Published online Jun 18, 2015. doi: 10.5312/wjo.v6.i5.439
Peer-review started: February 22, 2015
First decision: March 20, 2015
Revised: April 14, 2015
Accepted: May 8, 2015
Article in press: May 11, 2015
Published online: June 18, 2015
AIM: To identify the rate of non-responders to clopidogrel treatment in hip fracture patients and study how non-responders differ from controls.
METHODS: In a retrospective case-control study we included 28 cases of acute proximal femoral fracture with clopidogrel treatment 2011 to 2013. Eighty-four controls from the same time period were included. Data collected included response to clopidogrel measured with multiple electrode aggregometry (MEA), intraoperative bleeding, erythrocyte transfusion, time to surgery and the incidence of adverse events up to 3 mo after surgery.
RESULTS: Eight (29%) of the 28 cases were non-responders. The median intraoperative bleeding was 300 mL (range, 0-1500), and was lower for non-responders (50 mL) but did not reach statistical significance. Erythrocyte transfusions did not differ between responders, non-responders and controls. Forty-five (40%) of 112 patients had adverse events postoperatively but the rate did not differ between patients with and without clopidogrel treatment.
CONCLUSION: Almost one-third of patients with clopidogrel treatment and an acute proximal femoral fracture are non-responders to antiplatelet therapy and can be operated without delay.
Core tip: In this pilot study, almost one-third of patients with clopidogrel treatment and an acute proximal femoral fracture are non-responders to antiplatelet therapy. Analysis of variability in platelet aggregation can be used when fast tracking patients and we recommend this for emergency hospitals treating patients with acute proximal femoral fractures.
- Citation: Clareus A, Fredriksson I, Wallén H, Gordon M, Stark A, Sköldenberg O. Variability of platelet aggregation in patients with clopidogrel treatment and hip fracture: A retrospective case-control study on 112 patients. World J Orthop 2015; 6(5): 439-445
- URL: https://www.wjgnet.com/2218-5836/full/v6/i5/439.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i5.439
An increasing number of elderly patients are managed with long term antiplatelet therapy after cardiovascular and cerebrovascular events[1]. Clopidogrel is a frequently used antiplatelet drug which irreversibly inhibits ADP-induced platelet aggregation through blockade of the platelet P2Y12 receptor. Although the drug has been shown to be very effective in large clinical trials, there is a considerable inter-individual response to this drug. Depending on the platelet function method used and cut-off values set, between 5%-44% of patients have been shown to have reduced platelet inhibiting effect of the drug. The reason for this variability is likely multifactorial and include, e.g., genotype, drug interactions and compliance to drug treatment[2-4].
Approximately 1 in 5 of patients with clopidogrel treatment will need non-cardiac surgery within two years[5]. It is well known that patients with clopidogrel treatment undergoing cardiovascular surgery have an increased risk of bleeding events during and after surgery. They also have a higher percentage of post-operative hemorrhagic complications and transfusions[6,7]. Clinical guidelines recommend that patients on clopidogrel treatment should interrupt their therapy 5-7 d before surgery to avoid increased intraoperative bleeding, even though recent studies of patients undergoing hip fracture surgery is inconsistent if the risk of perioperative bleeding is increased or not[8].
Patients suffering a hip fracture are elderly, and because of multiple co-morbidities attributed to age, they are one of the most fragile patient-groups in orthopedics, with a high morbidity and mortality following surgical treatment. Delayed surgery is associated with both increased frequency of medical complications and increased mortality[9,10]. Thus, the demand for rapid surgery is, for hip fracture patients with simultaneous clopidogrel treatment, contrasted against the bleeding risk for these patients.
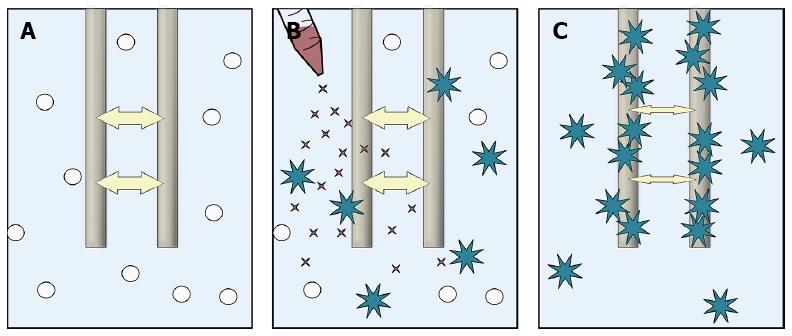
Laboratory tests have recently been developed in order to examine platelet function bedside. One of these methods is multiple electrode aggregometry (MEA)[11]. This method can be used to assess platelet aggregation during treatment with platelet inhibiting agents such as clopidogrel, aspirin and other new platelet inhibitors in a venous whole blood sample (Figure 1)[11].
The rate of non-responders and responders for clopidogrel treated patients in hip fracture patients has, to the best of our knowledge, not been published in peer-reviewed literature. The aim of this study was to identify the rate of non-responders to clopidogrel treatment in hip fracture patients and to study if responders and non-responders differ from patients without clopidogrel in intraoperative bleeding and adverse events.
The study was conducted in accordance with the ethical principles of the Helsinki declaration and was approved by Ethics Committee of the Karolinska Institute. This retrospective case-control study was performed at the Orthopedic Department of Danderyd Hospital in collaboration with the Karolinska Institute (Department of Clinical Sciences at Danderyd Hospital) in Stockholm, Sweden. Danderyd Hospital is 1 of the 5 major emergency hospitals in Stockholm, providing medical care with a catchment area of approximately 500000 inhabitants.
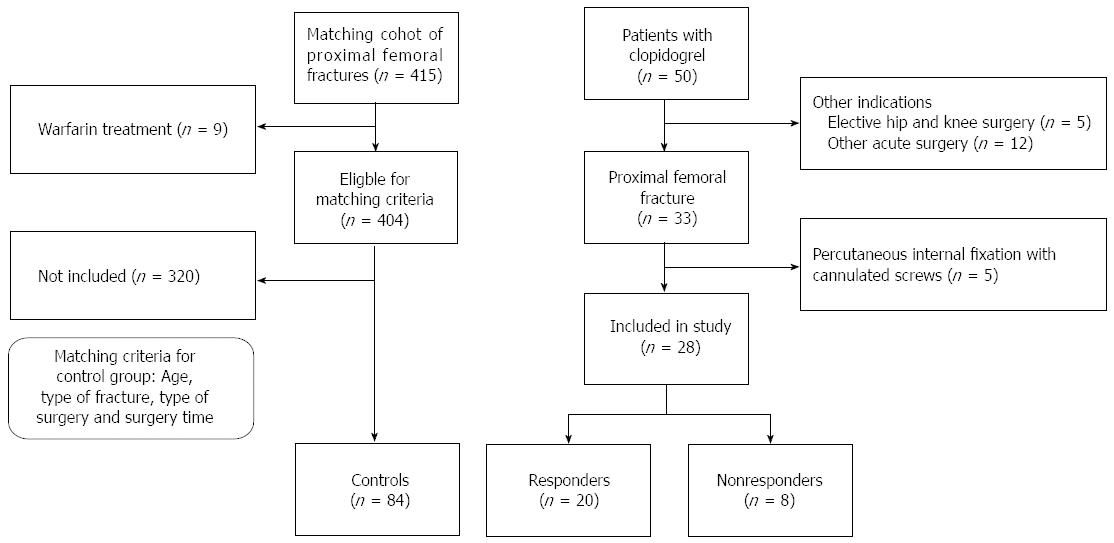
At our department, we fast-track hip fracture patients and operate > 80% of patients within 24 h from arrival to the hospital[12]. We included patients undergoing treatment with clopidogrel who had a concomitant primary hip fracture or periprosthetic fracture that required acute surgery between 2011-2013. From this group, we excluded patients with non-displaced femoral neck fracture who underwent percutaneous internal fixation with cannulated screws because of the minimal risk of bleeding through this type of surgery. We also excluded patients with other acute orthopedic injuries and patients planned for elective surgery. Three matched controls for every included patient were identified from our department including all patients who had been treated at the clinic for hip fracture during 2010-2013. Patients with warfarin treatment were excluded from the control group. The controls were then matched according to age, type of fracture (inter- or subtrochanteric/femoral neck/periprosthetic), type of surgery (sliding hip screw or intramedullary nail/hip arthroplasty/plate osteosynthesis/femoral stem revision) and operation time.
The outcome variables were the rate of non-responders in patients with clopidogrel treatment, perioperative bleeding in millilitre and the occurrence of adverse events up to 3 mo after surgery. Other variables collected included sex, age, American Society of Anesthesiologists (ASA)-classification[13], type of surgery, type of fracture and surgery time.
Blood samples for MEA were taken from an antecubital vein and collected in hirudin tubes (Refludan, Dynabyte). Test tubes were kept at room temperature until analysis 30-179 min after collection. MEA measured by Multiplate™ (Dynabyte, Munich, Germany) has been described elsewhere[14]. In brief, 300 μL of whole-blood is diluted 1:1 with 0.9% NaCl solution in cuvettes and heated to 37 °C under stirring for 3 min. After addition of a platelet agonist, platelets adhere to and aggregate on two pairs of silver-coated copper electrodes. The increase in electrical impedance between electrodes due to platelet aggregation is recorded in arbitrary units (AU) during 6 min (Figure 1). The MEA-value is the average area under the curve (AUC) for the two electrode pairs (AU*min) in the cuvette. For each patient 2 cuvettes were used. Adenosine diphosphate (ADP) was added at a final concentration of 6.4 μmol/L in each cuvette. We used the previously established cut off value to define clopidogrel responders[15]. Thus, responders were those who had a mean MEA ADP value of the readings obtained in the two cuvettes below 47 AU*min; non-responders were those with a value above this level[15].
From digital patient records the following parameters were collected: Type of hip fracture, ASA grade[13], indication for clopidogrel treatment, time to surgery in hours (arrival at the emergency department to skin incision), choice of anesthesia (general/spinal), method of surgery, preoperative treatment with platelet transfusion, intraoperative bleeding (assessed from suctions, drainage and swabs used during surgery) and peri- and post-operative transfusion with erythrocyte units and plasma. The intra-operative blood loss was calculated by measuring the fluid in collection containers subtracting the amount of lavage and by weighing surgical swabs. The total amount of transfusions given with platelets, erythrocytes and fresh-frozen plasma was recorded. The occurrence of adverse events (AEs) was recorded. The World Health Organization (WHO) definitions were used. An AE is defined as any unfavourable or unintended sign, symptom or disease associated with the use of a medical treatment or procedure, regardless of whether it is considered related to the medical treatment or procedure[16]. The Swedish personal identity number in conjunction with the Swedish Death Register and electronic hospital records was used to identify and verify all AEs as well as mortality up to 3 mo postoperatively.
Descriptive statistics were used. ANOVA and chi-square test were used to compare the groups. Bonferroni correction was used to correct for multiple comparison. The study size was derived from the number of available responders and non-responders during the study period. A P-value < 0.05 was considered significant. The statistical analysis was performed using SPSS Statistics software 22.0 for Mac (SPSS Inc., Chicago, IL).
One hundred and twelve patients were included in the study, 28 patients undergoing treatment with clopidogrel and 84 controls [male/females: 36/76, mean age 84 (range, 56-99) years] (Figure 2, Table 1). 20 patients where under clopidogrel treatment because of cerebral insult, 6 because of myocardial infarction with following artery stenting, and in 2 cases the indication of clopidogrel treatment could not be found in referral. Nineteen (17%) of the patients died during the study, mortality rate did not differ between the groups.
| Variable | None (n = 84) | Responder (n = 20) | Non-responder (n = 8) |
| Sex | |||
| Male | 22 (26) | 9 (45) | 5 (62) |
| Female | 62 (74) | 11 (55) | 3 (38) |
| Age | 85 (± 7) | 82 (± 9) | 88 (± 4) |
| ASA class | |||
| 1-2 | 27 (32) | 1 (5) | 0 (0) |
| 3-4 | 57 (68) | 19 (95) | 8 (100) |
| Fracture type | |||
| Femoral neck | 23 (27) | 8 (40) | 0 (0) |
| Per/subthrochanteric | 46 (55) | 8 (40) | 7 (88) |
| Periprostethic | 15 (18) | 4 (20) | 1 (12) |
| Surgery | |||
| Hemi/total arthroplasty | 23 (27) | 8 (40) | 0 (0) |
| Sliding hips screw or intramedullary nail | 46 (55) | 8 (40) | 7 (88) |
| Locking plate osteosynthesis | 6 (7) | 1 (5) | 1 (12) |
| Revision of implants | 9 (11) | 3 (15) | 0 (0) |
Twenty of the patients with clopidogrel treatment showed results of MEA as responders to the drug and 8 (29%) patients were non-responders. The median intraoperative bleeding was 300 mL (range, 0-1800). The bleeding was lower for non-responders but this did not reach statistical significance (P = 0.8). The responder group did not have more intraoperative bleeding than controls (Table 2). We found no significant difference in erythrocyte transfusion between the groups, with a median number of 2 units for controls and non-responders and 1 unit for responders (Table 2). The anaesthesia, plasma and platelet transfusions given differed between the groups (Table 3). Although tranexamic acid was given to the majority of all patients, platelet and plasma transfusions were more frequently given to patients with clopidogreal treatment, especially the responder group. Notably, platelet transfusion was mainly given to those of the patients with the most pronounced platelet inhibiting effect of clopidogrel. In contrast, in the non-responder group transfusion was given to 1 of the 8 patients (Table 3). The mean (SD) time to surgery was 26 ± 19 h and differed between the groups (P = 0.001). Responders waited on average almost one day more for their surgery compared to the controls and non-responders (Table 2). In 7 patients in the responder group, the reason for prolonged time to surgery was pronounced effect of clopidogrel treatment with significanty lower values than the other responders. In these cases the risk of major bleeding was considered higher than the risk of delayed hip fracture surgery, and it was recommended from the cardiologist or anesthesiologist consulted to wait with surgery if possible. These patients were re-tested before surgery with multiple electrode aggregometry. Data showed that the antiplatelet effect of clopidogrel had decreased to a ADP value of over 47 (i.e., a non-reponder value).
| Variable | Control (n = 84) | Responder (n = 20) | Non-responder (n = 8) | P-value |
| Peroperative bleedning (mean ± SD) | 350 (0-1800) | 300 (50-1500) | 150 (50-550) | 0.82 |
| Any erythrocyte transfusion, n (%) | 54 (64) | 13 (65) | 6 (75) | |
| Number of transfusions, median (range) | 2 (0-10) | 1 (0-6) | 2 (0-6) | 1.02 |
| Time to surgery (h), mean ± SD | 21 (± 12) | 45 (± 26) | 28 (± 33) | 0.0032 |
| Adverse events | ||||
| Any AE, n (%) | 31 (37) | 9 (45) | 5 (62) | 1.03 |
| Type of AE, n1 | ||||
| Deceased, n | 14 | 2 | 3 | |
| Hip related | 6 | 2 | 0 | |
| Cardiovascular | 8 | 1 | 3 | |
| Infection | 14 | 9 | 4 | |
| Other | 24 | 6 | 3 |
| Variable | Control (n = 84) | Responder (n = 20) | Non-responder (n = 8) | P-value |
| Anaesthesia | ||||
| Spinal | 82 (98) | 5 (25) | 7 (88) | 1 |
| General | 2 (2) | 15 (75) | 1 (12) | |
| Tranexamic acid | ||||
| No | 9 (11) | 3 (15) | 0 (0) | |
| Yes | 75 (89) | 17 (85) | 8 (100) | 0.003 |
| Plasma transfusion transfusion | ||||
| No | 83 (99) | 15 (75) | 7 (88) | |
| Yes | 1 (1) | 5 (25) | 1 (12) | 0.003 |
| Thrombocyte transfusion | ||||
| No | 84 (100) | 9 (45) | 7 (88) | |
| Yes | 0 (0) | 11 (55) | 1 (12) | 0.003 |
In this retrospective case-control study on patients with a proximal femoral fracture and concurrent clopidogrel treatment almost one third of patients with clopidogrel were non-responders, indicating that they had no effect of this treatment, or that they were not compliant to medication. Thus, the incidence of non-responsiveness is similar to what we found in patients with ischemic stroke or TIA treated with clopidogrel at our institution[17]. By continuously using MEA at our department, we were able to fast-track non-responders to surgery within the same time as the control group (Table 2).
In previous publications, anti-platelet treated hip fracture patients have been delayed to surgery, often with negative effects on complication rate and mortality. Harty et al[18] included 21 patients on clopidogrel with acute hip fracture in a case-control study and found that patients on clopidogrel in mean waited 7 d for surgery, and 30-d mortality for these patients were 29%, compared to the control group who had surgery within 2 d and had a 30-d mortality of 4%. The authors conclude that surgery should not be postponed. A high rate of complications due to prolonged time to surgery was also observed in a study made of Johansen et al[19], where clopidogrel treated patients who waited 5 d for surgery had a higher rate of complications and they also found an increased intraoperative bleeding for patients who had surgery immediately. This result is in line with Chechik et al[20] study of 44 patients where clopidogrel was continued throughout surgery. In contrast to these findings, there are reports that have failed to find a difference in bleeding and complication rate between patients on clopidogrel and controls[21,22]. These inconsistences between existing studies is possibly, as in our study, due to the fact that a large proportion of patients are non-responders[23,24]. These relatively recent studies of hip fracture patients with clopidogrel treatment have not considered the individual responsiveness to the drug, which may be an important reason for the inconsistency in their results[18,20-22]. If almost one third of the patients with clopidogrel (as in our study) have no effect of treatment the non-responders could even out the results a group level and hide the actual bleeding risk for responders. More research is needed in this area but clearly observational and interventional studies on hip fracture patients with concurrent antiplatelet therapy need to take this into consideration for bleeding endpoints.
The clinical recommendation to discontinue clopidogrel treatment 5-7 d ahead of surgery is based on studies made for cardiological interventions. They have reported that continuation, or late (i.e., 1 d) discontinuation of clopidogrel treatment is associated with increased intra- and postoperative bleeding and increased need for transfusions after coronary bypass surgery, compared to earlier discontinuation (i.e., 3-5 d)[6,7,25]. For hip fracture patients, this discontinuation is in stark contrast to the need for rapid surgery. Delayed surgery is associated with both increased morbidity and mortality[12,26]. Both the 30-d all-cause mortality as well as minor and major medical complications are significant higher in hip fracture patients with surgical delay over 48 h[9,10].
When patients with increased risk of bleeding are identified, preoperative treatment can be customized. In the above mentioned studies of hip fracture surgery and clopidogrel treatment, it is not reported if preoperative treatment differ between clopidogrel treated patients and controls. Wallace and Hossain report that all patients were medically optimized before surgery but do not mention if preoperative transfusion with platelets were given[22,27]. In our study patients with pronounced effect of the drug received platelet transfusions to higher extent than controls and those patients with none or low effect, and intraoperative bleeding did not differ between the groups. Neither did post-operative erythrocyte transfusion differ between the groups. Platelet transfusions should be administered with care, as whole blood transfusions, due to transfusion related complications such as infections, allergic reactions and febrile non-heamolytic transfusion[28]. This is why we find it important to identify patients that are at high risk for bleeding, so that we do not treat patients with platelet transfusion preoperatively if not needed.
In our study, non-responders were operated within the same time as controls, which likely reduces the risk of complications caused by delayed surgery. Patients with good response to the drug could either be optimized with platelet transfusion and operated immediately, or if their medical condition allowed, wait for surgery until a second analysis showed regression of clopidogrel effect. This is the reason why responders wait for surgery in mean 45 h compared to non-responders and controls who have surgery approximately within a day.
Analysis of platelet aggregation variability was also for help when planning for anaesthetics. Regional anaesthetics is associated with less risk for the patient compared to general anaesthetics and shortens operation time[29,30]. Hossain et al[27] reported in their study of 50 hip fractures and clopidogrel treatment that 88% of the patients had surgery in general anaesthesia, compared to controls were only 6% had general anaesthesia. In our study, patients eligible for spinal anaesthesia despite anti-platelet therapy could be identified preoperatively.
Variability of platelet function was, in the present study, evaluated with the MEA method. It is a standardized method to determine platelet function with high sensitivity and reproducibility[11]. The analysis is, compared to many other platelet function methods, a simple and rapid assay that can be used bedside in every day clinical practice. No centrifugation step which may influence platelet function is needed, as the analysis is made in a whole blood sample. Compared to template bleeding time which has previously been used to assess the risk of increased bleeding, the risk for user dependent variation is low since the method is easier to perform[11,14,31,32].
This is, to the best of our knowledge, the first published study where analysis of variability in platelet aggregation is used for patients with an acute proximal femoral fracture. We were able to obtain sound data on all studied outcome variable and found well matched controls for our cases. The main limitations of the study are the small sample size, retrospective design and relatively short follow-up. Thus, even though our groups did not differ in the incidence of adverse events or mortality rate, the study is not sufficiently powered for these outcomes. We have used intraoperative bleeding as a proxy for this, and most surgeons would agree that it is important to minimize blood loss for hip fracture patients. The study is also limited by intervention bias; MEA test was used to make clinical decisions such as platelet transfusions and surgical timing. These interventions could clearly have an effect on the results such as intraoperative bleeding as well as outcomes and differences between groups. This is however inherent in the method when using MEA and we believe that it is therefore this analysis is helpful for clinicians when making decision on timing of surgery.
In this pilot study, almost one-third of patients with clopidogrel treatment and a acute proximal femoral fracture are non-responders to antiplatelet therapy when presenting at the hospital. Analysis of variability in platelet aggregation can be used when fast tracking patients and we recommend this for emergency hospitals treating patients with acute proximal femoral fractures.
To identify the rate of non-responders to clopidogrel treatment in hip fracture patients and study how non-responders differ from controls.
The incidence of non-responsiveness to clopidogrel treatment for hip-fracture patients is unknown. By continuously using multiple electrode aggregometry (MEA) at the authors’ department they can fast-track non-responders to surgery despite clopidogrel treatment.
In this retrospective case-control study on patients with a proximal femoral fracture and concurrent clopidogrel treatment almost one third of patients with clopidogrel were non-responders, indicating that they had no effect of this treatment, or that they were not compliant to medication.
Analysis of variability in platelet aggregation using MEA can be used to identify non-responders and responders to clopidogrel treatment in hip fracture patients and they recommend this for emergency hospitals treating patients with acute proximal femoral fractures.
Responders: (i.e., to clopidogrel treatment): patients with anti-platelet effect of clopidogrel; Non-responders: Patients with no effect of treatment; MEA: A method to measure the effect of anti-platelet therapy in vivo.
The peer-reviewers pointed out that the study is also limited by intervention bias; MEA test was used to make clinical decisions such as platelet transfusions and surgical timing. These interventions could clearly have an effect on the results such as intraoperative bleeding as well as outcomes and differences between groups. The reviewers also pointed out the small sample and the retrospective design as limitations.
P- Reviewer: Guerado E, Hartshorn TA, Metzger PD, Wang Y, Zak L S- Editor: Tian YL L- Editor: A E- Editor: Zhang DN
| 1. | Brinker AD, Swartz L. Growth in clopidogrel-aspirin combination therapy. Ann Pharmacother. 2006;40:1212-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gurbel PA, Tantry US. Clopidogrel resistance? Thromb Res. 2007;120:311-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Cattaneo M. Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. J Thromb Haemost. 2007;5 Suppl 1:230-237. [PubMed] [Cited in This Article: ] |
| 4. | Järemo P, Lindahl TL, Fransson SG, Richter A. Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med. 2002;252:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Brilakis ES, Banerjee S. Patient with coronary stents needs surgery: what to do? JAMA. 2013;310:1451-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Mehta RH, Roe MT, Mulgund J, Ohman EM, Cannon CP, Gibler WB, Pollack CV, Smith SC, Ferguson TB, Peterson ED. Acute clopidogrel use and outcomes in patients with non-ST-segment elevation acute coronary syndromes undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2006;48:281-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, Yusuf S. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110:1202-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Manaqibwala MI, Butler KA, Sagebien CA. Complications of hip fracture surgery on patients receiving clopidogrel therapy. Arch Orthop Trauma Surg. 2014;134:747-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55:146-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91:922-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Tóth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96:781-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Eriksson M, Kelly-Pettersson P, Stark A, Ekman AK, Sköldenberg O. ‘Straight to bed’ for hip-fracture patients: a prospective observational cohort study of two fast-track systems in 415 hips. Injury. 2012;43:2126-2131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Owens WD, Felts JA, Spitznagel EL. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1437] [Cited by in F6Publishing: 1429] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 14. | Sibbing D, Braun S, Jawansky S, Vogt W, Mehilli J, Schömig A, Kastrati A, von Beckerath N. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb Haemost. 2008;99:121-126. [PubMed] [Cited in This Article: ] |
| 15. | Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schömig A, Kastrati A, von Beckerath N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53:849-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 486] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 16. | World Health Organization. WHO guidelines for reporting adverse events. [accessed 2005]. Available from: http://www.who.int.proxy.kib.ki.se/patientsafety/events/05/Reporting_Guidelines.pdf. [Cited in This Article: ] |
| 17. | Lundström A, Laska AC, Von Arbin M, Jörneskog G, Wallén H. Glucose intolerance and insulin resistance as predictors of low platelet response to clopidogrel in patients with minor ischemic stroke or TIA. Platelets. 2014;25:102-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Harty JA, McKenna P, Moloney D, D’Souza L, Masterson E. Anti-platelet agents and surgical delay in elderly patients with hip fractures. J Orthop Surg (Hong Kong). 2007;15:270-272. [PubMed] [Cited in This Article: ] |
| 19. | Johansen A, White J, Turk A. Clopidogrel therapy--implications for hip fracture surgery. Injury. 2008;39:1188-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Chechik O, Thein R, Fichman G, Haim A, Tov TB, Steinberg EL. The effect of clopidogrel and aspirin on blood loss in hip fracture surgery. Injury. 2011;42:1277-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Feely MA, Mabry TM, Lohse CM, Sems SA, Mauck KF. Safety of clopidogrel in hip fracture surgery. Mayo Clin Proc. 2013;88:149-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Wallace HC, Probe RA, Chaput CD, Patel KV. Operative treatment of hip fractures in patients on clopidogrel: a case-control study. Iowa Orthop J. 2012;32:95-99. [PubMed] [Cited in This Article: ] |
| 23. | Mahla E, Suarez TA, Bliden KP, Rehak P, Metzler H, Sequeira AJ, Cho P, Sell J, Fan J, Antonino MJ, Tantry US, Gurbel PA. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ Cardiovasc Interv. 2012;5:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Gurbel PA, Mahla E, Tantry US. Peri-operative platelet function testing: the potential for reducing ischaemic and bleeding risks. Thromb Haemost. 2011;106:248-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Firanescu CE, Martens EJ, Schönberger JP, Soliman Hamad MA, van Straten AH. Postoperative blood loss in patients undergoing coronary artery bypass surgery after preoperative treatment with clopidogrel. A prospective randomised controlled study. Eur J Cardiothorac Surg. 2009;36:856-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87:483-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 27. | Hossain FS, Rambani R, Ribee H, Koch L. Is discontinuation of clopidogrel necessary for intracapsular hip fracture surgery? Analysis of 102 hemiarthroplasties. J Orthop Traumatol. 2013;14:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Katus MC, Szczepiorkowski ZM, Dumont LJ, Dunbar NM. Safety of platelet transfusion: past, present and future. Vox Sang. 2014;107:103-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Rashid RH, Shah AA, Shakoor A, Noordin S. Hip fracture surgery: does type of anesthesia matter? Biomed Res Int. 2013;2013:252356. [PubMed] [Cited in This Article: ] |
| 30. | Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84:450-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 286] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Velik-Salchner C, Maier S, Innerhofer P, Streif W, Klingler A, Kolbitsch C, Fries D. Point-of-care whole blood impedance aggregometry versus classical light transmission aggregometry for detecting aspirin and clopidogrel: the results of a pilot study. Anesth Analg. 2008;107:1798-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Ridefelt P, Egberg N, Hillarp A, Lethagen S, Tengborn L. [New in vitro analysis tested: bleeding time is still the best method for evaluation of primary hemostasis]. Lakartidningen. 2001;98:3922-3924. [PubMed] [Cited in This Article: ] |