Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.343
Peer-review started: September 3, 2023
First decision: October 16, 2023
Revised: December 4, 2023
Accepted: December 20, 2023
Article in press: December 20, 2023
Published online: February 15, 2024
The controlling nutritional status (CONUT) score effectively reflects a patient’s nutritional status, which is closely related to cancer prognosis. This study investigated the relationship between the CONUT score and prognosis after radical surgery for colorectal cancer, and compared the predictive ability of the CONUT score with other indexes.
To analyze the predictive performance of the CONUT score for the survival rate of colorectal cancer patients who underwent potentially curative resection.
This retrospective analysis included 217 patients with newly diagnosed colorectal. The CONUT score was calculated based on the serum albumin level, total lymphocyte count, and total cholesterol level. The cutoff value of the CONUT score for predicting prognosis was 4 according to the Youden Index by the receiver operating characteristic curve. The associations between the CONUT score and the prognosis were performed using Kaplan-Meier curves and Cox regression analysis.
Using the cutoff value of the CONUT score, patients were stratified into CONUT low (n = 189) and CONUT high groups (n = 28). The CONUT high group had worse overall survival (OS) (P = 0.013) and relapse-free survival (RFS) (P = 0.015). The predictive performance of CONUT was superior to the modified Glasgow prognostic score, the prognostic nutritional index, and the neutrophil-to-lymphocyte ratio. Meanwhile, the predictive performances of CONUT + tumor node metastasis (TNM) stage for 3-year OS [area under the receiver operating characteristics curve (AUC) = 0.803] and 3-year RFS (AUC = 0.752) were no less than skeletal muscle mass index (SMI) + TNM stage. The CONUT score was negatively correlated with SMI (P < 0.01).
As a nutritional indicator, the CONUT score could predict long-term outcomes after radical surgery for colorectal cancer, and its predictive ability was superior to other indexes. The correlation between the CONUT score and skeletal muscle may be one of the factors that play a predictive role.
Core Tip: The controlling nutritional status (CONUT) score was significantly associated with the postoperative prognosis of colorectal cancer patients. Compared with other nutritional scores, CONUT score could serve as an optimal prognostic nutritional index to predict the long-term outcome after radical surgery for colorectal cancer.
- Citation: Liu LX, Wang H, Gao B, Xu TT, Yuan QG, Zhou SZ, Ding C, Miao J, Guan WX. Preoperative controlling nutritional status as an optimal prognostic nutritional index to predict the outcome for colorectal cancer. World J Gastrointest Oncol 2024; 16(2): 343-353
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/343.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.343
Colorectal cancer is the third most common cancer in the world and ranks second in cancer-related deaths despite improvements in treatment[1,2]. Therefore, identifying effective tools in prognostic prediction for colorectal cancer is warranted. Especially after radical colorectal cancer surgery. Early risk stratification contributes to individualized treatment and overall prognosis.
Several studies have shown that a decline in nutritional status hinders recovery after surgery, thus leading to poor prognosis[3,4]. The decline of nutritional status leads to the disorder of net protein balance in the body, when protein synthesis is insufficient to compensate for protein decomposition, thus affecting the muscle content of the body[5]. Sarcopenia occurs when the muscle content is reduced and has been confirmed as an independent risk factor for multiple cancers in several studies[6-9]. Currently, sarcopenia is mainly diagnosed by analyzing computed tomography (CT) images of the third vertebral slice. However, the CT method of skeletal muscle measurement has many drawbacks, including high expense, radiation exposure, and sophisticated manipulation, which largely restricts its wide application. Therefore, a series of nutrition-related scoring tools, such as controlling nutritional status (CONUT), modified Glasgow prognostic score (mGPS), prognostic nutritional index (PNI), neutrophil-to-lymphocyte ratio (NLR), and others have been developed to replace muscle measurement depending on serological indicators. Moreover, studies have shown that these nutrition-related scores can be used to evaluate the prognosis of patients with colorectal cancer[10-13]. However, their predictive performance varies between studies. Therefore, we need a convenient and relatively accurate nutrition-related scoring tool to evaluate the prognosis of patients with colorectal cancer.
A new nutritional scoring tool proposed in recent years, the CONUT score has been proven effective in assessing the nutritional status of patients[14]. The calculation of the CONUT score is based on serum albumin level, total peripheral lymphocyte counts, and total cholesterol level as previously described[15]. These serum indicators are readily available and convenient for clinical evaluation. According to recent studies, the CONUT score can be used to predict the outcome of a variety of cancers including gastric cancer[16], urinary system cancer[17], and lung cancer[18]. Although the predictive value of the CONUT score for the long-term prognosis of colorectal cancer after radical surgery has been reported, the mechanism of the CONUT score’s predictive power remains unclear. At the same time, compared with other nutrition-related scoring tools that rely on serological indicators, the accuracy of its predictive power has not been clearly reported.
This study aimed to analyze the predictive performance of the CONUT score for the survival rate of colorectal cancer patients who underwent potentially curative resection. Meanwhile, we also compared the prognostic ability of the CONUT score with other nutritional scoring systems in predicting the long-term outcome after radical surgery for colorectal cancer and explored the mechanism of the CONUT score’s predictive power.
A database comprised of 373 patients newly diagnosed with colorectal cancer who underwent surgery in Nanjing Drum Tower Hospital or General Hospital of Eastern Theater Command between January 2015 and January 2019 was analyzed retrospectively. Patients with pre-operative neoadjuvant chemotherapy, absent of pre-operative CT images, tumor node metastasis (TNM) stage IV, incomplete medical records, or lost during routine follow-up were excluded. This observational study was approved by the Clinical Research Ethics Committee of Nanjing Drum Tower Hospital and General Hospital of Eastern Theater Command. Written informed consent was obtained.
Laboratory tests were based on fasting blood collected on the morning of the first day of admission. American Joint Committee on Cancer stage was assessed according to the eighth version of the TNM staging system. Comorbidities included tuberculosis, hepatitis, diabetes, hypertension, coronary heart disease, hyperlipidemia, and other chronic diseases. Postoperative complications were defined as intestinal obstruction, intestinal paralysis, anastomotic leakage, abdominal infection, and other setbacks during the perioperative period.
The CONUT score was calculated as previously described and illustrated in Table 1[14]. The CONUT is assessed using serum albumin, lymphocyte counts, and total cholesterol. A CONUT score ≥ 5 was considered a state of moderate to severe malnutrition.
| CONUT | ||||
| Low | High | |||
| Serum albumin (g/dL) | 3.5-4.5 | 3.0-3.49 | 2.5-2.99 | < 2.5 |
| ALB score | 1 | 2 | 4 | 6 |
| Total lymphocyte (count/mm3) | ≥ 1600 | 1200-1599 | 800-1199 | < 800 |
| TLC score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | > 180 | 140-180 | 100-139 | < 100 |
| T-cho score | 0 | 1 | 2 | 3 |
| CONUT total score | 0-1 | 2-4 | 5-8 | 9-12 |
| Assessment | Normal | Light | Moderate | Severe |
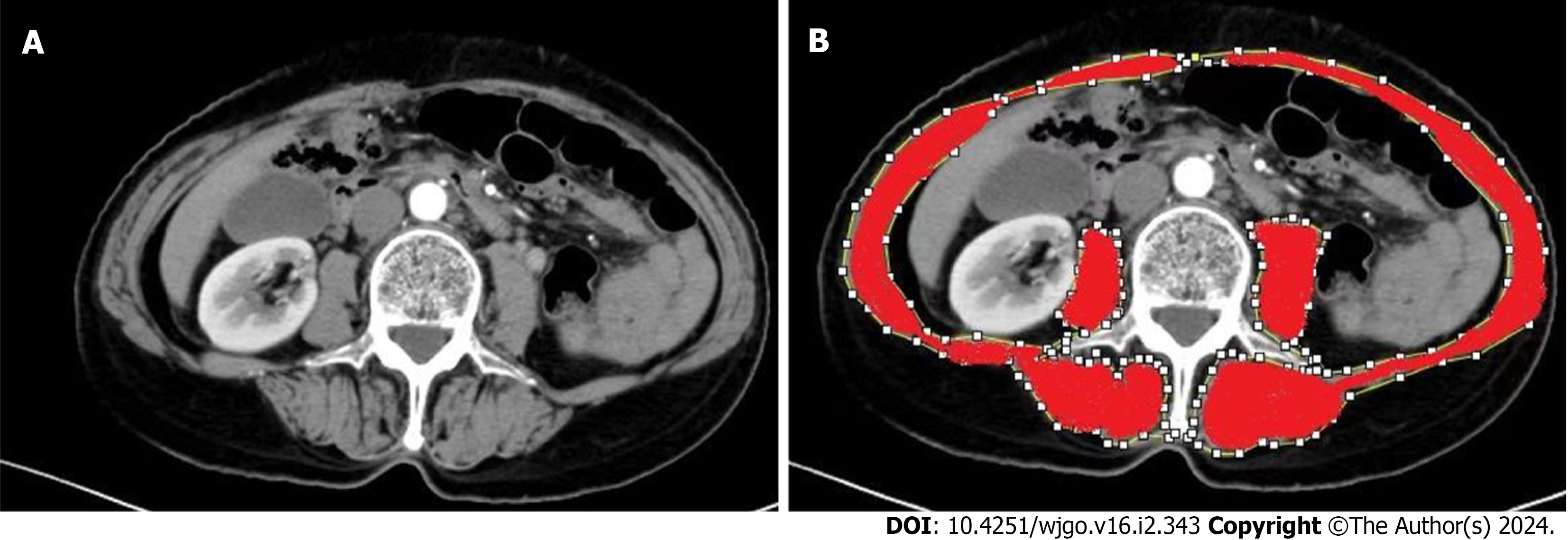
The skeletal muscle area (SMA) was measured using ImageJ software to measure the areas of the psoas major, paraspinal muscles, transverse abdominis, external oblique, internal oblique, and rectus abdominis in the cross-section of the third lumbar vertebrae on CT[19] (Figure 1). The skeletal muscle mass index (SMI) was calculated by dividing SMA (cm2) by the square of height (m). Sarcopenia was defined as SMI < 40.8 (cm2/m2) in men and SMI < 34.9 (cm2/m2) in women[8].
The NLR was calculated as the ratio of pre-operative neutrophil and lymphocyte counts[12]. The mGPS was scored using pre-operative C-reactive protein (CRP) and albumin levels. Patients were divided into three categories by mGPS: 0 (CRP ≤ 10 mg/L and albumin ≥ 35 g/L), 1 (CRP >10 mg/L or albumin < 35 g/L), and 2 (CRP > 10 mg/L and albumin < 35 g/L)[10]. The PNI was calculated based on preoperative albumin and lymphocyte counts: 10 × albumin (g/dL) + 0.005 × total lymphocyte counts (per mm3)[20]. The scores listed above are nutritional evaluation systems considered useful in cancer prognostic risk stratification[10-13].
Patients who underwent colorectal cancer surgery and were discharged from the hospital were followed by telephone every three months. Regular return and follow-up at the hospital were also required. The primary endpoint was death from all causes of illness. The secondary endpoint was medically proven recurrence.
IBM SPSS Statistics for Windows, Version 26.0 (Armonk, NY: IBM Corp) was used for statistical analysis. The correlations between parameters were examined using Pearson's or Spearman's correlation coefficient when appropriate. Differences between groups were assessed by χ2 test or analysis of variance. The Kaplan-Meier curve was used to analyze 3-year overall survival (OS) and 3-year relapse-free survival (RFS). Cox proportional hazards models were utilized to identify variables with significant independent associations with OS and RFS. The time-dependent receiver operating characteristic (ROC) curve was used to evaluate the accuracy of prognosis prediction and was compared using the DeLong test between different scores. P < 0.05 was considered significantly different.
Among the 217 patients with colorectal cancer, 92 (42.4%) were men and 125 (57.6%) were women, with a mean age of 58.4 years (range 24–88). Based on the time-dependent ROC, the best cutoff value of the CONUT score for predicting 3-year OS was 4. Therefore, patients were divided into CONUT low (CONUT ≤ 4) (n = 189, 87.1%) and CONUT high groups (CONUT ≥ 5) (n = 28, 12.9%). Characteristics were compared between the two groups.
The age, sex, tumor site, rates of comorbidities, pre-operative white blood cell count, platelet count, serum carcinoembryonic antigen, and carbohydrate antigen 19-9 levels between the two groups were not significantly different. Patients in the CONUT HIGH group showed a later tumor TNM stage (P = 0.044), had a higher incidence of postoperative complication rates (P = 0.044), higher CRP levels, and lower body mass index, hemoglobin, albumin, and cholesterol levels. The incidence of sarcopenia in the CONUT HIGH group was also higher (Table 2).
| Variable | All (217) | The CONUT score | P value | |
| CONUT low (189) | CONUT high (28) | |||
| Age (yr) | 58.4 ± 12.7 | 58.4 ± 12.4 | 58.9 ± 15.1 | 0.842 |
| Male sex, n (%) | 125 (57.6) | 113 (59.8) | 12 (42.9) | 0.091 |
| BMI (kg/m2) | 22.9 ± 3.2 | 23.1 ± 3.1 | 21.5 ± 3.9 | 0.015 |
| Tumor site, n (%) | 0.787 | |||
| Colon | 122 (56.2) | 105 (55.6) | 17 (60.7) | |
| Rectum | 95 (43.8) | 84 (44.4) | 11 (39.3) | |
| Comorbidities, yes, n (%) | 69(31.8) | 60(31.7) | 9(32.1) | 0.966 |
| Sarcopenia, yes, n (%) | 49 (22.6) | 32 (16.9) | 17 (60.7) | < 0.01 |
| WBC (× 109/L) | 5.84 ± 1.92 | 5.92 ± 1.92 | 5.27 ± 1.86 | 0.093 |
| Platelet (× 109/L) | 218 ± 85 | 222 ± 89 | 191 ± 46 | 0.068 |
| Hemoglobin (g/L) | 122.5 ± 24.6 | 125.6 ± 23.2 | 101.6 ± 24.1 | < 0.01 |
| Albumin (g/L) | 41.3 ± 4.4 | 42.1 ± 3.7 | 35.7 ± 4.5 | < 0.01 |
| Lymphocyte (× 109/L) | 1.6 ± 0.7 | 1.7 ± 0.6 | 0.9 ± 0.3 | < 0.01 |
| Cholesterol (mg/dL) | 166.6 ± 41.5 | 173.0 ± 39.2 | 123.7 ± 30.0 | < 0.01 |
| CRP (mg/L) | 8.9 ± 20.3 | 7.5 ± 19.7 | 18.5 ± 21.9 | 0.007 |
| CEA (ng/ml) | 5.9 ± 10.1 | 5.9 ± 10.5 | 6.6 ± 7.1 | 0.706 |
| CA199 (U/ml) | 25.5 ± 55.4 | 25.7 ± 56.8 | 24.5 ± 45.6 | 0.920 |
| Postoperative complications, yes, n (%) | 34 (15.7) | 26 (13.8) | 8 (28.6) | 0.044 |
| TNM stage, n (%) | 0.044 | |||
| Ⅰ | 36 (6.6) | 35 (18.5) | 1 (3.6) | |
| Ⅱ | 91 (1.9) | 74 (39.2) | 17 (60.7) | |
| Ⅲ | 90 (41.5) | 80 (42.3) | 10 (35.7) | |
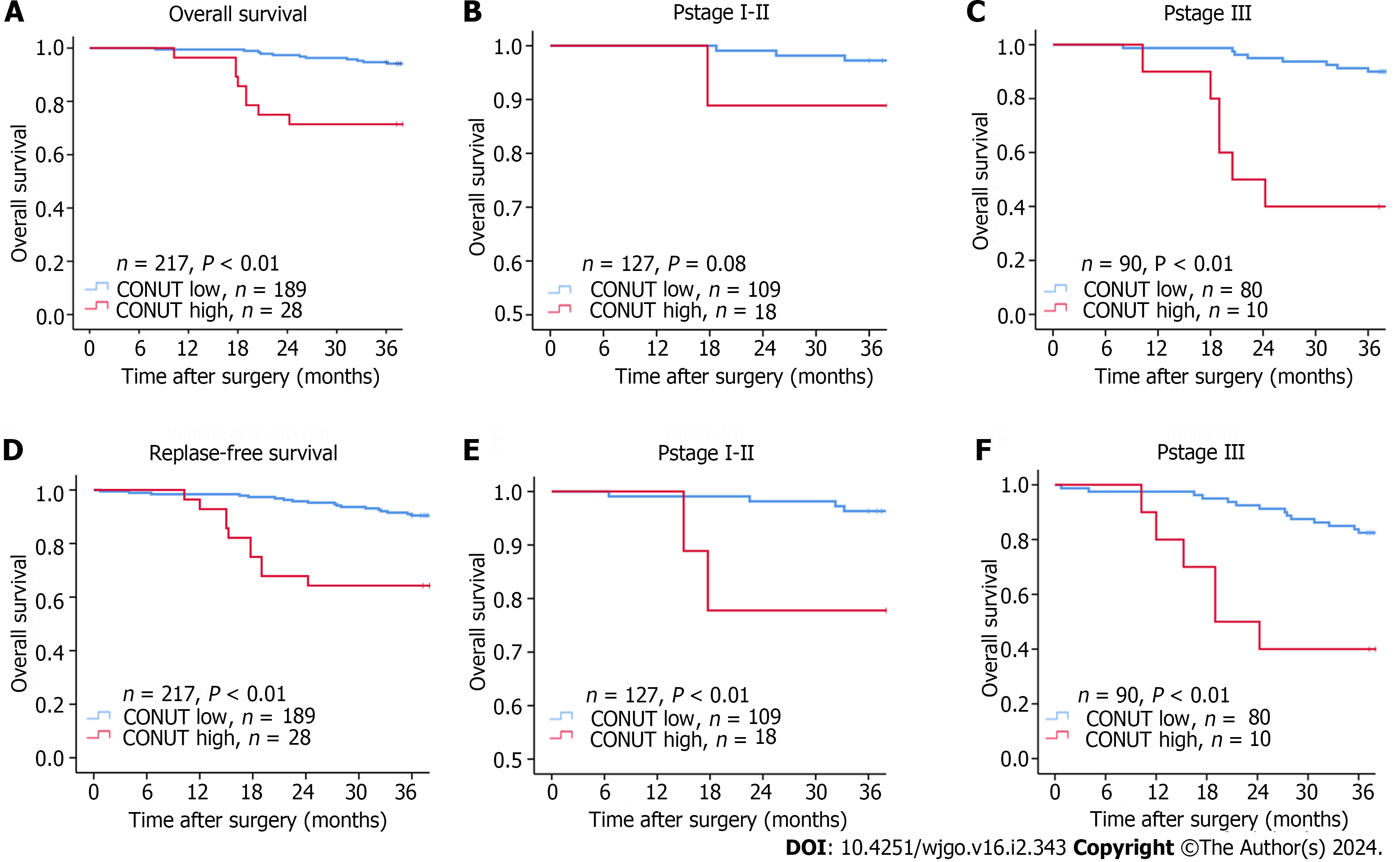
Median follow-up was 49.6 mo (8–85 mo). The 3-year OS (94.1% vs 71.4%, P <0.01) and RFS (89.9% vs 64.2%, P < 0.01) of the CONUT low group were significantly higher than those in the CONUT high group. Due to the high mortality and recurrence rate of colon cancer in stage III patients, we analyzed stage III patients separately to better explore the relationship between the CONUT score and patient prognosis. In patients with TNM stage III, the 3-year OS and RFS in the CONUT low were also significantly higher than those in the CONUT high group (90% vs 40%, P < 0.01; 83.7% vs 40%, P <0.01). There was a significant difference in the 3-year RFS between CONUT low and high in patients with TNM stages I-II (P < 0.01), but not with 3-year OS (P = 0.08; Figure 2). This may be due to lower mortality and a relatively short follow-up period for participants in stage I-II.
Baseline factors without linear inter-correlations were included in univariate Cox regression analysis. Statistically significant factors were included in multivariate analysis which showed sarcopenia [hazard ratio (HR) = 3.640, 95% confidence interval (95%CI): 1.182–11.207, P = 0.024], TNM tumor stage (Ⅲ vs Ⅱ, Ⅰ HR = 4.396, 95%CI: 1.534–12.598, P = 0.006) and the CONUT score (HR = 3.920, 95%CI: 1.330–11.547, P = 0.013) were associated with 3-year OS (Table 3). Sarcopenia (HR = 4.122, 95%CI: 1.678–10.125, P = 0.002), TNM tumor stage (Ⅲ vs Ⅱ, Ⅰ HR = 3.603, 95%CI: 1.612–8.053, P = 0.002), and the CONUT score (HR = 2.992, 95%CI: 1.241–7.212, P = 0.015) were associated with 3-year RFS (Table 4).
| Variable | Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Age (yr) | ≥ 65/< 65 | 1.815 | 0.738-4.468 | 0.194 | |||
| Sex | Female/male | 1.577 | 0.641-3.881 | 0.321 | |||
| BMI (kg/m2) | ≥ 24/ < 24 | 0.202 | 0.047-0.873 | 0.032 | 0.506 | 0.103-2.474 | 0.400 |
| Tumor site | Colon/rectum | 0.931 | 0.374-2.314 | 0.877 | |||
| Comorbidities | Yes/no | 2.557 | 0.745-8.777 | 0.136 | |||
| Sarcopenia | Yes/no | 8.012 | 3.151-20.372 | < 0.01 | 3.640 | 1.182-11.207 | 0.024 |
| WBC (× 109/L) | ≥ 10/< 10 | 1.228 | 0.164-9.202 | 0.841 | |||
| Platelet (× 109/L) | ≥ 300/< 300 | 0.798 | 0.184-3.454 | 0.763 | |||
| Hemoglobin (g/L) | ≥ 90/< 90 | 0.367 | 0.132-1.019 | 0.054 | 0.696 | 0.234-2.071 | 0.515 |
| CRP (mg/L) | > 10/≤ 10 | 1.647 | 0.381-7.130 | 0.504 | |||
| CEA (ng/mL) | ≥ 3.4/< 3.4 | 2.319 | 0.881-6.101 | 0.088 | 1.970 | 0.708-5.480 | 0.194 |
| CA199 (U/mL) | ≥ 37/< 37 | 1.903 | 0.254-14.257 | 0.531 | |||
| Postoperative complications | Yes/no | 1.028 | 0.300-3.529 | 0.965 | |||
| TNM stage | Ⅲ/Ⅰ,Ⅱ | 4.167 | 1.501-11.571 | 0.006 | 4.396 | 1.534-12.598 | 0.006 |
| CONUT score | ≥ 5/≤ 4 | 5.939 | 2.386-14.785 | < 0.01 | 3.920 | 1.330-11.547 | 0.013 |
| Variable | Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Age (yr) | ≥ 65/< 65 | 1.625 | 0.781-3.380 | 0.194 | |||
| Sex | Female/male | 1.503 | 0.724-3.118 | 0.274 | |||
| BMI (kg/m2) | ≥ 24/ < 24 | 0.354 | 0.135-0.928 | 0.035 | 0.792 | 0.269-2.337 | 0.673 |
| Tumor site | Colon/rectum | 0.986 | 0.469-2.074 | 0.970 | |||
| Comorbidities | Yes/no | 1.462 | 0.624-3.428 | 0.382 | |||
| Sarcopenia | Yes/no | 6.795 | 3.240-14.250 | < 0.01 | 4.122 | 1.678-10.125 | 0.002 |
| WBC (× 109/L) | ≥ 10/< 10 | 1.728 | 0.410-7.283 | 0.490 | |||
| Platelet (× 109/L) | ≥ 300/< 300 | 1.294 | 0.392-4.276 | 0.673 | |||
| Hemoglobin (g/L) | ≥ 90/< 90 | 0.492 | 0.199-1.214 | 0.124 | |||
| CRP (mg/L) | > 10/≤ 10 | 1.625 | 0.491-5.382 | 0.427 | |||
| CEA (ng/mL) | ≥ 3.4/< 3.4 | 2.356 | 1.072-5.177 | 0.033 | 2.220 | 0.989-4.984 | 0.053 |
| CA199 (U/mL) | ≥ 37/< 37 | 1.483 | 0.352-6.243 | 0.591 | |||
| Postoperative complications | Yes/no | 1.028 | 0.300-3.529 | 0.965 | |||
| TNM stage | Ⅲ/Ⅰ,Ⅱ | 3.477 | 1.581-7.650 | 0.002 | 3.603 | 1.612-8.053 | 0.002 |
| CONUT score | ≥ 5/≤ 4 | 4.724 | 2.177-10.254 | < 0.01 | 2.992 | 1.241-7.212 | 0.015 |
Time-dependent ROCs were utilized to predict 3-year OS in overall patients. The area under the ROCs curve (AUC) of the CONUT, NLR, mGPS, and PNI were 0.728 (95%CI: 0.607–0.850, P < 0.01), 0.585 (95%CI: 0.433–0.736, P = 0.222), 0.585 (95%CI: 0.445–0.726, P = 0.219) and 0.414 (95%CI: 0.262–0.566, P = 0.215), respectively (Figure 3). The CONUT score showed higher accuracy than NLR, mGPS, and PNI for predicting the 3-year OS in overall patients. The predictive accuracy was significantly different between CONUT and other scores (all P < 0.05) by the DeLong test. The best cutoff value of CONUT in predicting the 3-year OS was four as mentioned above. The CONUT score (AUC = 0.662, 95%CI: 0.546–0.778, P = 0.005) was also more effective in predicting the 3-year RFS in general patients enrolled (Figure 3).
Time-dependent ROCs were utilized to predict 3-year OS in all patients. The AUC of CONUT + TNM stage (AUC = 0.803, 95%CI: 0.703–0.904, P < 0.01) was higher than SMI + TNM stage (AUC = 0.760, 95%CI: 0.624–0.896, P < 0.01). When predicting the 3-year RFS, the AUC of CONUT + TNM stage (AUC = 0.752, 95%CI: 0.661–0.843, P < 0.01) was slightly lower than SMI + TNM stage (AUC = 0.766, 95%CI: 0.660–0.871, P < 0.01). Spearman correlation analysis was used to evaluate the correlations between the CONUT score and SMI. The CONUT score was negatively correlated with SMI (Figure 4). The relationships indicated that CONUT score could reflect the patient’s SMI to a certain extent and the predictive ability of postoperative prognosis of colorectal cancer is not weaker than SMI.
This study explored the associations between preoperative CONUT score and tumor prognosis among colorectal cancer patients. A pre-operative CONUT ≥ 5 was independently correlated with a 3.92-fold increased risk of poor OS and a 2.99-fold increased risk of poor RFS. By comparison, we found that the predictive power of the CONUT score was more accurate than that of other nutritional indexes. In addition, we observed a parallel relationship between the CONUT score and the patient’s skeletal muscle index, which strongly represents body protein reserve and nutritional state[21]. Therefore, the CONUT score may serve as the optimal PNI to predict the long-term outcome after radical surgery for colorectal cancer.
Many studies have examined nutritional status and tumor prognosis in recent years. Low levels of skeletal muscle mass are often associated with malnutrition[22]. Xiao et al[9] found that preoperative levels of skeletal muscle could predict cancer prognosis. As reported, colorectal cancer patients with a pre-operative diagnosis of sarcopenia had a 1.1–1.2-fold higher incidence of postoperative complications and a 2-fold increased risk of death within 30 d after surgery than those without sarcopenia[6-9]. Nonetheless, skeletal muscle measurement methods are not widely promoted because it is expensive, time and power consuming, carry radiation risks, and have other disadvantages. Therefore, a relatively convenient and safe early prediction indicator is needed. Although Golder et al[23] and Nozoe et al[24] found that mGPS, NLR, and PNI indexes could serve as prediction tools for prognosis in multiple cancers, the predictive power of these indicators remained unsatisfactory. In our study, the accuracy of the CONUT score was higher than that of mGPS, NLR, and PNI and showed satisfactory prediction performance. This objective, safe, simple, and easily accessible tool is more conducive to early and rapid prediction and has clinical application value.
As a nutritional status score, the effective role of the CONUT score has been clinically proven to evaluate nutrition[14]. The ability of CONUT to predict the prognosis of colon cancer may be related to its three components. First, serum albumin is a biomarker for systemic inflammation. Tumor prognosis is highly correlated with an inflammation state. Excessive inflammatory activation affects the tumor microenvironment[25] and suppresses normal immune reactions[26]. Second, Zhang et al[27], and Zhao et al[28] demonstrated that colon cancer patients with reduced lymphocyte counts had worse prognoses than patients with normal lymphocyte counts. Lymphocytes, as immune cells, can recognize and kill tumor cells through humoral and cellular immunity pathways, thus playing a significant role in inhibiting tumor cell proliferation and metastasis[29]. Third, cholesterol is mainly synthesized in the liver and exists in blood in the form of low-density lipoprotein (LDL). LDL receptor (LDLR) is expressed on the surface of both normal and tumor cells. Through the binding of LDLR to LDL, the serum LDL enters cells through endocytosis[30]. Unlike normal tissues, LDLR does not limit intracellular cholesterol transportation through feedback regulation in cancer cells[31], allowing for unrestricted cholesterol transport into tumor cells to provide energy. Moreover, cancer cells can also use cholesterol metabolites to synthesize substances that promote their growth and development[32,33]. Therefore, low serum cholesterol levels could be the result of an increased risk of cancer, indicating a poor prognosis. In conclusion, the ability of the CONUT score to predict prognosis may be due to its comprehensive reflection of the body's nutritional, inflammatory, and immune status, as well as tumor metabolism.
In addition, our study found a correlation between the CONUT score and skeletal muscle levels (R2 = 0.137, correlation coefficient -0.319, P < 0.01), possibly because both scores reflect the protein reserves in the body. Patients with cancer, especially those who undergo surgery procedures, could experience a larger demand for energy and protein intake than healthy people[34]. Inadequate energy or protein intake would accelerate glycogenolysis leading to glycogen depletion, further promoting gluconeogenesis and leading to skeletal protein decomposition[35]. Patients would be unable to tolerate surgery, delay recovery, and be even more prone to tumor recurrence, resulting in a poor prognosis when protein reserves are insufficient. This may be one of the factors contributing to the predictive power of CONUT scores.
The present study had several limitations. First, this study was a retrospective single-center study comprised of patients from the same region, and homogeneous in race. Second, the sample size was small, and the follow-up time was short. Last, the post-operative CONUT was not followed up and the nutritional status of patients could not be assessed dynamically. Therefore, prospective studies are warranted further to confirm the predictive significance of the CONUT score.
To conclude, the CONUT score was significantly associated with the postoperative prognosis of colorectal cancer patients. Compared with other nutritional scores, the CONUT score could be an optimal PNI to predict the long-term outcome after radical surgery for colorectal cancer. In addition, the predictive power of the CONUT score may be related to skeletal muscle reserves.
At present, the nutritional status of cancer patients is closely related to the survival prognosis of patients.
The controlling nutritional status (CONUT) score can effectively evaluate the nutritional status of cancer patients, so we explored the relationship between CONUT score and prognosis of cancer patients.
To explore the relationship between CONUT score and postoperative prognosis of colorectal cancer patients. To study the prognostic performance of CONUT score in colorectal cancer patients and compare it with other nutritional scores.
IBM SPSS Statistics for Windows, Version 26.0 (Armonk, NY: IBM Corp) was used for statistical analysis. The correlations between parameters were examined using Pearson's or Spearman's correlation coefficient when appropriate. Differences between groups were assessed by χ2 test or analysis of variance. The Kaplan-Meier curve was used to analyze 3-year overall survival (OS) and 3-year relapse-free survival (RFS). Cox proportional hazards models were utilized to identify variables with significant independent associations with OS and RFS. The time-dependent receiver operating characteristic curve was used to evaluate the accuracy of prognosis prediction and was compared using the DeLong test between different scores. P < 0.05 was considered significantly different.
The CONUT score can effectively predict postoperative prognosis of patients with colorectal cancer, and its predictive function may be related to skeletal muscle reserve. The predictive effective of the CONUT score is not inferior to that of the other nutritional scores.
The CONUT score was significantly associated with the postoperative prognosis of colorectal cancer patients. Compared with other nutritional scores, the CONUT score could be an optimal prognostic nutritional index to predict the long-term outcome after radical surgery for colorectal cancer. In addition, the predictive power of the CONUT score may be related to skeletal muscle reserves.
Further improvement of the CONUT score makes it more effective in predicting the prognosis of cancer patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabbri N, Italy; Shalaby MN, Egypt S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 795] [Article Influence: 265.0] [Reference Citation Analysis (0)] |
| 2. | Fan A, Wang B, Wang X, Nie Y, Fan D, Zhao X, Lu Y. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci. 2021;17:3837-3849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 3. | Haraga J, Nakamura K, Omichi C, Nishida T, Haruma T, Kusumoto T, Seki N, Masuyama H, Katayama N, Kanazawa S, Hiramatsu Y. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol. 2016;5:567-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 353] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 5. | Deldicque L. Protein Intake and Exercise-Induced Skeletal Muscle Hypertrophy: An Update. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients With Stage I to III Colorectal Cancer. Dis Colon Rectum. 2019;62:549-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Olmez T, Karakose E, Bozkurt H, Pence HH, Gulmez S, Aray E, Bulut CI, Sert OZ, Polat E, Duman M. Sarcopenia is associated with increased severe postoperative complications after colon cancer surgery. Arch Med Sci. 2021;17:361-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Su H, Ruan J, Chen T, Lin E, Shi L. CT-assessed sarcopenia is a predictive factor for both long-term and short-term outcomes in gastrointestinal oncology patients: a systematic review and meta-analysis. Cancer Imaging. 2019;19:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Xiao J, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA, Peng PD, Baracos VE, Lee VS, Ely S, Gologorsky RC, Weltzien E, Kroenke CH, Kwan ML, Alexeeff SE, Castillo AL, Prado CM. Association of Low Muscle Mass and Low Muscle Radiodensity With Morbidity and Mortality for Colon Cancer Surgery. JAMA Surg. 2020;155:942-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 10. | Hirashima K, Watanabe M, Shigaki H, Imamura Y, Ida S, Iwatsuki M, Ishimoto T, Iwagami S, Baba Y, Baba H. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49:1040-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688-2692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 12. | Rossi S, Basso M, Strippoli A, Schinzari G, D'Argento E, Larocca M, Cassano A, Barone C. Are Markers of Systemic Inflammation Good Prognostic Indicators in Colorectal Cancer? Clin Colorectal Cancer. 2017;16:264-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Yamamoto T, Kawada K, Obama K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 14. | Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38-45. [PubMed] [Cited in This Article: ] |
| 15. | Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, Baba Y, Sakamoto Y, Yamashita Y, Yoshida N, Chikamoto A, Baba H. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Zhang X. Controlling nutritional status score, a promising prognostic marker in patients with gastrointestinal cancers after surgery: A systematic review and meta-analysis. Int J Surg. 2018;55:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Niu X, Zhu Z, Bao J. Prognostic significance of pretreatment controlling nutritional status score in urological cancers: a systematic review and meta-analysis. Cancer Cell Int. 2021;21:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Toyokawa G, Kozuma Y, Matsubara T, Haratake N, Takamori S, Akamine T, Takada K, Katsura M, Shimokawa M, Shoji F, Okamoto T, Maehara Y. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J Thorac Dis. 2017;9:2942-2951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 20. | Yamamoto M, Saito H, Uejima C, Tanio A, Tada Y, Matsunaga T, Sakamoto T, Honjo S, Ashida K, Fujiwara Y. Combination of Serum Albumin and Cholinesterase Levels as Prognostic Indicator in Patients ith Colorectal Cancer. Anticancer Res. 2019;39:1085-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Barazzoni R, Jensen GL, Correia MITD, Gonzalez MC, Higashiguchi T, Shi HP, Bischoff SC, Boirie Y, Carrasco F, Cruz-Jentoft A, Fuchs-Tarlovsky V, Fukushima R, Heymsfield S, Mourtzakis M, Muscaritoli M, Norman K, Nyulasi I, Pisprasert V, Prado C, de van der Schuren M, Yoshida S, Yu Y, Cederholm T, Compher C. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. 2022;41:1425-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 94] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 22. | Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. 2020;11:366-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 23. | Golder AM, McMillan DC, Park JH, Mansouri D, Horgan PG, Roxburgh CS. The prognostic value of combined measures of the systemic inflammatory response in patients with colon cancer: an analysis of 1700 patients. Br J Cancer. 2021;124:1828-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol. 2020;119:199-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 922] [Cited by in F6Publishing: 1752] [Article Influence: 350.4] [Reference Citation Analysis (0)] |
| 27. | Zhang YY, Li WQ, Li ZF, Guo XH, Zhou SK, Lin A, Yan WH. Higher Levels of Pre-operative Peripheral Lymphocyte Count Is a Favorable Prognostic Factor for Patients With Stage I and II Rectal Cancer. Front Oncol. 2019;9:960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Zhao J, Huang W, Wu Y, Luo Y, Wu B, Cheng J, Chen J, Liu D, Li C. Prognostic role of pretreatment blood lymphocyte count in patients with solid tumors: a systematic review and meta-analysis. Cancer Cell Int. 2020;20:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1645] [Cited by in F6Publishing: 1944] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 30. | Deng CF, Zhu N, Zhao TJ, Li HF, Gu J, Liao DF, Qin L. Involvement of LDL and ox-LDL in Cancer Development and Its Therapeutical Potential. Front Oncol. 2022;12:803473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Lum DF, McQuaid KR, Gilbertson VL, Hughes-Fulford M. Coordinate up-regulation of low-density lipoprotein receptor and cyclo-oxygenase-2 gene expression in human colorectal cells and in colorectal adenocarcinoma biopsies. Int J Cancer. 1999;83:162-166. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91:41-45. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2: 132-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 365] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 34. | Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, Greenhaff PL, Hiesmayr M, Hjort Jakobsen D, Klek S, Krznaric Z, Ljungqvist O, McMillan DC, Rollins KE, Panisic Sekeljic M, Skipworth RJE, Stanga Z, Stockley A, Stockley R, Weimann A. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr. 2020;39:3211-3227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18:248-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |