd A, Byrne S, Middleton RJ, Banati RB, Liu GJ. Control of Neuroinflammation through Radiation-Induced Microglial Changes. Cells. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Hansen JB, Dos Santos LRB, Liu Y, Prentice KJ, Teudt F, Tonnesen M, Jonas JC, Wheeler MB, Mandrup-Poulsen T. Glucolipotoxic conditions induce β-cell iron import, cytosolic ROS formation and apoptosis. J Mol Endocrinol. 2018;61:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 65. | Lei L, Lin Z, Wang L, Zhao D, Wang Y, Li F. The dynamics mechanism of islet inflammation during type 2 diabetes progress. Chinese Science Bulletin. 2020;65:4139. [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 66. | Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia M, Misso G. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol. 2020;98:139-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 254] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 67. | Kim SS, Jang HJ, Oh MY. Quercetin Enhances the Function and Reduces Apoptosis of Mouse Islets. Transplant Proc. 2019;51:1451-1457. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 68. | Wang JY, Nie YX, Dong BZ, Cai ZC, Zeng XK, Du L, Zhu X, Yin XX. Quercetin protects islet β-cells from oxidation-induced apoptosis via Sirt3 in T2DM. Iran J Basic Med Sci. 2021;24:629-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 69. | Lee JS, Kim YR, Park JM, Kim YE, Baek NI, Hong EK. Cyanidin-3-glucoside isolated from mulberry fruits protects pancreatic β-cells against glucotoxicity-induced apoptosis. Mol Med Rep. 2015;11:2723-2728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Lee JS, Kim YR, Song IG, Ha SJ, Kim YE, Baek NI, Hong EK. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int J Mol Med. 2015;35:405-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Nizamutdinova IT, Jin YC, Chung JI, Shin SC, Lee SJ, Seo HG, Lee JH, Chang KC, Kim HJ. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res. 2009;53:1419-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 72. | Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, Ahmad B, Bawazeer S, Atif M, Peters DG, Mubarak MS. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother Res. 2019;33:263-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 73. | Zhang Y, Zhen W, Maechler P, Liu D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. J Nutr Biochem. 2013;24:638-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670:325-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 75. | He C, Wang Z, Shi J. Pharmacological effects of icariin. Adv Pharmacol. 2020;87:179-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 76. | Li X, Wang YX, Shi P, Liu YP, Li T, Liu SQ, Wang CJ, Wang LX, Cao Y. Icariin treatment reduces blood glucose levels in type 2 diabetic rats and protects pancreatic function. Exp Ther Med. 2020;19:2690-2696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Xin Y, Zhang H, Jia Z, Ding X, Sun Y, Wang Q, Xu T. Resveratrol improves uric acid-induced pancreatic β-cells injury and dysfunction through regulation of miR-126. Biomed Pharmacother. 2018;102:1120-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Rehman K, Akash MSH. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J Cell Biochem. 2017;118:3577-3585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 79. | Keshtkar S, Kaviani M, Jabbarpour Z, Al-Abdullah IH, Aghdaei MH, Nikeghbalian S, Shamsaeefar A, Geramizadeh B, Azarpira N, Ghahremani MH. Significant reduction of apoptosis induced via hypoxia and oxidative stress in isolated human islet by resveratrol. Nutr Metab Cardiovasc Dis. 2020;30:1216-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Li Y, Zhang T, Huang Q, Sun Y, Chang X, Zhang H, Zhu Y, Han X. Inhibition of tumor suppressor p53 preserves glycation-serum induced pancreatic beta-cell demise. Endocrine. 2016;54:383-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX, Liu XF, Chen X, Chen HG, Ke HY, Liu C. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J Mol Cell Cardiol. 2018;124:26-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 82. | Joshi T, Singh AK, Haratipour P, Sah AN, Pandey AK, Naseri R, Juyal V, Farzaei MH. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J Cell Physiol. 2019;234:17212-17231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 83. | Li J, Wu N, Chen X, Chen H, Yang X, Liu C. Curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets. 2019;11:152-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Li J, Du H, Zhang M, Zhang Z, Teng F, Zhao Y, Zhang W, Yu Y, Feng L, Cui X, Lu T, Guan F, Chen L. Amorphous solid dispersion of Berberine mitigates apoptosis via iPLA(2)β/Cardiolipin/Opa1 pathway in db/db mice and in Palmitate-treated MIN6 β-cells. Int J Biol Sci. 2019;15:1533-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Kobayashi M, Kurata T, Hamana Y, Hiramitsu M, Inoue T, Murai A, Horio F. Coffee Ingestion Suppresses Hyperglycemia in Streptozotocin-Induced Diabetic Mice. J Nutr Sci Vitaminol (Tokyo). 2017;63:200-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Bhattacharya S, Oksbjerg N, Young JF, Jeppesen PB. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes Metab. 2014;16:602-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 87. | Lee D, Kim YM, Jung K, Chin YW, Kang KS. Alpha-Mangostin Improves Insulin Secretion and Protects INS-1 Cells from Streptozotocin-Induced Damage. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Liu C, Hao Y, Yin F, Zhang Y, Liu J. Geniposide protects pancreatic β cells from high glucose-mediated injury by activation of AMP-activated protein kinase. Cell Biol Int. 2017;41:544-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Nopparat J, Nualla-Ong A, Phongdara A. Ethanolic extracts of Pluchea indica (L.) leaf pretreatment attenuates cytokine-induced β-cell apoptosis in multiple low-dose streptozotocin-induced diabetic mice. PLoS One. 2019;14:e0212133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Liu Y, Han J, Zhou Z, Li D. Paeoniflorin protects pancreatic β cells from STZ-induced damage through inhibition of the p38 MAPK and JNK signaling pathways. Eur J Pharmacol. 2019;853:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15:639-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 460] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 92. | Kuo CS, Chen JS, Lin LY, Schmid-Schönbein GW, Chien S, Huang PH, Chen JW, Lin SJ. Inhibition of Serine Protease Activity Protects Against High Fat Diet-Induced Inflammation and Insulin Resistance. Sci Rep. 2020;10:1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 93. | Nitta CF, Orlando RA. Crosstalk between immune cells and adipocytes requires both paracrine factors and cell contact to modify cytokine secretion. PLoS One. 2013;8:e77306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Massaro M, Scoditti E, Pellegrino M, Carluccio MA, Calabriso N, Wabitsch M, Storelli C, Wright M, De Caterina R. Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazar in attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol Res. 2016;107:125-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Padmavathi G, Roy NK, Bordoloi D, Arfuso F, Mishra S, Sethi G, Bishayee A, Kunnumakkara AB. Butein in health and disease: A comprehensive review. Phytomedicine. 2017;25:118-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 96. | Wang Z, Lee Y, Eun JS, Bae EJ. Inhibition of adipocyte inflammation and macrophage chemotaxis by butein. Eur J Pharmacol. 2014;738:40-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300:E877-E885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 98. | Yoshida H, Takamura N, Shuto T, Ogata K, Tokunaga J, Kawai K, Kai H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-alpha in mouse adipocytes. Biochem Biophys Res Commun. 2010;394:728-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Yoshida H, Watanabe W, Oomagari H, Tsuruta E, Shida M, Kurokawa M. Citrus flavonoid naringenin inhibits TLR2 expression in adipocytes. J Nutr Biochem. 2013;24:1276-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 100. | Yoshida H, Watanabe H, Ishida A, Watanabe W, Narumi K, Atsumi T, Sugita C, Kurokawa M. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochem Biophys Res Commun. 2014;454:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 101. | Tsuhako R, Yoshida H, Sugita C, Kurokawa M. Naringenin suppresses neutrophil infiltration into adipose tissue in high-fat diet-induced obese mice. J Nat Med. 2020;74:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S, Shulman GI. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235-3245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 103. | Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B, Liu J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1. J Lipid Res. 2014;55:363-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 104. | Zhao S, Liu H, Gu L. American cranberries and health benefits - an evolving story of 25 years. J Sci Food Agric. 2020;100:5111-5116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Kowalska K, Olejnik A, Rychlik J, Grajek W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015;185:383-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 106. | Kowalska K, Olejnik A. Cranberries (Oxycoccus quadripetalus) inhibit pro-inflammatory cytokine and chemokine expression in 3T3-L1 adipocytes. Food Chem. 2016;196:1137-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Bansode RR, Randolph P, Ahmedna M, Williams LL, Yu J. Bioavailability and hypolipidemic effects of peanut skin polyphenols. J Med Food. 2015;18:265-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Xiang L, Wu Q, Cheng L, Sun K, Li J, Yoshida M, Qi J. Leptin and Adiponectin Signaling Pathways Are Involved in the Antiobesity Effects of Peanut Skin Extract. Oxid Med Cell Longev. 2019;2019:2935315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Xiang L, Wu Q, Osada H, Yoshida M, Pan W, Qi J. Peanut skin extract ameliorates the symptoms of type 2 diabetes mellitus in mice by alleviating inflammation and maintaining gut microbiota homeostasis. Aging (Albany NY). 2020;12:13991-14018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 110. | Bansode RR, Randolph P, Hurley S, Ahmedna M. Evaluation of hypolipidemic effects of peanut skin-derived polyphenols in rats on Western-diet. Food Chem. 2012;135:1659-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr Metab (Lond). 2008;5:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 112. | Xiao N, Mei F, Sun Y, Pan G, Liu B, Liu K. Quercetin, luteolin, and epigallocatechin gallate promote glucose disposal in adipocytes with regulation of AMP-activated kinase and/or sirtuin 1 activity. Planta Med. 2014;80:993-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369-2380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1942] [Cited by in F6Publishing: 2062] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 114. | Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051-19059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 115. | Na HY, Lee BC. Scutellaria baicalensis Alleviates Insulin Resistance in Diet-Induced Obese Mice by Modulating Inflammation. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4273] [Cited by in F6Publishing: 4214] [Article Influence: 221.8] [Reference Citation Analysis (0)] |
| 117. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3146] [Cited by in F6Publishing: 3350] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 118. | Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 442] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 119. | Gratas-Delamarche A, Derbré F, Vincent S, Cillard J. Physical inactivity, insulin resistance, and the oxidative-inflammatory loop. Free Radic Res. 2014;48:93-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 120. | Aye IL, Jansson T, Powell TL. Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol Cell Endocrinol. 2013;381:46-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 753] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 122. | Belwal T, Nabavi SF, Nabavi SM, Habtemariam S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 123. | Yan F, Dai G, Zheng X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J Nutr Biochem. 2016;36:68-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 132] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 124. | Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 408] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 125. | Pu P, Wang XA, Salim M, Zhu LH, Wang L, Chen KJ, Xiao JF, Deng W, Shi HW, Jiang H, Li HL. Baicalein, a natural product, selectively activating AMPKα(2) and ameliorates metabolic disorder in diet-induced mice. Mol Cell Endocrinol. 2012;362:128-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 126. | Rebello CJ, Greenway FL, Lau FH, Lin Y, Stephens JM, Johnson WD, Coulter AA. Naringenin Promotes Thermogenic Gene Expression in Human White Adipose Tissue. Obesity (Silver Spring). 2019;27:103-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 127. | Gandhi GR, Jothi G, Antony PJ, Balakrishna K, Paulraj MG, Ignacimuthu S, Stalin A, Al-Dhabi NA. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. 2014;745:201-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 128. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1862] [Cited by in F6Publishing: 1912] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 129. | Xu N, Zhang L, Dong J, Zhang X, Chen YG, Bao B, Liu J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol Nutr Food Res. 2014;58:1258-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 130. | Kwon EY, Jung UJ, Park T, Yun JW, Choi MS. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015;64:1658-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 131. | Kwon EY, Kim SY, Choi MS. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 132. | Kim S, Jwa H, Yanagawa Y, Park T. Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. J Med Food. 2012;15:527-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Zhang DM, Lou LX, Wu AM, Lü XY, Hu ZJ, Zhang YH, Liu HF. [Effects of Astragalus membranaceus and Potentilla discolor mixture on insulin resistance and its related mRNA expressions in KKAy mice with type 2 diabetes]. Zhong Xi Yi Jie He Xue Bao. 2012;10:821-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 134. | Abutair AS, Naser IA, Hamed AT. Soluble fibers from psyllium improve glycemic response and body weight among diabetes type 2 patients (randomized control trial). Nutr J. 2016;15:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 135. | Noureddin S, Mohsen J, Payman A. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Complement Ther Med. 2018;40:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 136. | Gibb RD, McRorie JW Jr, Russell DA, Hasselblad V, D'Alessio DA. Psyllium fiber improves glycemic control proportional to loss of glycemic control: a meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am J Clin Nutr. 2015;102:1604-1614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 137. | Ziai SA, Larijani B, Akhoondzadeh S, Fakhrzadeh H, Dastpak A, Bandarian F, Rezai A, Badi HN, Emami T. Psyllium decreased serum glucose and glycosylated hemoglobin significantly in diabetic outpatients. J Ethnopharmacol. 2005;102:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | Schell J, Betts NM, Lyons TJ, Basu A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann Nutr Metab. 2019;74:165-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 139. | Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Haghighian HK. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement Ther Med. 2019;43:283-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 140. | Cortez-Navarrete M, Martínez-Abundis E, Pérez-Rubio KG, González-Ortiz M, Méndez-Del Villar M. Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J Med Food. 2018;21:672-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 141. | Yao Z, Gu Y, Zhang Q, Liu L, Meng G, Wu H, Xia Y, Bao X, Shi H, Sun S, Wang X, Zhou M, Jia Q, Wu Y, Song K, Gao W, Guo C, Niu K. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur J Nutr. 2019;58:819-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 142. | Hori Y, Fujisawa M, Shimada K, Oda A, Katsuyama S, Wada K. Rapid analysis of 4-O-methylpyridoxine in the serum of patients with Ginkgo biloba seed poisoning by ion-pair high-performance liquid chromatography. Biol Pharm Bull. 2004;27:486-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Wang X, Ma Y, Xu Q, Shikov AN, Pozharitskaya ON, Flisyuk EV, Liu M, Li H, Vargas-Murga L, Duez P. Flavonoids and saponins: What have we got or missed? Phytomedicine. 2023;109:154580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 144. | Kang MJ, Khanal T, Kim HG, Lee DH, Yeo HK, Lee YS, Ahn YT, Kim DH, Jeong HG, Jeong TC. Role of metabolism by human intestinal microflora in geniposide-induced toxicity in HepG2 cells. Arch Pharm Res. 2012;35:733-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 145. | Zhou Y, Liao Q, Lin M, Deng X, Zhang P, Yao M, Zhang L, Xie Z. Combination of ¹H NMR- and GC-MS-based metabonomics to study on the toxicity of Coptidis Rhizome in rats. PLoS One. 2014;9:e88281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 146. | Song J, He GN, Dai L. A comprehensive review on celastrol, triptolide and triptonide: Insights on their pharmacological activity, toxicity, combination therapy, new dosage form and novel drug delivery routes. Biomed Pharmacother. 2023;162:114705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 147. | Liu Y, Xin H, Zhang Y, Che F, Shen N, Cui Y. Leaves, seeds and exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, phytochemistry, pharmacology, resource utilization and toxicity. J Ethnopharmacol. 2022;298:115645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 148. | Raman A, Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine. 1996;2:349-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |