Published online Oct 18, 2017. doi: 10.5312/wjo.v8.i10.754
Peer-review started: February 15, 2017
First decision: March 27, 2017
Revised: June 13, 2017
Accepted: July 7, 2017
Article in press: July 10, 2017
Published online: October 18, 2017
To determine the effects of a cell sheet created from sheep bone marrow and tricalcium phosphate (TCP) on osteogenesis.
Bone marrow cells were harvested from a sheep and cultured in a minimal essential medium (MEM) containing ascorbic acid phosphate (AscP) and dexamethasone (Dex). After 2 wk, the formed osteogenic matrix cell sheet was lifted from the culture dish using a scraper. Additionally, harvested bone marrow cells were cultured in MEM only as a negative control group, and in MEM with AscP, Dex, and β-glycerophosphate as a positive control group. For in vitro evaluation, we measured the alkaline phosphatase (ALP) activity and osteocalcin (OC) content in the media of the cultured cells from each group. For in vivo analysis, a porous TCP ceramic was used as a scaffold. We prepared an experimental group comprising TCP scaffolds wrapped with the osteogenic matrix cell sheets and a control group consisting of the TCP scaffold only. The constructs were implanted subcutaneously into athymic rats and the cell donor sheep, and bone formation was confirmed by histology after 4 wk.
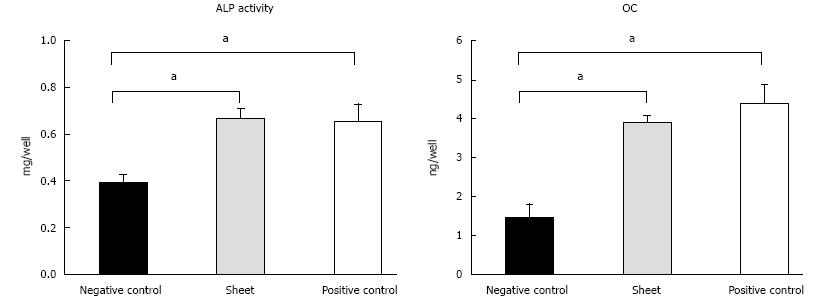
In the in vitro part, the mean ALP activity was 0.39 ± 0.03 mg/well in the negative control group, 0.67 ± 0.04 mg/well in the sheet group, and 0.65 ± 0.07 mg/well in the positive control group. The mean OC levels were 1.46 ± 0.33 ng/well in the negative control group, 3.92 ± 0.16 ng/well in the sheet group, and 4.4 ± 0.47 ng/well in the positive control group, respectively. The ALP activity and OC levels were significantly higher in the cell sheet and positive control groups than in the negative control group (P < 0.05). There was no significant difference in ALP activity or OC levels between the cell sheet group and the positive control group (P > 0.05). TCP constructs wrapped with cell sheets prior to implantation showed bone formation, in contrast to TCP scaffolds alone, which exhibited poor bone formation when implanted, in the subcutaneous layer both in athymic rats and in the sheep.
This technique for preparing highly osteoinductive TCP may promote regeneration in large bone defects.
Core tip: An osteogenic matrix cell sheet derived from sheep bone marrow enhances osteogenic differentiation. We found that the osteogenic matrix cell sheets on tricalcium phosphate discs efficiently promotes bone formation.
- Citation: Kira T, Akahane M, Omokawa S, Shimizu T, Kawate K, Onishi T, Tanaka Y. Bone regeneration with osteogenic matrix cell sheet and tricalcium phosphate: An experimental study in sheep. World J Orthop 2017; 8(10): 754-760
- URL: https://www.wjgnet.com/2218-5836/full/v8/i10/754.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i10.754
Massive bone defects that result from trauma or tumor resection, osteomyelitis, or osteonecrosis require bone grafting and represent a great burden in clinical practice. Although an autologous bone graft transferred as either vascularized or non-vascularized tissue remains the gold standard to treat bone defects, the graft procedure is associated with complications at donor sites[1,2]. Allografts carry high risks of infections, immunological rejection, and poor rate of bone-healing[3]. Although artificial bone material possesses some osteoinductive and osteoconductive activities, its osteogenic potential is limited[4].
Bone marrow mesenchymal stromal/stem cells (BMSCs) are capable of differentiating into osteoblasts, chondrocytes, or adipocytes in vitro and are widely applied in bone tissue engineering[5]. They are preferably combined with scaffolds to prevent the BMSCs from flowing out of the target site[6].
We previously developed a new technique of BMSC transplantation using osteogenic matrix cell sheets (OMCSs) derived from rat BMSCs to induce osteogenesis[7]. Because these OMCSs do not require a scaffold and maintain intercellular networks with the extracellular matrix that they produce, these sheets can be used in various graft sites in animal models[8,9]. Furthermore, these OMCSs produce growth factors, such as bone morphogenetic protein and vascular endothelial growth factor. Therefore the OMCSs represent an ideal candidate for promoting new bone formation. However, no studies have investigated in vivo osteogenesis of OMCSs in a large animal model.
This study aimed to investigate whether OMCSs could promote in vivo osteogenesis in a sheep model. The sheep is a frequently used model for orthopedic research for several reasons: The bone size is large enough to allow complex orthopedic procedures to be performed and for medical devices and biomaterials to be tested; the lifespan of the animal is short enough for age-related studies in diseases such as osteoarthritis and osteoporosis to be performed; and bone remodeling in sheep is comparable to that in humans[10,11].
BMSCs were obtained from the humeral head of a 2-year-old male Corriedale sheep (40.0 kg body weight; Japan Lamb, Hiroshima, Japan) by bone marrow aspiration under general anesthesia with intravenous atipamezole (20 μg/kg IV, ZENOAQ, Fukushima, Japan) and induction with intravenous ketamine (2 mg/kg IV, Daiichi Sankyo Propharma, Tokyo. Japan). The aspirated cells were collected in two 75-cm2 culture flasks (Falcon; BD Biosciences, Franklin Lakes, NJ, United States) containing 15 mL of regular medium comprising minimal essential medium (Nacalai Tesque, Kyoto, Japan) supplemented with 15% fetal bovine serum (Gibco Life Technologies, Carlsbad, CA, United States) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Nacalai Tesque). Cells were cultured in a humidified atmosphere of 95% air and 5% carbon dioxide at 37 °C. After reaching confluence (at approximately day 14), the primary cultured cells were released from the culture substratum using trypsin-EDTA (Nacalai Tesque).
To create OMCSs, the cells released from the primary culture were seeded at 2 × 103 cells/cm2 in culture dishes for subculture in regular medium containing 10 nmol/L dexamethasone (Dex, Sigma, St. Louis, MO, United States) and 0.28 mmol/L l-ascorbic acid phosphate magnesium salt n-hydrate (AscP, Wako Pure Chemical Industrials, Kyoto, Japan) until they reached confluence (at approximately day 14). After two rinses with PBS (Gibco), the cell sheet was lifted using a scraper. The cell sheet was easily detached from the culture dish by gentle scraping in PBS, starting from the periphery of the sheet (Figure 1A). As positive and negative controls for osteoblastic differentiation, respectively, released cells were also seeded at the same cell density and cultured in osteoinductive medium (containing 10 nmol/L Dex, 0.28 mmol/L AscP, and 10 mmol/L β-glycerophosphate) or in regular medium (without Dex, AscP or β-glycerophosphate) until they reached confluence.
Alkaline phosphatase activity measurement: Alkaline phosphatase (ALP) activity was measured in cells cultured in 12-well plates (Falcon), as reported previously[12]. For each condition, six wells were evaluated. ALP activity is represented as the amount of p-nitrophenol released after 30 min of incubation at 37 °C. The measurements were repeated twice.
Osteocalcin measurement: The Osteocalcin (OC) content of the culture medium was measured by an ELISA developed in a previous study[13]. Briefly, conditioned medium was collected at day 12 and an aliquot (100 μL) of 1:10 diluted medium was analyzed. The OC measurements evaluated four wells for each group, and the measurements were repeated twice.
Construction of tricalcium phosphate scaffold wrapped with a OMCS: Sterilized porous beta tricalcium phosphate (TCP) ceramics (Superpore; discs: 5 mm in diameter and 2 mm thick; 60% porosity) were purchased from Pentax (Tokyo, Japan). Constructs with OMCSs were prepared by wrapping the OMCS around the TCP immediately previous to transplantation (Figure 1B and C).
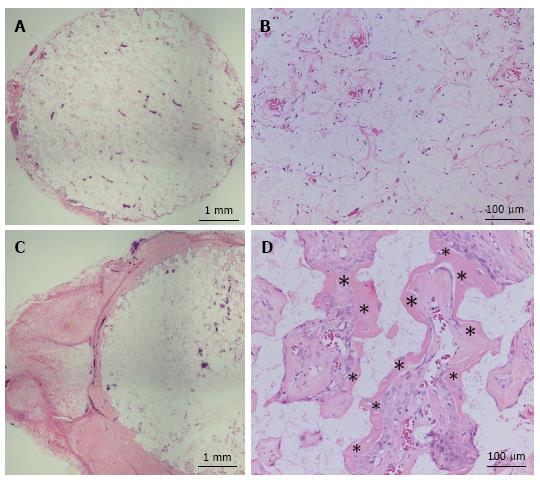
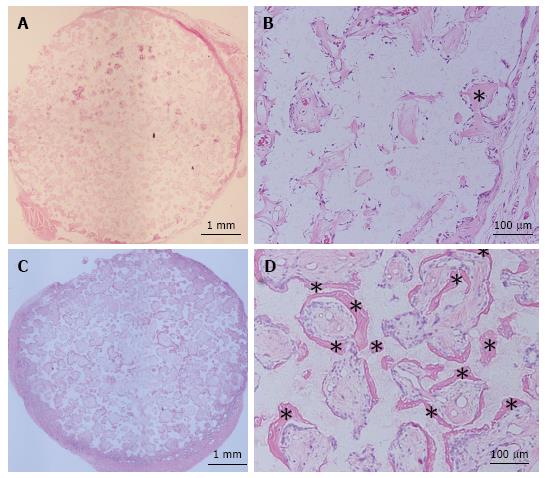
Construct implantation in the subcutaneous layer of athymic rats and the cell donor sheep: TCP constructs were implanted into the subcutaneous layer on the backs of athymic 7-wk-old male F344/NJcl-rnu/rnu rats (CLEA Japan Inc., Tokyo, Japan) as described previously[7,14]. Additionally, constructs were implanted subcutaneously into the abdomen of the cell donor sheep under the general anesthesia conditions described above. We also prepared control groups in which TCP discs were implanted subcutaneously into athymic rats and at a different site in the sheep. For each group, six constructs were implanted into one recipient rat to produce the subcutaneous implantation model, and all constructs were implanted into the cell donor sheep. After 4 wk, the implanted constructs were harvested and bone formation was histologically evaluated. The harvested discs were fixed in buffered formalin (Wako Pure Chemical Industries). Each disc was embedded in paraffin after decalcification, cut through the middle of the disk, and then stained with hematoxylin and eosin for histological evaluation.
The care and handling of the rats and the sheep used in this study was approved by the animal care committee of our institute and met the standards of the National Institutes of Health.
The ALP activity values and OC levels are presented as mean and SD. One-way analysis of variance with post-hoc multiple comparisons using Tukey’s test was conducted to determine statistical significance. Values of P < 0.05 were considered statistically significant.
In the in vitro study, the mean ALP activity was 0.39 ± 0.03 mg/well in the negative control group, 0.67 ± 0.04 mg/well in the sheet group, and 0.65 ± 0.07 mg/well in the positive control group (Figure 2). The mean OC levels were 1.46 ± 0.33 ng/well in the negative control group, 3.92 ± 0.16 ng/well in the sheet group, and 4.4 ± 0.47 ng/well in the positive control group, respectively. The ALP activity and OC levels were significantly higher in the cell sheet and positive control groups than in the negative control group (P < 0.05). There was no significant difference in ALP activity or OC levels between the cell sheet group and the positive control group (P > 0.05).
Figures 3 and 4 show representative histological sections of constructs subcutaneously implanted at 4 wk into athymic rats and the cell donor sheep, respectively. The low-magnification images show higher levels of bone formation in the TCP-cell sheet construct sections than in sections of TCP discs without the cell sheet, in the subcutaneous layer of both athymic rats and sheep.
Our study demonstrates that we can successfully use large animal models to derive and culture OMCS, and that TCP constructs wrapped with sheep OMCSs lead to bone formation after implantation. This is important, as sheep bone healing is more comparable to that of humans than rodents used in previous models.
Even though BMSCs alone have osteogenic potential, their implantation as a cell suspensions leads to an uneven distribution and weak adhesion of cells to the bone surface, potentially leading to cell defluvium from the target site[15]. In contrast, cell sheets bear intact cell-cell junctions and an extracellular matrix, which provide mechanical support to the cell and thereby maintain the viability of the cells at the transplanted site[16]. Furthermore, prior culture with medium that includes agents that potentiate bone formation, such as Dex and AscP, allows for already pre-determined cells to be implanted[17], which have a better chance of laying down additional matrix and integrating with the transplant site than cells that are still actively engaged in proliferation.
BMSCs including osteoblasts and osteoprogenitor cells are bone-forming cells that express various osteoblastic markers such as OC, and exhibit ALP activity[18,19]. ALP is considered a relevant biochemical marker in osteoblast differentiation. The activity and correct localization of ALP are necessary for bone development and differentiation[20,21]. OC is considered a late marker of osteogenic differentiation and its expression at high levels indicates maturation and terminal differentiation of osteoblasts[22]. Others have suggested that Dex may inhibit OC expression through direct binding of the Dex-activated glucocorticoid receptor to negative glucocorticoid response elements in the OC promoter[23]. However, we observed enhanced OC expression in OMCSs compared with the negative control group[23]. The effect of Dex is dependent on its concentration and on the stage of cellular differentiation. The Dex concentration used in this study (10 nmol/L) is considered an appropriate concentration for the differentiation of BMSCs committed to the osteogenic lineage. The ALP activity and OC levels observed in the OMCS suggest that the osteogenic differentiation ability of the BMSCs was enhanced by AscP and Dex.
OMCSs can be easily detached from the plastic dish and transplanted subcutaneously with or without a scaffold, and has osteogenic potential to form new bone tissue. The TCP scaffold also has osteoinductive properties in bone; however, minimal osteogenesis was observed inside the TCP without the cell sheet in athymic rats, presumably because of the small number of osteoprogenitor cells available for integration into the disc in the subcutaneous layer. Conversely, substantial amounts of new bone was found inside the TCP that had been wrapped with an OMCS. We suggest that these transplanted sheep cells from the OMCSs migrated into the TCP disc, proliferated, differentiated into osteoblasts, and mineralized, and thus induced bone formation.
Results similar to those for athymic rats were obtained for implantation in the cell donor sheep. We have previously investigated cell sheets derived from rodent bone marrow and reported their efficacy for osteogenesis, angiogenesis and reconstructive surgery[8,9,24,25]. We found that severe fracture and nonunion could be united by the implantation of OMCSs[9,24] and that OMCSs can enhance early bone tunnel healing in a tendon graft model[8]. Furthermore, we show that a vascularized tissue-engineered bone scaffold composed of OMCSs wrapped around vascular bundles within a TCP mediates abundant vascularization and osteogenesis[25]. Although these studies reported that OMCS transplantation is useful for bone reconstruction, large animal studies are required to further support the potential of OMSC transplantation for clinical application. Guo et al[26] created cell sheets using canine cells, and showed that cell sheets exhibited normal activity and a preserved extracellular matrix and multi-layer cell structure, and displayed osteogenic induction. They used AscP (vitamin C) to create cell sheets from bone marrow mesenchymal stem cells, whereas we used a combination of AscP and Dex to create our cell sheets. Culturing cell in the medium containing AscP and Dex can stimulate osteogenic differentiation and complete cell sheet formation; therefore, using induction medium containing AscP and Dex may be more suitable for creating cell sheets for application in bone reconstruction surgery[17].
We chose a sheep model because sheep have bone properties similar to those of humans, with similar bone turnover and remodeling activities[27-29]. Furthermore, their size allows for the simultaneous implantation of many TCP constructs[30-33], and the subcutaneous implantation approach used in this study permitted the implantation of multiple TCP constructs in one sheep. Thus, the results of these in vivo studies suggest that human OMCSs can induce high levels of osteogenesis in TCP.
There were a few limitations in our study. First, we only used 2-mm-thick TCP. In future studies, we aim to use thicker TCP discs to assess their osteogenic ability when transplanted with OMCSs. Second, the experimental period in the present study was relatively short. Therefore, a longer follow-up study is required to investigate whether the implanted TCP remains in the sheep or is broken down and resorbed by the newly formed tissue. Third, we did not confirm whether the bone that formed in the TCP construct was derived from host cells or donor cells. Finally, we need to verify osteoinductive and osteoconductive ability of human OMCSs. Concerning these points, further study will be necessary.
Osteogenic matrix cell sheets (OMCSs) created from bone marrow mesenchymal stromal/stem cells (BMSCs) can be used to aid fracture nonunion, delayed bone union, or bone defects in rodent models. However, there has been no report as to whether OMCSs can also successfully induce bone formation in a large animal model. Here, the authors investigated whether OMCSs could promote in vivo osteogenesis in a sheep model.
OMCSs are easily created from BMSCs and can be transplanted without the requirement for a scaffold. OMCSs have a high osteogenic potential, because the cells are supported by a rich extracellular matrix and various growth factors required for bone formation. As such, OMCSs are thought to be an ideal graft material for bone regenerative medicine.
Over the past decade, the utility of OMCSs for bone reconstruction has been verified in rat models of bone repair. However, rat bone does not contain Haversian canals, and thus the mode of remodeling differs to that of humans. In the current study, the authors used a sheep model to explore the osteoconductive and osteoinductive potential of sheep-drived OMCSs as sheep bone formation and repair is similar to that of human bone.
The authors suggest that OMCSs in combination with bone prostheses could be manipulated to intricately shape bony defects, and will be particularly relevant in areas where the osteogenic potential for regeneration is poor, such as in older patients or patients with bone disorders.
TCP: Tricalcium phosphate is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. TCP is grafted into the bone defect, but has lowered osteogenic potential; BMSCs: Bone marrow mesenchymal stromal/stem cells. BMSCs can be induced to differentiate into osteogenic, chondrogenic, adipogenic or other cell lineages with the appropriate media conditions. Furthermore, BMSCs secrete various growth factors, such as bone morphogenetic protein, basic fibroblast growth factor, transforming growth factor, vascular endothelial growth factor, as well as matrix proteins, including osteocalcin, and alkaline phosphatase. These cells are routinely used as a cell source for musculoskeletal tissue engineering purposes; OMCSs: Osteogenic Matrix Cell Sheets are BMSCs cultured with dexamethasone and ascorbic acid phosphate. The cells undergo differentiation and matrix production, producing a cell sheet structure that can be collected as a single cell sheet. These sheets offer in vitro osteogenic potential and in vivo bone formation with/without the aid of an additional scaffold.
The manuscript is an interesting biotechnological application of bone marrow-derived cell sheets to induce subcutaneous osteogenesis in a rat and a sheep model. The manuscript is of interest in its field and has novelty since it explores the use of these cell sheets in a large animal model, the sheep, which outcomes are fairly more comparable to humans than the usual rodent models.
We would like to thank to Ms Kunda F and Ms Matsumura M (Nara Medical University) for their technical assistance.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Emara KM, Peng BG, Scarfì S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Colen SR, Shaw WW, McCarthy JG. Review of the morbidity of 300 free-flap donor sites. Plast Reconstr Surg. 1986;77:948-953. [PubMed] [Cited in This Article: ] |
| 2. | Laurie SW, Kaban LB, Mulliken JB, Murray JE. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933-938. [PubMed] [Cited in This Article: ] |
| 3. | Agarwal R, García AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev. 2015;94:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 399] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 4. | Liu B, Lun DX. Current application of β-tricalcium phosphate composites in orthopaedics. Orthop Surg. 2012;4:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Jiang X, Zou S, Ye B, Zhu S, Liu Y, Hu J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone. 2010;46:1156-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Pneumaticos SG, Triantafyllopoulos GK, Chatziioannou S, Basdra EK, Papavassiliou AG. Biomolecular strategies of bone augmentation in spinal surgery. Trends Mol Med. 2011;17:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Akahane M, Nakamura A, Ohgushi H, Shigematsu H, Dohi Y, Takakura Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008;2:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Inagaki Y, Uematsu K, Akahane M, Morita Y, Ogawa M, Ueha T, Shimizu T, Kura T, Kawate K, Tanaka Y. Osteogenic matrix cell sheet transplantation enhances early tendon graft to bone tunnel healing in rabbits. Biomed Res Int. 2013;2013:842192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Shimizu T, Akahane M, Morita Y, Omokawa S, Nakano K, Kira T, Onishi T, Inagaki Y, Okuda A, Kawate K. The regeneration and augmentation of bone with injectable osteogenic cell sheet in a rat critical fracture healing model. Injury. 2015;46:1457-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1-10. [PubMed] [Cited in This Article: ] |
| 11. | Andreasen CM, Ding M, Overgaard S, Bollen P, Andersen TL. A reversal phase arrest uncoupling the bone formation and resorption contributes to the bone loss in glucocorticoid treated ovariectomised aged sheep. Bone. 2015;75:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ohgushi H, Dohi Y, Katuda T, Tamai S, Tabata S, Suwa Y. In vitro bone formation by rat marrow cell culture. J Biomed Mater Res. 1996;32:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15:169-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Tohma Y, Ohgushi H, Morishita T, Dohi Y, Tadokoro M, Tanaka Y, Takakura Y. Bone marrow-derived mesenchymal cells can rescue osteogenic capacity of devitalized autologous bone. J Tissue Eng Regen Med. 2008;2:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Yamato M, Okano T. Cell sheet engineering. Materials Today. 2004;7:42-47. [Cited in This Article: ] |
| 16. | Xie Q, Wang Z, Huang Y, Bi X, Zhou H, Lin M, Yu Z, Wang Y, Ni N, Sun J. Characterization of human ethmoid sinus mucosa derived mesenchymal stem cells (hESMSCs) and the application of hESMSCs cell sheets in bone regeneration. Biomaterials. 2015;66:67-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Akahane M, Shimizu T, Kira T, Onishi T, Uchihara Y, Imamura T, Tanaka Y. Culturing bone marrow cells with dexamethasone and ascorbic acid improves osteogenic cell sheet structure. Bone Joint Res. 2016;5:569-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 394] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int. 2003;14 Suppl 3:S35-S42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Miao D, Scutt A. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J Histochem Cytochem. 2002;50:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Sabokbar A, Millett PJ, Myer B, Rushton N. A rapid, quantitative assay for measuring alkaline phosphatase activity in osteoblastic cells in vitro. Bone Miner. 1994;27:57-67. [PubMed] [Cited in This Article: ] |
| 22. | Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731-2733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone. 2009;45:579-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T, Tanaka Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46:418-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Nakano K, Murata K, Omokawa S, Akahane M, Shimizu T, Kawamura K, Kawate K, Tanaka Y. Promotion of Osteogenesis and Angiogenesis in Vascularized Tissue-Engineered Bone Using Osteogenic Matrix Cell Sheets. Plast Reconstr Surg. 2016;137:1476-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Guo P, Zeng JJ, Zhou N. A novel experimental study on the fabrication and biological characteristics of canine bone marrow mesenchymal stem cells sheet using vitamin C. Scanning. 2015;37:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Martini L, Fini M, Giavaresi G, Giardino R. Sheep model in orthopedic research: a literature review. Comp Med. 2001;51:292-299. [PubMed] [Cited in This Article: ] |
| 28. | Gardel LS, Serra LA, Reis RL, Gomes ME. Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng Part B Rev. 2014;20:126-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Timmins NE, Scherberich A, Früh JA, Heberer M, Martin I, Jakob M. Three-dimensional cell culture and tissue engineering in a T-CUP (tissue culture under perfusion). Tissue Eng. 2007;13:2021-2028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Reichert JC, Woodruff MA, Friis T, Quent VM, Gronthos S, Duda GN, Schütz MA, Hutmacher DW. Ovine bone- and marrow-derived progenitor cells and their potential for scaffold-based bone tissue engineering applications in vitro and in vivo. J Tissue Eng Regen Med. 2010;4:565-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Braccini A, Wendt D, Jaquiery C, Jakob M, Heberer M, Kenins L, Wodnar-Filipowicz A, Quarto R, Martin I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells. 2005;23:1066-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Cheng M, Moretti M, Engelmayr GC, Freed LE. Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng Part A. 2009;15:645-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, Caplan AI, Guilak F, Freed LE. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials. 2010;31:2193-2200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |