Published online May 14, 2024. doi: 10.3748/wjg.v30.i18.2379
Revised: March 15, 2024
Accepted: April 19, 2024
Published online: May 14, 2024
Transarterial radioembolization or selective internal radiation therapy (SIRT) has emerged as a minimally invasive approach for the treatment of tumors. This percutaneous technique involves the local, intra-arterial delivery of radioactive microspheres directly into the tumor. Historically employed as a palliative mea
Core Tip: Transarterial radioembolization (TARE), with the concept of radiation segmentectomy, is undergoing a paradigm shift in its role in treating primary liver malignancies, particularly hepatocellular carcinoma. This editorial delves into the emerging applications of TARE and explores the rationale, technical aspects, and future perspectives in the field of radiation segmentectomy.
- Citation: Inchingolo R, Cortese F, Pisani AR, Acquafredda F, Calbi R, Memeo R, Anagnostopoulos F, Spiliopoulos S. Selective internal radiation therapy segmentectomy: A new minimally invasive curative option for primary liver malignancies? World J Gastroenterol 2024; 30(18): 2379-2386
- URL: https://www.wjgnet.com/1007-9327/full/v30/i18/2379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i18.2379
Transarterial radioembolization (TARE), synonymous with selective internal radiation therapy (SIRT) and radioembolization, represents a minimally invasive modality for localized cancer therapy, entailing the percutaneous, intra-arterial administration of radioactive microspheres directly into the tumor vasculature[1]. Pioneering work by Ariel[2] in 1965 described a technique for the intrahepatic infusion of yttrium-90 (Y-90) bonded to ceramic microspheres, delivered via the celiac artery, in four patients with hepatocellular carcinoma (HCC), achieving symptomatic improvement. In 1968, Simon et al[3] proved the efficacy of Y-90, in treating five symptomatic patients with neuroendocrine liver metastases. Subsequent decades witnessed numerous studies investigating the use of TARE for both primary and secondary hepatic malignancies. These studies, employing both resin[4,5] and glass microspheres[6-8], demonstrated the efficacy and safety profile of TARE in selectively delivering high tumoricidal radiation doses while minimizing adverse effects. Data from these investigations supported regulatory clearances. The Food and Drug Administration granted a humanitarian device exemption for glass microspheres in 1999, in patients with unresectable HCC. Subsequently, in 2002, premarket approval was obtained for resin microspheres targeting colorectal cancer liver metastases[9]. Historically employed as a palliative intervention for hepatic malignancies, TARE has undergone a significant therapeutic transformation in the last decade. The advent of radiation segmentectomy (RS) has fundamentally reshaped the treatment paradigm for TARE, with the potential for reclassification as a curative locoregional modality.
Three commercially available microsphere types currently exist for therapeutic applications: Y90-resin microspheres (Sir-Spheres, Sirtex Medical Ltd., Sydney, Australia), Y90-glass microspheres (TheraSphere, Boston Scientific, Boston, MA, United States) and 166Ho-poly-L-lactic acid microspheres (QuiremSpheres, Quirem Medical B.V., Deventer, The Netherlands)[10].
Y-90 is the most common radioisotope used in TARE. This pure β-emitter exhibits a 64.2-h half-life, and approximately 90% of its β-rays are emitted within 7 d, with a maximum β-ray energy of 2.27 MeV and a mean of 0.93 MeV. Β-rays emitted from microspheres achieve a mean tissue penetration depth of 2.5 mm, with a maximum depth of 11 mm. The two commercially available Y-90 microsphere types exhibit distinct physical characteristics and Y-90 binding methods, leading to important clinical implications[11-14].
There is an important clinical impact about the use of microspheres, due to the difference in specific gravity (75 Bq for resin vs 2500 Bq for glass). To achieve a desired level of activity, a larger quantity of resin microspheres is required compared to glass spheres, resulting in a potentially greater embolic effect, which may be advantageous in certain clinical scenarios. On the other hand, glass microspheres might be the preferred choice, due to their lower embolic effect, when avoiding early blood stasis or reflux is critical. This could be particularly relevant in situations such as HCC with portal vein invasion or when planning for very high radiation dose administration[11].
All local intra-arterial therapies are based on the principle that liver tumors are preferentially supplied by the hepatic arteries due to neo-angiogenesis, whereas the liver parenchyma is mainly supplied by the portal vein[15]. Although TARE and transarterial chemoembolization (TACE) are similar in terms of the technical aspects of the procedure, as both require selective catheterization of the tumor-feeding vessels, there are substantial differences between the mechanisms of action[16]. In TACE, chemotherapeutic-loaded microparticles occlude tumor-feeding vessels, ensuring a stagnant flow and an ischemic microenvironment, maximizing exposure to cytotoxic drugs, and promoting ischemic necrosis. Conversely, TARE, leverages β-particles to generate free radicals in the presence of oxygen, inducing DNA damage to one or both DNA strands, ultimately leading to tumor cell apoptosis. Optimal tumor blood flow is therefore imperative for TARE. Furthermore, sufficient microsphere distribution throughout the tumor nodule is required to achieve maximum radiation effect and to avoid a low total radiation dose within the tumor[10,17]. Consequently, TARE procedures typically utilize smaller particles compared to those employed in TACE (25-35 μm vs 75-300 μm in diameter, respectively)[16].
TARE treatment is indicated for patients with HCC, metastatic liver lesions, and cholangiocarcinoma. Notably, the therapeutic role of TARE has evolved beyond its use as a primarily palliative intervention, with increasing application in recent years for curative or locoregional control[12,18]. The recent revision of the BCLC HCC treatment algorithm incorporates TARE as a curative treatment option in selected cases[19]. Of particular interest, for patients classified within BCLC stages 0 and A, TARE performed with the intent of achieving segmentectomy-like outcomes is considered therapeutically equivalent to established local ablative methods. This approach assumes particular importance in clinical scenarios where surgical resection or conventional ablative techniques are deemed unfeasible. Currently, TARE is recommended in single nodules < 8 cm, based on the results of the LEGACY study[19-21]. In large tumors, radiation lobectomy (RL) by TARE may be considered when future liver remnant (FLR) is small; moreover, TARE (as well as ablation and TACE) may be considered for bridging in liver transplant candidates with a waiting time of > 6 months. On the other hand, the BCLC 2022 update does not endorse TARE for patients classified as BCLC-B or BCLC-C, due to negative outcomes reported in phase III trials[20]. Nevertheless, encouraging data from various authors suggest potential benefits of TARE in patients with portal vein invasion, warranting further investigation to establish its role in this specific patient population[1].
The latest ESMO Clinical Practice Guidelines for Cholangiocarcinoma assert that intra-arterial therapies, such as TARE, may be considered in combination with chemotherapy to improve response and disease control in patients with liver-limited intrahepatic cholangiocarcinoma (iCCA)[22]. Several studies have shown encouraging results involving the use of RS in unresectable iCCA[23,24], but no randomized data are available to date. Ness and Molvar[25] stated that TARE has the potential to improve survival while exhibiting a favorable safety profile with minimal side effects, and downstage selected patients, allowing curative resection, especially when used in conjunction with chemotherapy.
TARE encompasses three primary techniques: Sequential lobar palliative radioembolization or lobar dosing, RL, and RS. The initial approach has been primarily performed with palliative intent in advanced-stage disease not amenable to surgical resection, transplantation, thermal ablation, or TACE. It is noteworthy that TARE, due to its microembolic effect, has historically been a viable treatment option for patients presenting with portal vein thrombosis, a contraindication for TACE[18]. RL involves the lobar administration of Y90, inducing atrophy of the treated liver lobe and compensatory hypertrophy of the untreated lobe, thereby potentially facilitating subsequent surgical resection in HCC patients with a limited FLR at presentation. The average lobar radiation dose for RL using glass microspheres generally ranges from 120 to 150 Gy[18,26,27].
RS, first described in 2011[28], represents a more selective approach that aims to deliver very high radiation doses within one to three liver segments. The primary objective is to achieve a high tumoricidal dose within the targeted tumor itself, while concurrently establishing an adequate zone of necrosis to eradicate potential adjacent satellite lesions[29]. The high radiation dose maximizes the cytotoxic effect, while the focused delivery minimizes the risk of collateral injury to the surrounding healthy liver parenchyma[18,29].
Since RS is based on high-dose delivery, most studies concern glass microspheres because they accommodate a higher specific activity with a concomitantly lower particle count, reducing the embolic effect and the risk of early stasis associated with resin microsphere (2500 Bq per glass microsphere vs 75 Bq per resin microsphere)[30]. Pathologic evaluation of explanted livers from patients undergoing liver transplantation following downstaging with RS provided compelling evidence for the efficacy of high-dose TARE[31]. A tumor-absorbed dose > 190 Gy was demonstrated to achieve a higher rate of complete pathologic necrosis (CPN) on liver explants, compared to a tumour absorbed dose < 190 Gy, resulting in longer time to progression and overall survival[32]. Moreover, Gabr et al[33] recently reported that a targeted dose of 400 Gy was well tolerated and resulted in CPN in all patients, compared to prior thresholds of 190 Gy (complete response of 100% vs 65% respectively). The extent of necrosis exhibited a positive correlation with the absorbed radiation dose, whereas tumor size did not appear to be a significant influencing factor[33].
RS is assuming an increasingly prominent role, particularly in HCC treatment, due to the continuously amassing published data, with a trajectory beginning from the first investigation in 2011[28], followed by the 2016 PREMIERE randomized trial[34], and culminating in the 2021 LEGACY milestone study[21], cited by the 2022 BCLC[19] and the latest RASER study, further supporting the ablative potential of RS[35]. The LEGACY study demonstrated the efficacy of Y90 radiation segmentectomy as a neoadjuvant strategy to liver transplantation, surgical resection, or as a standalone treatment for lesions up to 8 cm, supporting the use of a volume absorbed dose > 400 Gy as a “threshold” dose for an ablative effect with an objective response rate over 24 months of 88% and median response duration of 11.8 months[21]. Furthermore, results from the RASER study demonstrated 100% CPN rate following RS (mean delivered dose: 776.5 Gy) for HCC ≤ 3 cm[35].
RS may offer a potential curative approach for lesions not amenable to percutaneous ablation or surgical resection, due to anatomical constraints, significant comorbidities, or low functional liver reserve[36]. Unlike percutaneous therapies, RS has no risk of needle-track seeding, liver injury due to challenging anatomical locations, or development of arterioportal fistulas. While thermal ablation is generally accepted as a curative treatment option for lesions < 3 cm, RS demonstrates promise for achieving curative intent even in lesions up to 8 cm. Obviously, TARE carries a higher economic burden compared to ablation, and its efficacy may be limited in hypovascular tumors[36]. Compared to TACE, RS has been reported to be safer and more effective for patients with moderate liver dysfunction and advanced disease[37,38]. Recent important studies have investigated personalized and maximal dosing, transforming TARE to a local curative treatment comparable to surgical resection or percutaneous ablative options[21,33,39].
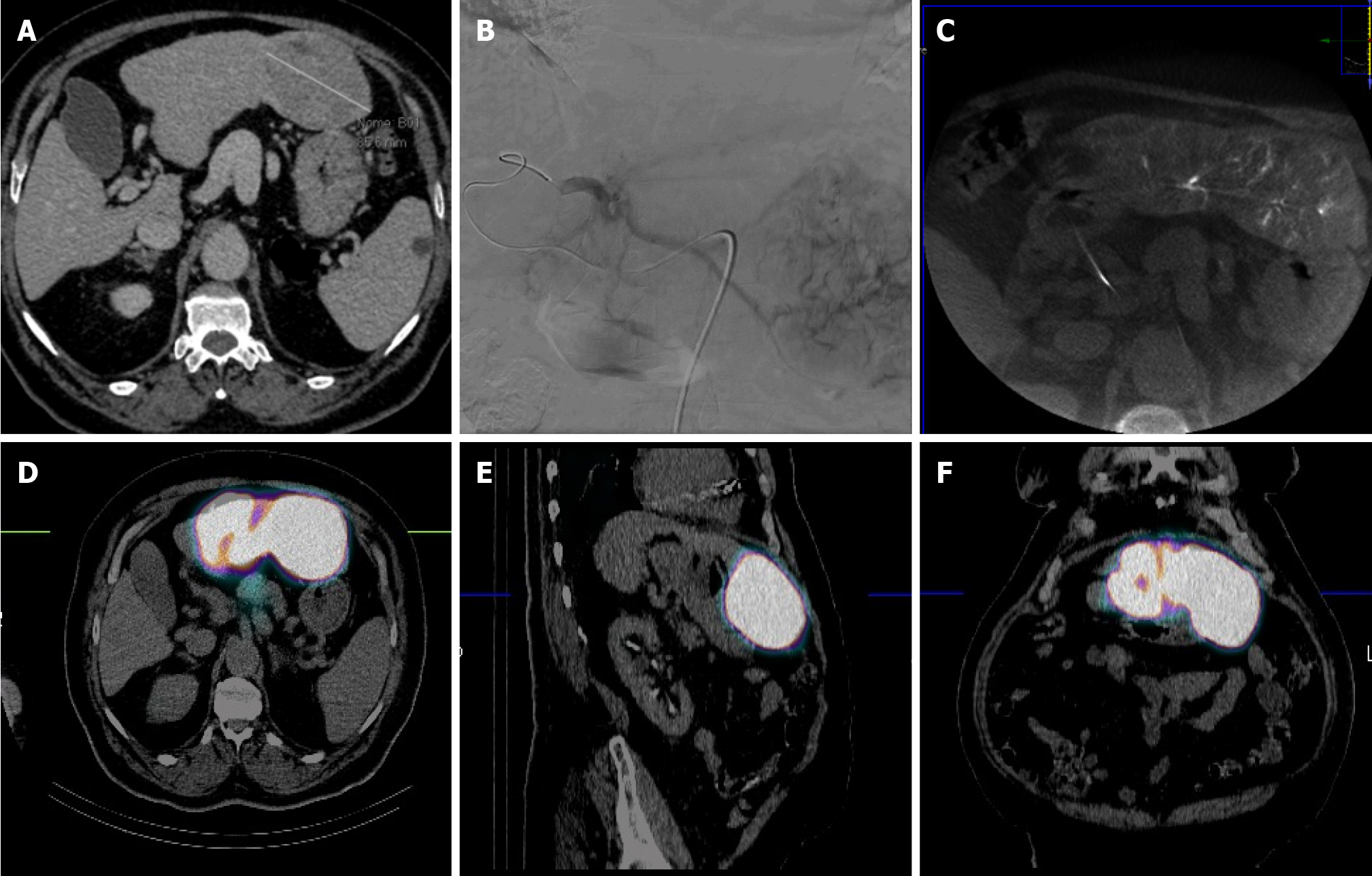
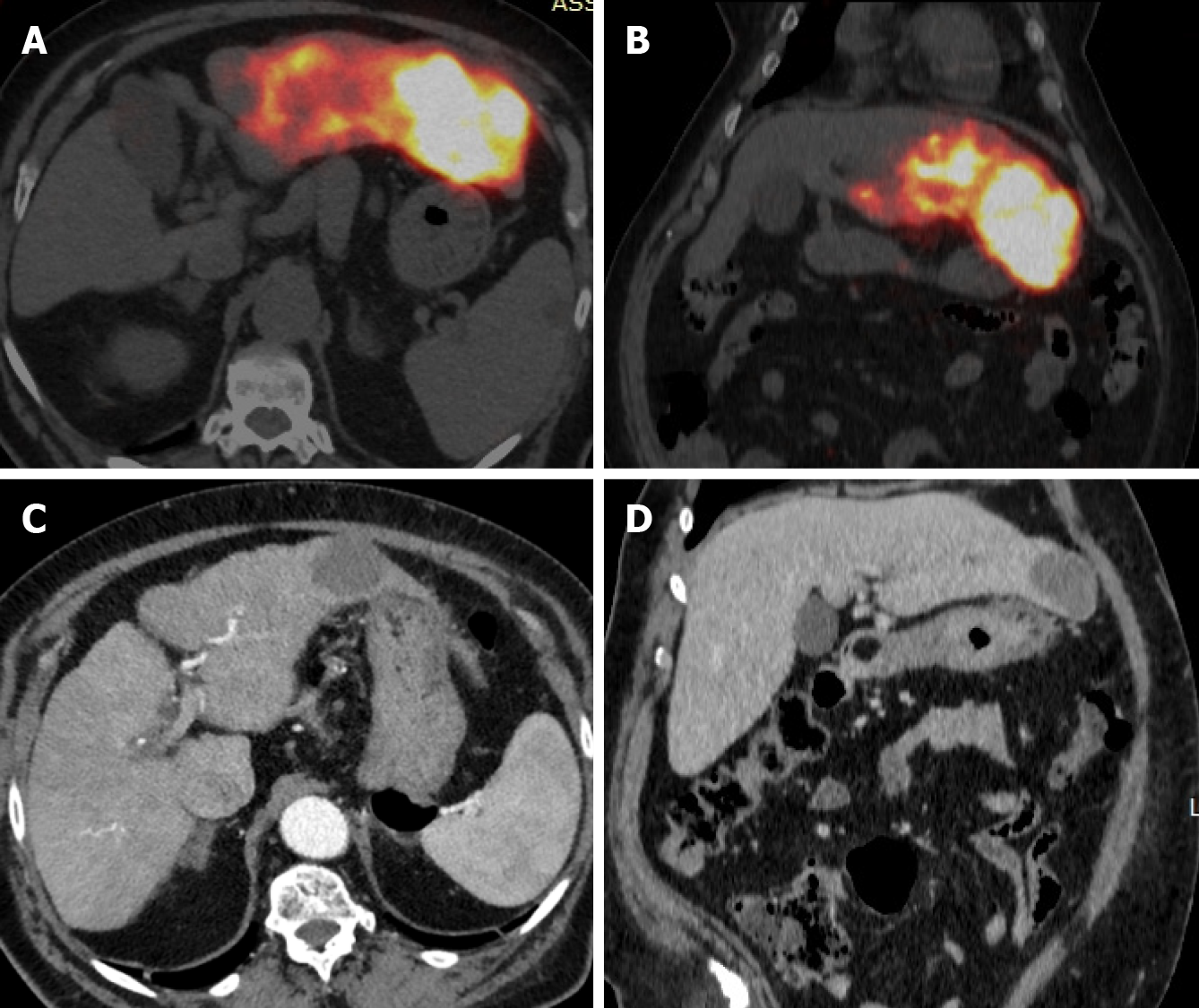
RS, as well as others RS techniques, entails a multi-step procedural approach, including pre-treatment angiography (with the possibility of embolization), followed by 99mTc-MAA administration, scintigraphy (Figure 1), dosimetry, Y90 treatment and post-treatment scintigraphy (Figure 2)[16,17,40]. As abovementioned, RS differs regarding dosimetry and extent of treated parenchyma. Personalised dosimetry is today routinely accepted and relies on mathematical models to estimate the absorbed radiation dose to target tissues, exploiting several different methods, each with its strengths and limitations. The Medical Internal Radiation Dose model assumes a uniform radiation distribution within the target liver volume, whilst the body surface area (BSA) method is based on a correlation between the BSA and liver size. Both do not take in account the difference in distribution between tumour tissue and the surrounding parenchyma. Partition model dosimetry, on the other hand, considers particle distribution ratio (tumour to normal, T:N ratio) between tumour and non-tumour compartments, as determined by software programs such as MIM (MIM Software, Beachwood, OH) and Simplicit90Y (Boston Scientific, Marlborough, MA). The T:N ratio is calculated from the 99mTc-MAA distribution in the target liver tissue or by evaluating tissue perfusion on intraprocedural cone beam CT[31].
Y90 treatment is performed by positioning the catheter within the selected arterial segment under fluoroscopic guidance and subsequently injecting the predetermined dose into the targeted perfused hepatic segment. TARE is typically confined to a maximum of two Couinaud hepatic segments to reduce the risk of radiation-induced complications[30]. The delivery system for 90Y administration is projected to minimize radiation exposure for personnel involved in the procedure[16,41]. Glass microspheres are delivered in saline, whereas resin microspheres are often delivered in sterile water or a 5% dextrose solution[42].
Over the past decade, TARE has evolved from a palliative lobar treatment for liver malignancies not amenable to other surgical or local treatments, to a selective, segmental therapy with curative intent for both primary and oligometastatic liver disease[43]. The fundamental principle underlying RS lies in the concept that segmental administration of a high radiation dose achieves a targeted ablative effect on tumors[29]. The effectiveness of RS is directly linked to the safe delivery of the maximum tolerated dose in a super-selective manner, while sparing the surrounding liver parenchyma. Therefore, future investigations on RS should prioritize optimizing the dosimetry and refining the technique for super-selective administration of the radiospheres within the designated hepatic segment(s).
Personalized, accurate dosimetry is the cornerstone of successful modern radiotherapeutic modalities. The field of dosimetry is undergoing advancements through the development of novel and improved dosimetry software, as well as with the integration of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT, facilitating the calculation of the metabolically active compound of the tumor, rather than the entire lesion visible on conventional axial imaging. Improvements in outcomes assessment, aiming for the prompt detection of recurrent disease are under investigation using 18F-FDG PET/CT protocols and the PERCIST criteria to evaluate the metabolic response[30]. Only recently, the dose limit for RS has been revisited and doses over 1000 Gy have been reported to be safe and effective in the treatment of HCC using glass microspheres[44].
State-of-the-art guidance using Angio-CT systems and fusion imaging, as well as novel anti-reflux catheter technology are currently under rigorous investigation for potential near-future incorporation into routine clinical practice, holding promise for optimizing targeting accuracy, achieving super-selective catheterization and enhancing delivery efficacy during RS[45,46].
Patient selection remains a critical determinant of treatment success and the influence of genetic factors, such as KRAS mutations and HCC genotypes, should be explored to provide a robust evidence base for personalized treatment regimens, incorporating combinations of different systemic and local treatments and aiming to further improve clinical outcomes[47,48].
The field of TARE microsphere technology is undergoing continuous development, as evidenced by the accumulating body of clinical data. 166Ηο microspheres have obtained the CE mark, with initial clinical data published[49], while 88Re - Rhenium microspheres (30 μm) are currently under investigation, offering the significant advantage of immediate availability via a dedicated Rhenium generator[50]. Finally, increasing experience, alongside the establishment of standardized protocols for catheterization and procedural planning, has the potential to propel widespread adoption and application of this promising percutaneous, locoregional therapeutic option for liver malignancies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Hashim Z, Pakistan S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Criss CR, Makary MS. Recent Advances in Image-Guided Locoregional Therapies for Primary Liver Tumors. Biology (Basel). 2023;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Ariel IM. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres). Ann Surg. 1965;162:267-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Simon N, Warner RR, Baron MG, Rudavsky AZ. Intra-arterial irradiation of carcinoid tumors of the liver. Am J Roentgenol Radium Ther Nucl Med. 1968;102:552-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Mantravadi RV, Spigos DG, Tan WS, Felix EL. Intraarterial yttrium 90 in the treatment of hepatic malignancy. Radiology. 1982;142:783-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 130] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Grady ED. Internal radiation therapy of hepatic cancer. Dis Colon Rectum. 1979;22:371-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Andrews JC, Walker SC, Ackermann RJ, Cotton LA, Ensminger WD, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637-1644. [PubMed] [Cited in This Article: ] |
| 7. | Dancey JE, Shepherd FA, Paul K, Sniderman KW, Houle S, Gabrys J, Hendler AL, Goin JE. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med. 2000;41:1673-1681. [PubMed] [Cited in This Article: ] |
| 8. | Houle S, Yip TK, Shepherd FA, Rotstein LE, Sniderman KW, Theis E, Cawthorn RH, Richmond-Cox K. Hepatocellular carcinoma: pilot trial of treatment with Y-90 microspheres. Radiology. 1989;172:857-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 98] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Saini A, Wallace A, Alzubaidi S, Knuttinen MG, Naidu S, Sheth R, Albadawi H, Oklu R. History and Evolution of Yttrium-90 Radioembolization for Hepatocellular Carcinoma. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | d'Abadie P, Hesse M, Louppe A, Lhommel R, Walrand S, Jamar F. Microspheres Used in Liver Radioembolization: From Conception to Clinical Effects. Molecules. 2021;26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Choi JW, Kim HC. Radioembolization for hepatocellular carcinoma: what clinicians need to know. J Liver Cancer. 2022;22:4-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Sivananthan G, Tabori NE. Principles of Radioembolization. Semin Intervent Radiol. 2021;38:393-396. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Boston Scientific Advancing science for life. TheraSphere™ Y-90 Glass Microspheres. [cited 15 November 2024]. Available from: https://www.bostonscientific.com/en-US/products/cancer-therapies/therasphere-y90-glass-microspheres.html. [Cited in This Article: ] |
| 14. | Bastiaannet R, Kappadath SC, Kunnen B, Braat AJAT, Lam MGEH, de Jong HWAM. The physics of radioembolization. EJNMMI Phys. 2018;5:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Sutphin PD, Lamus D, Kalva SP, Li J, Corbin IR. Interventional Radiologic Therapies for Hepatocellular Carcinoma: From Where We Began to Where We Are Going. 2019 Aug 6. In: Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet]. Cham (CH): Humana Press; 2019–. [PubMed] [Cited in This Article: ] |
| 16. | Cappelli A, Pettinato C, Golfieri R. Transarterial radioembolization using yttrium-90 microspheres in the treatment of hepatocellular carcinoma: a review on clinical utility and developments. J Hepatocell Carcinoma. 2014;1:163-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Mosconi C, Cappelli A, Pettinato C, Golfieri R. Radioembolization with Yttrium-90 microspheres in hepatocellular carcinoma: Role and perspectives. World J Hepatol. 2015;7:738-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Miller FH, Lopes Vendrami C, Gabr A, Horowitz JM, Kelahan LC, Riaz A, Salem R, Lewandowski RJ. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics. 2021;41:1802-1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 1457] [Article Influence: 728.5] [Reference Citation Analysis (41)] |
| 20. | Lucatelli P, Guiu B. 2022 Update of BCLC Treatment Algorithm of HCC: What's New for Interventional Radiologists? Cardiovasc Intervent Radiol. 2022;45:275-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021;74:2342-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 22. | Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 117] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 23. | Kumar P, Mhaskar R, Kim R, Anaya D, Frakes J, Hoffe S, Choi J, Kis B. Unresectable Intrahepatic Cholangiocarcinoma Treated with Radiation Segmentectomy/Lobectomy Using Yttrium 90-labeled Glass Microspheres. J Clin Exp Hepatol. 2022;12:1259-1263. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 24. | Yu Q, Patel M, Kwak D, Ungchusri E, Wang Y, Van Ha T, Zangan S, Marshall E, Little K, Baker T, Liao CY, Pillai A, Ahmed O. Segmental Yttrium-90 Radioembolization Using Glass Microspheres Greater than 400 Gray for the Treatment of Intrahepatic Cholangiocarcinoma: A Preliminary Experience. J Vasc Interv Radiol. 2023;34:1970-1976.e1. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 25. | Ness JR, Molvar C. Radioembolization of Intrahepatic Cholangiocarcinoma: Patient Selection, Outcomes, and Competing Therapies. Semin Intervent Radiol. 2021;38:438-444. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 26. | Entezari P, Gabr A, Kennedy K, Salem R, Lewandowski RJ. Radiation Lobectomy: An Overview of Concept and Applications, Technical Considerations, Outcomes. Semin Intervent Radiol. 2021;38:419-424. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 27. | Lewandowski RJ, Donahue L, Chokechanachaisakul A, Kulik L, Mouli S, Caicedo J, Abecassis M, Fryer J, Salem R, Baker T. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol. 2016;114:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, Baker T, Kulik L, Gupta R, Abecassis M, Benson AB 3rd, Omary R, Millender L, Kennedy A, Salem R. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, Kulik L, Ganger D, Desai K, Thornburg B, Mouli S, Hickey R, Caicedo JC, Abecassis M, Riaz A, Salem R. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287:1050-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Entezari P, Gabr A, Salem R, Lewandowski RJ. Yttrium-90 for colorectal liver metastasis - the promising role of radiation segmentectomy as an alternative local cure. Int J Hyperthermia. 2022;39:620-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Yu Q, Khanjyan M, Fidelman N, Pillai A. Contemporary applications of Y90 for the treatment of hepatocellular carcinoma. Hepatol Commun. 2023;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 32. | Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, Mulcahy M, Baker T, Abecassis M, Sato KT, Caicedo JC, Fryer J, Hickey R, Hohlastos E, Lewandowski RJ, Salem R. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, Salem R. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48:580-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 426] [Article Influence: 53.3] [Reference Citation Analysis (30)] |
| 35. | Kim E, Sher A, Abboud G, Schwartz M, Facciuto M, Tabrizian P, Knešaurek K, Fischman A, Patel R, Nowakowski S, Llovet J, Taouli B, Lookstein R. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol. 2022;7:843-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 36. | Prachanronarong K, Kim E. Radiation Segmentectomy. Semin Intervent Radiol. 2021;38:425-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 37. | Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, Vaidya S, Monsky WL, Park JO, Bhattacharya R, Hippe DS, Harris WP. Segmental Yttrium-90 Radioembolization versus Segmental Chemoembolization for Localized Hepatocellular Carcinoma: Results of a Single-Center, Retrospective, Propensity Score-Matched Study. J Vasc Interv Radiol. 2017;28:777-785.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (37)] |
| 38. | Biederman DM, Titano JJ, Korff RA, Fischman AM, Patel RS, Nowakowski FS, Lookstein RA, Kim E. Radiation Segmentectomy versus Selective Chemoembolization in the Treatment of Early-Stage Hepatocellular Carcinoma. J Vasc Interv Radiol. 2018;29:30-37.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, Assenat E, Tacher V, Robert C, Terroir-Cassou-Mounat M, Mariano-Goulart D, Amaddeo G, Palard X, Hollebecque A, Kafrouni M, Regnault H, Boudjema K, Grimaldi S, Fourcade M, Kobeiter H, Vibert E, Le Sourd S, Piron L, Sommacale D, Laffont S, Campillo-Gimenez B, Rolland Y; DOSISPHERE-01 Study Group. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 40. | Malhotra A, Liu DM, Talenfeld AD. Radiation Segmentectomy and Radiation Lobectomy: A Practical Review of Techniques. Tech Vasc Interv Radiol. 2019;22:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Padia SA, Lewandowski RJ, Johnson GE, Sze DY, Ward TJ, Gaba RC, Baerlocher MO, Gates VL, Riaz A, Brown DB, Siddiqi NH, Walker TG, Silberzweig JE, Mitchell JW, Nikolic B, Salem R; Society of Interventional Radiology Standards of Practice Committee. Radioembolization of Hepatic Malignancies: Background, Quality Improvement Guidelines, and Future Directions. J Vasc Interv Radiol. 2017;28:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Boas FE, Bodei L, Sofocleous CT. Radioembolization of Colorectal Liver Metastases: Indications, Technique, and Outcomes. J Nucl Med. 2017;58:104S-111S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Lewandowski RJ, Salem R. Implementation of radiation segmentectomy for early-stage hepatocellular carcinoma. Lancet Gastroenterol Hepatol. 2022;7:783-784. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 44. | Mourad SN, De la Garza-Ramos C, Toskich BB. Radiation Segmentectomy Above 1,000 Gy for the Treatment of Hepatocellular Carcinoma: Is There a Dose Limit? J Vasc Interv Radiol. 2023;34:1458-1462. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 45. | Piron L, Le Roy J, Cassinotto C, Delicque J, Belgour A, Allimant C, Beregi JP, Greffier J, Molinari N, Guiu B. Radiation Exposure During Transarterial Chemoembolization: Angio-CT Versus Cone-Beam CT. Cardiovasc Intervent Radiol. 2019;42:1609-1618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | d'Abadie P, Walrand S, Goffette P, Amini N, Maanen AV, Lhommel R, Jamar F. Antireflux catheter improves tumor targeting in liver radioembolization with resin microspheres. Diagn Interv Radiol. 2021;27:768-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Cherri S, Melocchi L, Gandolfi L, Rossi G, Zaniboni A. Integrated Decision-Making in the Treatment of Colon-Rectal Cancer: The Case of KRAS-Mutated Tumors. Life (Basel). 2023;13. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 48. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1343] [Cited by in F6Publishing: 2551] [Article Influence: 850.3] [Reference Citation Analysis (1)] |
| 49. | Bastiaannet R, van Roekel C, Smits MLJ, Elias SG, van Amsterdam WAC, Doan D, Prince JF, Bruijnen RCG, de Jong HWAM, Lam MGEH. First Evidence for a Dose-Response Relationship in Patients Treated with (166)Ho Radioembolization: A Prospective Study. J Nucl Med. 2020;61:608-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Shukla J, Goyal A, Chhabra A, Rathore Y, Bansal K, Pandey S, Parmar M, Singhal S, Kalra N, Duseja A, Mittal BR. Cold kit for Rhenium-188 microspheres based selective intra-arterial therapy (SIRT): Preparation, characterization and feasibility study. Appl Radiat Isot. 2022;190:110423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |