Published online Nov 18, 2021. doi: 10.5312/wjo.v12.i11.816
Peer-review started: May 9, 2021
First decision: June 16, 2021
Revised: June 27, 2021
Accepted: September 30, 2021
Article in press: September 30, 2021
Published online: November 18, 2021
Paraspinal muscle strength and fatigue are considered important in low back pain (LBP) prevention and rehabilitation. High reliability of paraspinal strength and electromyographic (EMG)-fatigue parameters has not been universally reported. Moreover, the discriminative validity of these parameters requires further exploration, under the threat of potentially poor reliability of the methods examined.
To investigate the reliability and discriminative validity of paraspinal strength and EMG-related fatigue in subjects with recurrent LBP and healthy participants.
Test-retest measurements were performed in 26 healthy and 66 LBP volunteers, for reliability. Paraspinal isometric maximal and mean strength were determined with a maximum voluntary isometric contraction (MVIC) protocol, performed in a custom-made device. For the fatigue test, participants performed a 60% MIVC level continuous isometric contraction of the paraspinals, in conjunction with EMG analysis from 4 muscle sites of the lumbar spine. Initial median frequency (IMF), the median frequency slope (MFslope), as well as the root mean square (RMS) slope EMG parameters were used as fatigue measures. Data were analysed with repeated measures ANOVA for test-retest differences. For reliability, the intraclass correlation coefficient (ICC3,1), standard error of the measurement (SEM) and the smallest detectable difference (SDD) were reported. Group-related differences for fatigue measures were analysed with a Multivariate Analysis of Covariance, with age, weight and strength as covariates.
Isometric strength presented statistically significant between-day differences (P < 0.01), however these did not exceed 10% (healthy: 7.2%/LBP-patients: 9.7%) and ICC reliability values were excellent, yet test-retest error was increased for the patient group (healthy: ICC3,1: 0.92-0.96, SEM: 5.72-5.94 Hz, SDD: 18.51%-18.57%/LBP-patients: ICC3,1: 0.91-0.96, SEM: 6.49-6.96, SDD: 30.75%-31.61%). For the frequency data, IMF reliability was excellent (healthy: ICC3,1: 0.91-0.94, SEM: 3.45-7.27 Hz, SDD: 9.56%-20.14%/patients: ICC3,1: 0.90-0.94, SEM: 6.41-7.59 Hz, SDD: 17.75%-21.02%) and of MF raw and normalised slopes was good (healthy: ICC3,1: 0.78-0.82, SEM: 4.93-6.02 Hz, SDD: 13.66-16.67%/LBP-patients: ICC3,1: 0.83-0.85, SEM: 6.75-7.47 Hz, SDD: 18.69%-20.69%). However, the reliability for RMS data presented unacceptably high SDD values and were not considered further. For discriminative validity, less MVIC and less steep MFslopes were registered for the patient group (P < 0.01).
Reliability and discriminative ability of paraspinal strength and EMG-related frequency parameters were demonstrated in healthy participants and patients with LBP.
Core Tip: Patients with low back pain (LBP) frequently exhibit muscle strength and fatigue impairments. Sixty-six patients with sub-acute recurrent LBP, able to perform a short duration isometric maximal strength evaluation, followed by a brief submaximal endurance performance test of the paraspinals, demonstrated strength deficits, as well as electromyographic (EMG)-fatigue differences in relation to a group of healthy participants. Test-retest reliability examining the level of accuracy of strength and EMG-fatigue measures, and the discriminative validity of frequency data were also reported. There were no adverse effects of the methodology followed. Paraspinal muscle re-training to improve the identified deficits should be emphasised.
- Citation: Koumantakis GA, Oldham JA. Paraspinal strength and electromyographic fatigue in patients with sub-acute back pain and controls: Reliability, clinical applicability and between-group differences. World J Orthop 2021; 12(11): 816-832
- URL: https://www.wjgnet.com/2218-5836/full/v12/i11/816.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i11.816
Low back pain (LBP) is drawing a lot of research effort worldwide, due to the disability and work loss associated with this health care condition[1]. For approximately 80% of LBP cases labeled as non-specific LBP a precise diagnosis cannot be established and only 15% of cases can be attributed to a specific pathology[1]. The “non-specific” category is the one that presents the greatest challenge, as it forms the largest group but also as there seems to be no apparent etiologic link between pain and structure[2].
Due to the episodic nature of LBP, the condition has been labelled as recurrent, if present on less than half the days (< 6 mo) in a 12-month period, occurring in multiple episodes over the year[3]. Around 2/3 of people who ever had back pain will have some recurrence each year[4]. The causes of recurrence are not clear and may vary for different populations, however both biomechanical and psychosocial factors have been proposed as contributors to LBP disability[1,5], with alterations in muscle structure and function being more evident in chronic LBP (CLBP) than in recurrent LBP (RLBP)[6,7].
The trunk muscle activity functional alterations already evident in people with RLBP even during periods of remission of symptoms compared to healthy controls, have been recently summarized as greater co-contraction, different redistribution of muscle activity, and delayed postural control of deeper trunk muscles[8]. Redistribution of the pattern of activity between different parts of the paraspinal muscles, synergistically contracting in response to the functional demands of spinal movement and stability, has also been described to vary between the upper and lower spinal segments in CLBP and healthy controls, rendering the lower spinal segments of patients with CLBP relatively unprotected upon sustained contractions, registering in parallel deficits in timed endurance[9]. Additionally, patients with RLBP compared to those with CLBP demonstrated a generalised lack of activation ability of the paraspinals while performing a low-load lumbar extension task, corresponding to a lower metabolic activity at both the erector spinae and multifidus and combined with less perceived exertion following completion of the task, possibly due to the lower activation levels of those muscles[6].
Considering the anti-gravity functional role of the paraspinals muscles, good paraspinal muscle endurance (fatigue-resistance), assessed with an isometric time to complete exhaustion test was found to prevent first-time occurrence of back pain in men only[10] and in both men and women, however only for subjects in the lowest performance tertile[11]. Measuring paraspinal fatigue to complete exhaustion possesses inherent limitations, as measurements can be affected by patients’ psychology[12,13], depending on their willingness to perform a test that is physically demanding and potentially having a pain-provocation effect during its execution and afterwards.
Given the significant role of paraspinal muscle fatigue in LBP progression[14,15], alternative fatigue assessment techniques were required, to overcome validity issues in the determination of paraspinal endurance with the classic Sorensen test performed to complete exhaustion, especially in pain populations[16]. Significant metabolic processes within the muscle, associated with a decreasing pattern of motor unit firing frequencies can be detected with electromyographic (EMG) monitoring from the beginning of a contraction, much earlier than the time of mechanical inability to sustain the contraction, with accurate methods required to assess the pattern of these processes[17]. Therefore, brief paraspinal muscle testing, performed at set percentages of a maximum voluntary isometric contraction (MVIC), estimating the fatigue characteristics of contracting muscles from EMG-related parameters have been developed[18]. Indeed, EMG-fatigue data were more reliable under a task performed at a set percentage of an MVIC than a modified Sorensen test of 1 min duration, when directly compared in healthy participants[19].
Besides the brevity of the contractions required and the non-invasive nature of the surface EMG methods involved, it is of high importance to ascertain the reliability and validity level of the EMG-related spectrum and amplitude parameters of the paraspinal muscles, therefore providing an accessible monitoring method for clinicians[17]. However, the random nature of the EMG signal in general, as well as the EMG activity redistribution differences between healthy controls and patients with LBP[9], render these measurement properties difficult to achieve[20-22]. Additionally, the safety of paraspinal muscle maximal strength assessment in healthy and patient populations is important, due to the intense contractions involved. Due to the different physiological and structural changes identified between patients with recurrent and CLBP[6], between patients and healthy controls[9,23] and a possible role of paraspinal EMG-determined fatigue in the prediction of LBP development[15], the measurement of EMG-fatigue parameters of these muscles in different LBP patient subgroups and subjects without LBP requires systematic study.
The aims of this study were to investigate the reliability and discriminative validity of paraspinal strength and EMG-related endurance spectrum and amplitude parameters in subjects with RLBP at the sub-acute stage of symptoms in relation to healthy participants.
Adult subjects (> 18 years), without LBP (n = 26) and patients with LBP (n = 66), participated in this study, between January-September 2000. Subjects without LBP were either students or University employees. Patients were recruited from the orthopaedic clinic of a local hospital and several local general practices. Patients were eligible for the study if they had a history of RLBP (repeated episodes of pain in past year collectively lasting for less than 6 mo)[24] of a nonspecific nature, defined as back pain complaints occurring without identifiable specific anatomical or neuro
| Age (yr) | Height (cm) | Body mass (kg) | BMI (kg/m2) | |
| Healthy | ||||
| Male (n = 13) | 27.0 ± 6.8a | 178.4 ± 5.5 | 78.9 ± 8.7 | 24.7 ± 2.3 |
| Female (n = 13) | 24.6 ± 5.3b | 163.0 ± 8.0 | 56.8 ± 5.7b | 21.5 ± 3.5b |
| Total (n = 26) | 25.8 ± 6.1b | 170.7 ± 10.4 | 67.8 ± 13.4b | 23.1 ± 3.3b |
| Patients | ||||
| Male (n = 34) | 35.7 ± 10.2 | 177.5 ± 6.4 | 81.1 ± 10.6 | 25.7 ± 2.6 |
| Female (n = 32) | 39.2 ± 10.9 | 166.2 ± 5.8 | 74.5 ± 13.6 | 26.9 ± 4.4 |
| Total (n = 66) | 37.4 ± 10.6 | 172.1 ± 8.4 | 77.9 ± 12.5 | 26.3 ± 3.6 |
The local National Health Service (NHS) Trust and University Ethical Committees granted ethical approval for all experiments. All research assessments were conducted in a local research centre. All subjects gave informed consent prior to their participation. All rights of participants were protected at all times, according to the declaration of Helsinki.
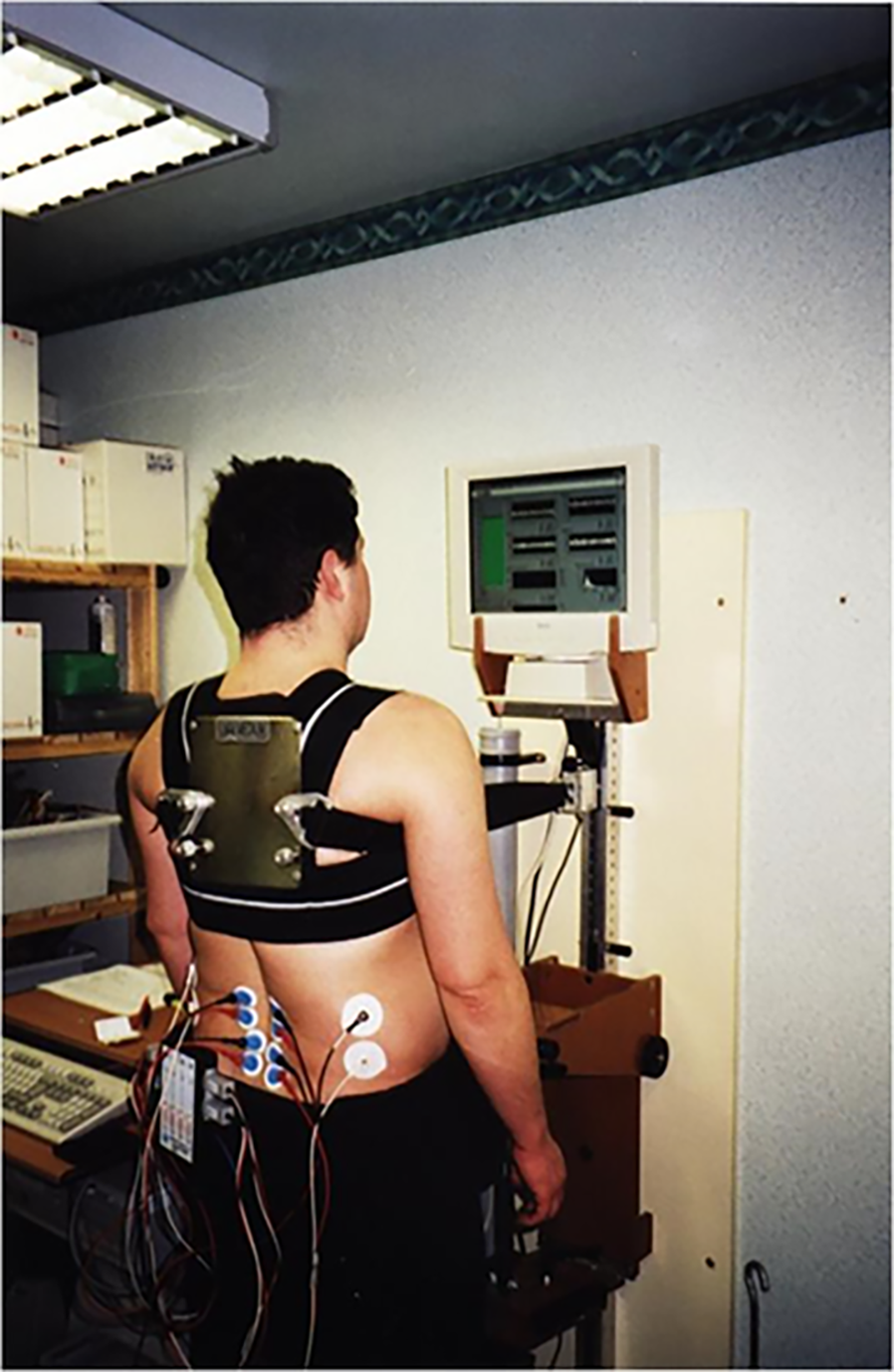
Muscle strength was assessed in a custom-made isomyometer designed and manufactured by the Medical Physics Department (St Mary’s Hospital, Central Manchester Healthcare Trust-CMHT). Its design was based on a very similar type of myometer developed in the Boston Neuromuscular Research Centre, the “Back Analysis System”[18,26]. Subjects were put in a standing position in the myometer, with appropriate stabilisation of the lower limbs. Subjects had to pull maximally backwards performing an isometric contraction of their paraspinal muscles and force was registered on an S-type load cell (250-kg Tedea Huntleigh, United Kingdom), positioned directly in front of their chest. A special built-in calibration system of the back myometer was also employed, in order to regularly test the transducer’s linear response between 0-200 kg, with known weights (Medical Physics Dept). An inextensible strap made of nylon linked the transducer to the subjects. The strap was securely fixed on the subjects’ back around the T6-T7 level, through a chest harness (Figure 1). In order to be able to comfortably generate paraspinal muscle strength, the hip, knees and ankle joints were placed in mid-range functional positions. The force transducer was interfaced to a computer and by means of a graphical programming analysis system (LabVIEW 5.0TM, National Instruments, Texas, United States), its output was online displayed on a flat-screen computer monitor placed at participants eye-level for visual feedback purposes.
Paraspinal muscle MVIC was determined in the upright position, in the following manner: three or more MVIC attempts were requested until the efforts were within 5-10% of each other. The best effort was taken as the MVC. Contraction duration was kept as short as possible (3-5 s), to minimise any reduced motivation or pain arising from prolonged contractions. Rest intervals between repeat MVICs were set at 60-s[27,28].
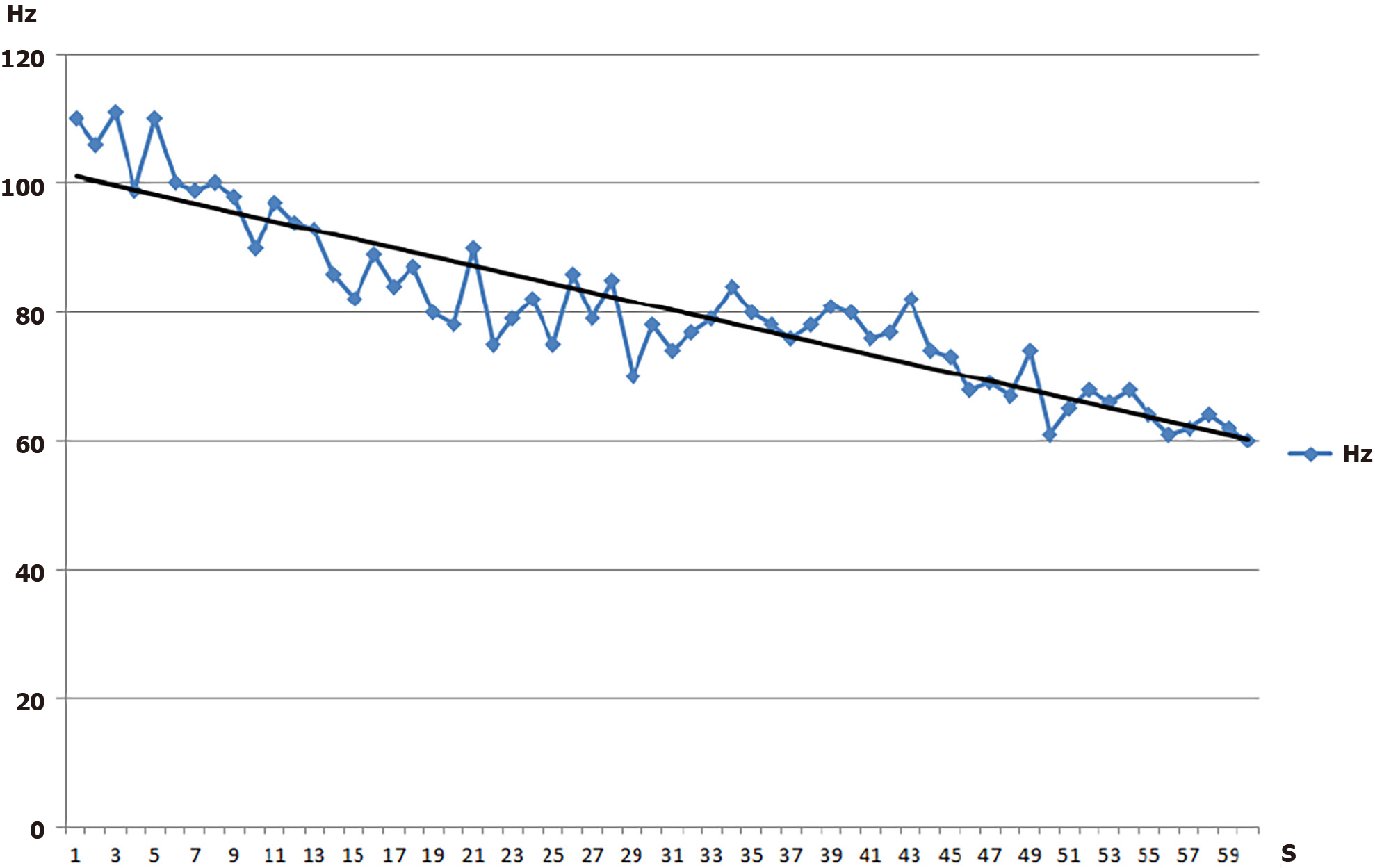
A four-channel EMG recorder (MP100 WSW, BIOPAC Systems), was used to collect EMG signals from 2 different back muscles bilaterally, the erector spinae (L2/3) and multifidus (L4/5). EMG signals were high-pass and low-pass filtered at 8 and 500 Hz respectively, amplified (× 10, CMRR: 110 dB min, SNR: 65 dB min) and analogue-to-digital converted at a sampling rate of 1024 Hz. An additional sharp 50 Hz notch filter was applied for DC noise removal. The waveforms collected were online analysed by a graphical programming analysis system (LabVIEWTM 5.0) in order to continuously derive the median frequency (MF) of the power density spectrum every second, using a Fast Fourier Transform algorithm. Also, the root mean square (RMS) was calculated every second. A linear regression line was fitted through the MF and RMS 60-s history, to obtain a measure of the rate of MF decrease (Figure 2) and RMS increase.
The bipolar electrode technique was utilised for the acquisition of the EMG signal. Appropriate skin preparation methods were used (light abrasion with fine sandpaper and wiping contact areas with cotton-wool soaked with surgical spirit) to reduce skin resistance to acceptable levels for recording, below 10 KOhms[19]. Four pairs of disposable pregelled surface electrodes (Ag/AgCl, Blue Sensor M-00-S, Medicotest, Ltd.) were applied in the direction of the muscle fibres, according to previous anatomic specifications[27,29]. As no significant differences have been identified between male and female subjects in the fibre orientation of both muscles[30], a uniform procedure was followed for electrode placement in both genders. The reference electrodes for each of the channels were placed on the skin surface, overlying an electrically unrelated tissue to the one the bipolar configuration was recording from[19]. Inter-electrode distance for the recording electrodes was set at 20 mm. The electrode location was reproduced in follow-up assessments by tracing their initial location onto a transparent A4 sheet, along with natural skin blemishes and distinctive marks, at the first assessment[19]. Skin impedance was checked right after the application of electrodes and was generally kept below 10 KOhms. In very few occasions appropriate skin impedance was not achieved and those electrodes had to be replaced and the skin preparation technique to be repeated.
The principal investigator conducted all experiments (test-retest reliability) in healthy participants and patients with LBP, to minimise any between-rater variance. Participants’ paraspinal muscle strength and fatigue performance were assessed on two separate occasions, with a week’s interval between measurements for normal subjects and a 3-5 d interval for patients. During the time interval between the 2 measurements, subjects were asked to maintain normal activities.
Muscle fatigue measurements followed the strength measurements on each of the 2 testing days. All subjects performed a 60% MVIC back muscle contraction in the isomyometer for 60 s, while EMG signal was acquired and online transformed to collect all EMG-fatigue related measurements for subsequent analysis.
Sample size was based on an a priori power calculation, estimating that at least 26 participants per group would be required to detect a 6.6%/min between-group difference in normalized MF slopes (MFslopes), at 80% power and a significance level of a = 0.05 (nQuery Advisor, v.3.0, Statistical Solutions, Saugus, MA, United States)[27].
The normality of distribution of all continuous variables was examined with the Kolmogorov-Smirnov test. Demographics were compared between-groups (independent samples t-test), to identify possible significant differences in factors known to affect the EMG-fatigue measures. Significant systematic between-sides differences were examined for the EMG-fatigue parameters (paired samples t-test).
For the reliability study, repeated measures ANOVA analysis was performed to detect any significant between-day systematic differences for each population separately. Test-retest reliability was established with 3 different measures, one of relative reliability, the intraclass correlation coefficient (ICC3,1)[31] and two of absolute reliability, the standard error of measurement (SEM)[32,33] derived from the ANOVA error components and the smallest detectable difference (SDD)[33], to determine the magnitude of change that exceeds the threshold of measurement error at the 95% confidence level and is not to be attributed to test-retest error. The ICC3,1 was chosen, as only one rater was involved in all measurements[31]. ICC values less than 0.50 indicate poor reliability, between 0.50 and 0.75 moderate, between 0.75 and 0.90 good and values greater than 0.90 excellent reliability[31]. The SEM is based only on the within-subject error/variability, it is a measure of the “precision” of measurement, expressed in the same units as the original measurement and can be directly compared against subjects’ values. SDD is considered a “clinical applicability” index, derived from the SEM (SDD = 1.96√2SEM), expressed either in raw terms (actual units of measurement) or as a percentage of the parameter’s grand mean. It is a useful index for diagnostic tests, indicating the level of change in a parameter attributed with 95% certainty to a true change in a subject’s condition, instead of being caused by test-retest errors. A small SDD associated with repeated test application renders a measurement more responsive to change.
For paraspinal strength, two force variables were derived: mean strength (the mean of 3 MVICs within 5%-10% of each other) and maximum strength (the highest of the 3 MVICs) to establish whether any of the two presented an advantage over the other. If EMG-fatigue reliability indices were of good-excellent level, measures were averaged across bilateral muscles within a session, as this practice has been previously reported to increase the reliability of these measures further[34].
For discriminative validity, preliminary analysis was conducted (independent samples t-tests), to determine which of those EMG-fatigue variables that displayed good-excellent reliability also presented significant between-group differences. Subsequently, a one-way Multivariate Analysis of Covariance (MANCOVA) was conducted to determine whether the initially detected statistically significant between-group differences in EMG-fatigue parameters would remain significant, after controlling for factors that could have influenced in parallel the EMG-fatigue parameters. Such factors, according to previous studies, were age[35-38], participants’ body mass[37] and the MVIC levels used for EMG-fatigue testing, with higher force levels resulting in higher fatigue rates[26] and were introduced as covariates. The assumptions required for conducting ANCOVA analysis were checked with relevant statistical procedures[39]. The statistical review of the study was performed by a biomedical statistician.
All data satisfied the normality of distribution criterion and were therefore summarised as means ± SD and analyzed with parametric statistics. The demographic characteristics of each group are presented in Table 1. Significant between-group differences were identified for age (P < 0.001), weight (P = 0.002) and body mass index (P = 0.001). Male and female participants were equally distributed in both groups, thus the effect of gender on EMG parameters was controlled.
Raw data from the two testing occasions are presented in Tables 2 and 3. For strength, the repeated measures ANOVA revealed statistically significant between-day differences in both groups, due to generally better performance on day 2 (Table 2). In clinical terms though, the mean % increase detected was rather insignificant (6.5%-7.2% for healthy and 9.4%-9.7% for patients). Relative reliability ICC indices were excellent for both mean and maximum MVIC, however the SDD was around 18.5% for healthy and 31.0%-31.5% for patients with LBP (Table 4).
| Healthy (n = 26) | LBP (n = 66) | |||||
| Parameter | Day 1 | Day 2 | P value | Day 1 | Day 2 | P value |
| IMF (Hz) | ||||||
| L2/3 R | 60.3 ± 10.9 | 61.0 ± 11.1 | 0.48 | 66.5 ± 17.6 | 66.1 ± 15.8 | 0.82 |
| L2/3 L | 58.2 ± 9.0 | 59.1 ± 12.3 | 0.52 | 63.5 ± 17.2 | 61.9 ± 13.2 | 0.18 |
| L4/5 R | 89.3 ± 20.3 | 91.0 ± 16.4 | 0.49 | 83.2 ± 24.3 | 83.0 ± 21.3 | 0.89 |
| L4/5 L | 90.9 ± 20.1 | 92.3 ± 17.3 | 0.59 | 82.7 ± 26.0 | 83.5 ± 20.8 | 0.59 |
| L2/3 | 59.24 ± 9.7 | 60.1 ± 11.4 | 0.44 | 65.0 ± 16.4 | 64.0 ± 13.8 | 0.39 |
| L4/5 | 90.1 ± 19.4 | 91.6 ± 15.9 | 0.49 | 82.9 ± 24.3 | 83.2 ± 20.0 | 0.83 |
| MF slopes (raw, Hz/s) | ||||||
| L2/3 R | -0.30 ± 0.14 | -0.35 ± 0.12 | 0.02a | -0.12 ± 0.16 | -0.12 ± 0.17 | 0.77 |
| L2/3 L | -0.30 ± 0.11 | -0.31 ± 0.10 | 0.52 | -0.10 ± 0.18 | -0.11 ± 0.14 | 0.41 |
| L4/5 R | -0.48 ± 0.16 | -0.54 ± 0.22 | 0.19 | -0.23 ± 0.25 | -0.22 ± 0.20 | 0.62 |
| L4/5 L | -0.56 ± 0.21 | -0.61 ± 0.19 | 0.19 | -0.21 ± 0.25 | -0.23 ± 0.25 | 0.53 |
| L2/3 | -0.30 ± 0.11 | -0.33 ± 0.10 | 0.08 | -0.11 ± 0.16 | -0.11 ± 0.15 | 0.51 |
| L4/5 | -0.52 ± 0.17 | -0.57 ± 0.19 | 0.13 | -0.22 ± 0.23 | -0.22 ± 0.20 | 0.84 |
| MF slopes (normalised, %/min) | ||||||
| L2/3 R | -28.8 ± 11.5 | -31.5 ± 9.0 | 0.14 | -9.0 ± 15.2 | -9.9 ± 15.8 | 0.55 |
| L2/3 L | -29.9 ± 9.3 | -29.8 ± 8.4 | 0.95 | -8.1 ± 15.9 | -10.5 ± 14.8 | 0.11 |
| L4/5 R | -34.0 ± 11.3 | -34.2 ± 11.9 | 0.90 | -15.0 ± 15.7 | -15.0 ± 12.0 | 0.98 |
| L4/5 L | -36.4 ± 10.7 | -37.7 ± 10.4 | 0.58 | -13.2 ± 14.0 | -17.1 ± 13.3 | 0.01a |
| L2/3 | -29.3 ± 9.8 | -30.6 ± 7.9 | 0.39 | -8.6 ± 14.8 | -10.2 ± 14.5 | 0.21 |
| L4/5 | -35.2 ± 9.6 | -36.0 ± 10.2 | 0.67 | -14.1 ± 13.7 | -16.1 ± 11.7 | 0.10 |
| RMS slopes (normalised, %/min) | ||||||
| L2/3 R | 43.4 ± 45.0 | 37.4 ± 35.2 | 0.35 | 10.4 ± 21.4 | 11.3 ± 18.5 | 0.72 |
| L2/3 L | 27.4 ± 30.8 | 29.0 ± 28.3 | 0.73 | 11.3 ± 17.3 | 13.9 ± 22.8 | 0.17 |
| L4-5 R | 42.6 ± 35.9 | 32.7 ± 29.2 | 0.19 | 14.3 ± 21.2 | 12.6 ± 22.2 | 0.43 |
| L4/5 L | 37.1 ± 36.2 | 38.4 ± 34.1 | 0.85 | 10.3 ± 19.3 | 13.9 ± 21.2 | 0.07 |
| Controls (n = 26) | Patients (n = 66) | |||||
| Parameter | ICC3,1 | SEM | SDD% | ICC3,1 | SEM | SDD% |
| MVIC (kg) | ||||||
| Maximum | 0.92 (0.83-0.97) | 5.94 | 18.57 | 0.91 (0.86-0.94) | 6.96 | 31.61 |
| Mean | 0.96 (0.90-0.98) | 5.72 | 18.51 | 0.96 (0.93-0.97) | 6.49 | 30.75 |
For the EMG-fatigue measures (Table 3), only the L2/3 MFslope on the right side (raw) in healthy and L4/5 MFslope on the L side (normalized) in patients were significantly steeper on the second day. Data between sides were pooled, as no apparent R/L differences were present in general. EMG-fatigue reliability was similar in both populations (Table 5). The ICCs for the initial MF (IMF) data from individual channels and for the merged R/L values were excellent and the SDDs were between 9.5%-20.0% for healthy and 17.7%-21.0% for LBP patients. The ICCs for the raw and normalised MFslopes were good and the SDDs were between 13.7%-16.7% for healthy and 18.7%-20.7% for LBP patients. All 3 reliability indices (ICC, SEM, SDD) generally improved for the pooled data. However, the ICCs for the amplitude data (RMSslopes) were poor-moderate and the SDDs were between 41.1%-67.0% for healthy and 30.0%-40.1% for LBP patients, deemed as unacceptably high for clinical applications.
| Controls (n = 26) | Patients (n = 66) | |||||
| Parameter | ICC3,1 | SEM | SDD | ICC3,1 | SEM | SDD |
| IMF (Hz) | ||||||
| L2/3 R | 0.91 (0.80-0.96) | 3.32 | 9.19 | 0.80 (0.69-0.87) | 7.56 | 20.95 |
| L2/3 L | 0.82 (0.63-0.92) | 4.57 | 12.66 | 0.80 (0.69-0.87) | 6.88 | 19.06 |
| L4/5 R | 0.82 (0.61-0.92) | 7.99 | 22.13 | 0.82 (0.73-0.89) | 9.64 | 26.70 |
| L4/5 L | 0.81 (0.60-0.91) | 8.31 | 23.02 | 0.86 (0.79-0.92) | 8.61 | 23.85 |
| L 2/3 | 0.94 (0.87-0.98) | 3.45 | 9.56 | 0.90 (0.84-0.94) | 6.41 | 17.75 |
| L 4/5 | 0.91 (0.79-0.96) | 7.27 | 20.14 | 0.94 (0.90-0.96) | 7.59 | 21.02 |
| MF slopes (raw, Hz/s) | ||||||
| L2/3 R | 0.61 (0.27-0.81) | 0.07 | 0.19 | 0.75 (0.62-0.84) | 0.08 | 0.22 |
| L2/3 L | 0.67 (0.36-0.85) | 0.06 | 0.17 | 0.66 (0.50-0.78) | 0.09 | 0.25 |
| L4/5 R | 0.52 (0.14-0.77) | 0.13 | 0.36 | 0.73 (0.60-0.83) | 0.12 | 0.33 |
| L4/5 L | 0.55 (0.18-0.78) | 0.13 | 0.36 | 0.62 (0.45-0.75) | 0.15 | 0.41 |
| L 2/3 | 0.77 (0.46-0.90) | 0.06 | 0.17 | 0.86 (0.77-0.91) | 0.08 | 0.22 |
| L 4/5 | 0.73 (0.35-0.89) | 0.11 | 0.30 | 0.88 (0.80-0.92) | 0.10 | 0.28 |
| MF slopes (normalised, %/min) | ||||||
| L2/3 R | 0.67 (0.36-0.85) | 5.79 | 16.03 | 0.69 (0.55-0.80) | 8.59 | 23.79 |
| L2/3 L | 0.65 (0.33-0.84) | 5.36 | 14.85 | 0.69 (0.54-0.80) | 8.44 | 23.38 |
| L4/5 R | 0.64 (0.31-0.83) | 7.11 | 19.69 | 0.62 (0.45-0.75) | 8.59 | 23.79 |
| L4/5 L | 0.50 (0.11-0.75) | 7.60 | 21.05 | 0.57 (0.38-0.71) | 8.66 | 23.99 |
| L 2/3 | 0.82 (0.57-0.92) | 4.93 | 13.66 | 0.85 (0.75-0.91) | 7.47 | 20.69 |
| L 4/5 | 0.78 (0.49-0.91) | 6.02 | 16.67 | 0.83 (0.73-0.90) | 6.75 | 18.69 |
| RMS slopes (normalised, %/min) | ||||||
| L2/3 R | 0.73 (0.46-0.88) | 21.00 | 58.17 | 0.48 (0.27-0.65) | 14.48 | 40.11 |
| L2/3 L | 0.76 (0.51-0.89) | 14.83 | 41.08 | 0.71 (0.56-0.81) | 10.83 | 30.00 |
| L4/5 R | 0.44 (0.04-0.72) | 24.07 | 66.67 | 0.73 (0.60-0.83) | 11.26 | 31.19 |
| L4/5 L | 0.54 (0.17-0.78) | 24.20 | 67.03 | 0.61 (0.44-0.74) | 12.44 | 34.46 |
For the discriminative validity, no significant differences were identified for the IMF, therefore this parameter was not tested further, while all MFslopes presented significant between-group differences (P < 0.001). A one-way MANCOVA analysis determined that there was a statistically significant difference between the 2 groups of participants on the combined dependent variables, after controlling for age, weight and MVIC, F(4,84) = 3.95, P = 0.006, Wilks’ Lambda = 0.835, partial η2 = 0.165. Follow up univariate tests revealed that differences between healthy and participants with RLBP were highly significant (P < 0.009) for all the EMG-frequency slope data (raw MFslopes and normalised MFslopes), when controlling for age, weight and MVIC (Table 6). Assumptions for running a one-way MANCOVA were systematically checked prior to its conduct. Linear relationships between pairs of dependent variables and between pairs of dependent variables and covariates within each group of the independent variables were examined with scatterplot matrices. Homogeneity of regression slopes and homogeneity of variances and covariances were equal in all groups of the independent variable (Box’s M Test of equality of covariance matrices, P = 0.06). Homogeneity of error variances of the dependent variables within each group were also equal (Levene’s test of equality of error variances, P > 0.05). No significant univariate outliers were detected in the groups of independent variables for each of the dependent variables, by inspection of the standardised residuals.
Back pain is a very prevalent musculoskeletal pathology of recurrent nature[4], and has been associated with a variety of possible causative factors[5]. ‘Previous LBP’ significantly contributes to the condition’s recurrence, having an odds ratio between 1.5-4.5[5]. However, many of the remaining physical impairments from ‘previous LBP’ episodes that possibly contribute to symptoms’ recurrence remain speculative, as in their majority these are not apparent with radiological methods or are masked during clinical assessment by co-existing pain, disability or psychological parameters[1]. Thus LBP is labelled in many instances as ‘non-specific’[2].
Nearly all (14/15) clinical guidelines on the effective management of non-specific LBP in primary care recommend the use of exercise, among other treatment options[40]. The type of exercise should be adapted according to the specific requirements of each LBP stage, however endurance re-training of the trunk muscles is well-placed within the ‘muscle re-education’ algorithm proposed[41]. Periods of pain remission between recurrences should be viewed as opportune timeframes to assess and functionally re-train the neuromuscular and anatomical deficits of trunk muscles, than being periods of rest that progressively lead to deconditioning of the neuromuscular system[8].
Under this framework, the present study aimed to assess the reliability of MVIC and of EMG time-dependent frequency and amplitude parameters of the paraspinals during an isometric fatigue test at 60% MVIC level, in patients with RLBP and healthy controls. Group-related performance differences were also examined.
Many factors need to be carefully considered in a maximal performance test such as the assessment of isometric strength. The upright position of the subjects was selected, as it has been successfully used before in multiple studies of different research centres[23,42,43], without any known contra-indications reported in the literature (safety of test ensured). Also, the intention was to avoid any lifting-type strength assessment activities where the trunk is in forward flexion[44]. Trunk forward flexion may have been the position that some of the LBP participants had “injured” themselves in the past and also it is not a comfortable position for many LBP patients[25], so on these grounds it was avoided. Additionally, no significant benefit over the upright position in EMG-fatigue reliability has been demonstrated with the latter type of experiment[44]. Confirming previous observations, MVIC measurement with the methods used was not associated with any new low back injuries. Only slight discomfort was reported by some participants from the patient group during the performance of the MVICs and for a short period (around 10 min) afterwards.
Similar trends with other strength experiments[43] were observed for the non-LBP as well as the RLBP population used for the paraspinal strength repeated measures, reflecting a learning effect between the 2 measurement sessions. Both groups of participants generally demonstrated increased strength output on the second testing occasion. However, due to the standardised methodology employed[28], the average increase was kept below 10% (between 6.5%-9.7%), a value clinically rather insignificant. Indeed, a similar previous study that conducted 3 measurement sessions, reported no further improvement in isometric MVIC after the second session[34]. In view of this learning effect though, for the discriminative validity analysis, only data from the second testing occasion were utilised.
This reliability study in the patient group was the largest so far in the literature[20]. Results clearly indicate that the technique employed for healthy participants and patients, considering the relative (ICCs) and absolute reliability indices (SEMs/SDDs) together[32,33], demonstrated good-excellent reliability for all the frequency-related parameters and that clinical differences can be reliably detected for values of EMG-fatigue MFslopes that exceed 18.7%-20.7%/min of initial values. Averaging data between sides, as previously suggested[34], increased reliability and decreased measurement error even further. The general trend in participants of this study was that no between-sides imbalances were present. It has to be emphasized, though, that the method of averaging data between-sides renders the technique insensitive in detecting between-sides differences present in some individuals.
Conversely, amplitude related RMS slopes presented with poor-moderate test-retest reliability and were not processed further. It might have been that these indices were either more sensitive to the differences in MVIC levels between-sessions or that they present more variability in day-to-day testing, due to load sharing phenomena present in sustained contractions[9]. However, as a general trend, it can be attested that RMS increases were lower in general for the patients with RLBP than the healthy par
Similar findings for poor reliability in RMS amplitude slopes have been previously reported for isometric fatigue testing at 60% MVIC level[19,44], however for MFslopes variable reliability levels have been reported[20-22,34].
For discriminative validity, a one-way MANCOVA determined that there was a statistically significant difference between the 2 groups of participants regarding the EMG-frequency raw or normalised MFslopes from both upper and lower muscle sites, after controlling for age, weight and MVIC. Interestingly, while the EMG time-dependent frequency parameters presented highly significant differences between the two groups, less decline in MFslopes was demonstrated in patients with RLBP.
The rate of decrease in MF, as expressed by the least squares linear regression from the beginning to the end of a sustained contraction (MFslope) has been initially proposed to be mainly related to the endurance capacity of a muscle (Figure 2), with steeper slopes indicating greater muscle fatigue almost invariably present in patients with CLBP[18,45,46]. The rate of fatigue with this type of experiment depends on the level of sub-maximal contraction it is performed, in this instance at 60% MVIC level. Therefore, if patients under-perform during MVIC generation, they will be performing the fatigue test under a lower load level. Thus, in this study, the MVIC was introduced as a covariate in the ANCOVA analysis, to statistically control against this eventuality.
The paraspinals are a characteristic example of a multi-layer multiple muscle system, synergistically activated to perform a variety of tasks under different conditions[8,9] in combination with muscles from adjacent body parts[47]. The initial distribution of activation in their various parts and the progressive re-distribution of activation during sustained activities[6,9], is organised and continuously monitored by the central nervous system (CNS). However, various factors relative to cognitive[12,48] or physiologically-controlled peripheral requirements like inter-individual characteristics[37], task biomechanical demands[19,49], the presence of atrophy related mainly to ongoing disability[7] or pain frequency characteristics (recurrent or continuous)[6] can lead to either steeper MFslopes (in case of selective atrophy of type II fibres[50] or a ‘confronter’ type of patient with LBP[48]) or less steep MFslopes (due to generalised inhibition[6,51], redistribution of muscle activity phenomena[23,28] or ‘avoider’ type of patient with LBP[48]), or even a mixed picture[38,42] compared to healthy participants.
EMG signal estimating the rate of muscle fatigue, through analysis of the frequency and time domain of the signal is rather complex, affected by the anatomical and physiological properties of muscles[6], the control scheme of the CNS[9] and the characteristics of the equipment used to collect the signal[26]. EMG fatigue measures, collected at a certain level of maintained contraction according to the methodology of the experiment conducted, are considered relatively independent of subjects’ volitional effort, as the firing frequency of motor units cannot be perceived nor regulated[18].
However, it can also be logically derived that non-volitional alterations in the organization of the motor commands controlled by the CNS[52], can influence the manifestations of EMG-fatigue time-dependent indices[23,42]. A point to consider in the interpretation of paraspinal muscle behaviour under maximal (strength) or prolonged (fatigue-related) contractions is the present and past history of LBP episodes. If patients are in an acute or even at a sub-acute remission stage of symp
Additionally, two studies on the redistribution of paraspinal muscle activity under sustained contractions offer complementary support to the findings of this study. The first showed that in healthy participants there was an increase in paraspinal level of contraction at lower spinal segments, whereas patients with CLBP did not demon
Another issue to consider is the direction of a ‘desired improvement’ in the patient group which is a debatable point, as the MFslopes in this group were significantly less steep than in the healthy participants (P < 0.002) across all muscle sites monitored, even after controlling for age, weight and different MVIC levels between the groups (Table 6). Indeed, opposite changes in MF slopes post rehabilitation have been reported, possibly reflecting better activation of paraspinal muscles post-rehabilitation[51]. Pain-related muscle inhibition phenomena and different load-sharing patterns in the back muscles of patients, which may be a possible CNS strategy (non-volitional) to distribute the load “evenly” between all muscle groups involved in the contraction[23,42,48,54,55] may potentially limit the applicability of the frequency spectrum EMG indices as endurance indicators. Alternatively, they may expand the definition of power spectrum frequency parameters, as indicators of neural motor control strategies[42].
A single rater experienced in the measurement methods employed was only involved in all measurements, to eliminate any between-rater error. Also, due to the variability of the EMG signal, only isometric examination methods of the paraspinal muscles were employed. However, the test-retest reliability, clinical applicability and discriminative validity of the methods involved was thoroughly examined in an adequate sample of healthy volunteers and in patients with recurrent non-specific LBP at a sub-acute stage of symptoms. An a priori sample size calculation was also performed to ensure sufficient power of the study based on expected between-group differences. Future studies could further examine the inter-rater reliability of the techniques already presented or expand the testing methods to either examine different exercise tasks (intermittent isometric contractions, different MVIC levels, dynamic contractions), including additional muscle groups (abdominals, gluteals) and patients with LBP with a range of neuromuscular deconditioning, disability and cognitive characteristics that affect functional performance.
Reliability, clinical applicability and discriminative ability of paraspinal strength and EMG-related frequency parameters were demonstrated in healthy participants and patients with non-specific recurrent sub-acute LBP. The EMG-related amplitude parameters did not present adequate reliability and the IMF parameter did not present significant differences between the groups examined. Further examination of those methods is endorsed.
A significant predictor of low back pain (LBP) recurrence is ‘previous LBP’. Partly, this may be due to persisting neuromuscular system activation deficits linked to strength deficits, as well as endurance deficits in patients with recurrent LBP (RLBP) even during periods of symptoms remission. LBP management clinical guidelines propose muscle re-conditioning as a prerequisite for successful management of recurrences.
Paraspinal muscle strength and endurance deficits require reliable monitoring. To overcome patient motivation or cognitive-related concerns affecting maximal strength testing, as well as endurance testing with prolonged contractions to complete exhaustion, alternative methods have been proposed in patients with RLBP, in order to establish the contribution of those parameters in neuromuscular deconditioning, to limit further recurrences.
As electromyographic (EMG)-based frequency and amplitude domain time dependent alterations, linked to the endurance characteristics of the muscles monitored have not been universally obtained for the paraspinals, a primary objective of this study was to determine the reliability of those measures. The reliability level of maximal paraspinal muscle strength performance was also examined. Furthermore, the discriminative validity of paraspinals muscle strength and time-dependent EMG frequency and amplitude domain alterations was tested.
A custom-made isomyometer was utilised to initially assess the maximum voluntary isometric contraction (MVIC) of the paraspinals in the upright trunk position. Subsequently, short duration (60-s) isometric contractions at a submaximal level of contraction (60% of MVIC) were employed, to determine the EMG-time dependent frequency [initial median frequency (IMF) and median frequency (MF) slopes] and amplitude changes [root mean square (RMS) slopes] of the paraspinal muscles with recording electrodes placed at 4 muscle sites (L2/3 and L4/5, bilaterally). The most reliable parameters were used further to test the between populations discriminative ability of the method.
For both groups, MVIC presented excellent intraclass correlation coefficient (ICC) reliability values, although statistically significant between-day increases (P < 0.01) were recorded, within a margin of 10%; test-retest error was increased for patients compared to healthy participants. The EMG reliability of the frequency parameters was good (MF slopes) to excellent (IMF), however for the amplitude parameter (RMS slope) it was poor, for both groups. Statistically significant less MVIC and less steep MF slopes were registered for the patient group. These findings confirm previous research in the field, however in a larger population of participants with a history of RLBP and a sufficiently large comparison group of healthy participants.
Although EMG time-dependent frequency parameters presented highly significant differences between the two groups, these were in the opposite than the expected direction. The validity of this finding is enhanced for two reasons; the between-group differences in MF slopes remained after statistically controlling for possible confounders and these differences were confirmed at all muscle sites monitored. Apparently, alterations in the organization of the motor commands in patients with RLBP can additionally influence the manifestations of EMG-related time-dependent indices. Therefore, the alterations in the EMG-frequency spectrum under sustained contractions cannot only be considered as indicators of peripheral fatigue or peripheral muscle atrophy.
This methodology of EMG-related alterations followed in the current experiment is reliable. The validity of the between-group differences obtained between patients with RLBP and healthy participants requires further study. In order to explain the significance of the current findings, the history of LBP has to be taken into consideration. Therefore, results from patients with varying amounts of LBP-related disability and disease duration are required, in conjunction with detailed imaging methods of peripheral muscle state and recording of the different patterns of activation utilised under controlled experimental conditions or less controlled functional tasks. Furthermore, the effect of exercise on EMG-related frequency parameters and whether the alterations registered post-exercise in the frequency domain correspond to less LBP recurrences requires examination from a clinical viewpoint.
We thank all healthy volunteers and patients with low back pain who agreed to participate in this study. We are also grateful to Ms. Lalou P for her advice on the statistics of this paper.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Orthopedics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ibrahim AA S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M; Lancet Low Back Pain Series Working Group. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356-2367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1769] [Cited by in F6Publishing: 2012] [Article Influence: 335.3] [Reference Citation Analysis (0)] |
| 2. | Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389:736-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1098] [Cited by in F6Publishing: 1207] [Article Influence: 172.4] [Reference Citation Analysis (0)] |
| 3. | Stanton TR, Latimer J, Maher CG, Hancock MJ. How do we define the condition 'recurrent low back pain'? Eur Spine J. 2010;19:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | da Silva T, Mills K, Brown BT, Pocovi N, de Campos T, Maher C, Hancock MJ. Recurrence of low back pain is common: a prospective inception cohort study. J Physiother. 2019;65:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Parreira P, Maher CG, Steffens D, Hancock MJ, Ferreira ML. Risk factors for low back pain and sciatica: an umbrella review. Spine J. 2018;18:1715-1721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Goubert D, De Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, Van Oosterwijck J, Dhondt E, Danneels L. Lumbar muscle structure and function in chronic vs recurrent low back pain: a cross-sectional study. Spine J. 2017;17:1285-1296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural Changes of Lumbar Muscles in Non-specific Low Back Pain: A Systematic Review. Pain Physician. 2016;19:E985-E1000. [PubMed] [Cited in This Article: ] |
| 8. | Devecchi V, Rushton AB, Gallina A, Heneghan NR, Falla D. Are neuromuscular adaptations present in people with recurrent spinal pain during a period of remission? PLoS One. 2021;16:e0249220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Sanderson A, Martinez-Valdes E, Heneghan NR, Murillo C, Rushton A, Falla D. Variation in the spatial distribution of erector spinae activity during a lumbar endurance task in people with low back pain. J Anat. 2019;234:532-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Biering-Sørensen F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine (Phila Pa 1976). 1984;9:106-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 885] [Cited by in F6Publishing: 775] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 11. | Alaranta H, Luoto S, Heliövaara M, Hurri H. Static back endurance and the risk of low-back pain. Clin Biomech (Bristol, Avon). 1995;10:323-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Mannion AF, O'Riordan D, Dvorak J, Masharawi Y. The relationship between psychological factors and performance on the Biering-Sørensen back muscle endurance test. Spine J. 2011;11:849-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Applegate ME, France CR, Russ DW, Leitkam ST, Thomas JS. Determining Physiological and Psychological Predictors of Time to Task Failure on a Virtual Reality Sørensen Test in Participants With and Without Recurrent Low Back Pain: Exploratory Study. JMIR Serious Games. 2018;6:e10522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Steele J, Bruce-Low S, Smith D. A reappraisal of the deconditioning hypothesis in low back pain: review of evidence from a triumvirate of research methods on specific lumbar extensor deconditioning. Curr Med Res Opin. 2014;30:865-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Heydari A, Nargol AV, Jones AP, Humphrey AR, Greenough CG. EMG analysis of lumbar paraspinal muscles as a predictor of the risk of low-back pain. Eur Spine J. 2010;19:1145-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Demoulin C, Vanderthommen M, Duysens C, Crielaard JM. Spinal muscle evaluation using the Sorensen test: a critical appraisal of the literature. Joint Bone Spine. 2006;73:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Farina D, Gazzoni M, Merletti R. Assessment of low back muscle fatigue by surface EMG signal analysis: methodological aspects. J Electromyogr Kinesiol. 2003;13:319-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Roy SH, De Luca CJ, Casavant DA. Lumbar muscle fatigue and chronic lower back pain. Spine (Phila Pa 1976). 1989;14:992-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 330] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Koumantakis GA, Arnall F, Cooper RG, Oldham JA. Paraspinal muscle EMG fatigue testing with two methods in healthy volunteers. Reliability in the context of clinical applications. Clin Biomech (Bristol, Avon). 2001;16:263-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Mohseni Bandpei MA, Rahmani N, Majdoleslam B, Abdollahi I, Ali SS, Ahmad A. Reliability of surface electromyography in the assessment of paraspinal muscle fatigue: an updated systematic review. J Manipulative Physiol Ther. 2014;37:510-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Peach JP, Gunning J, McGill SM. Reliability of spectral EMG parameters of healthy back extensors during submaximum isometric fatiguing contractions and recovery. J Electromyogr Kinesiol. 1998;8:403-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Elfving B, Németh G, Arvidsson I, Lamontagne M. Reliability of EMG spectral parameters in repeated measurements of back muscle fatigue. J Electromyogr Kinesiol. 1999;9:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Peach JP, McGill SM. Classification of low back pain with the use of spectral electromyogram parameters. Spine (Phila Pa 1976). 1998;23:1117-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Von Korff M, Saunders K. The course of back pain in primary care. Spine (Phila Pa 1976). 1996;21:2833-7; discussion 2838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 329] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | O'Sullivan P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man Ther. 2005;10:242-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 587] [Cited by in F6Publishing: 555] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 26. | Oddsson LI, Giphart JE, Buijs RJ, Roy SH, Taylor HP, De Luca CJ. Development of new protocols and analysis procedures for the assessment of LBP by surface EMG techniques. J Rehabil Res Dev. 1997;34:415-426. [PubMed] [Cited in This Article: ] |
| 27. | Koumantakis GA, Watson PJ, Oldham JA. Supplementation of general endurance exercise with stabilisation training vs general exercise only. Physiological and functional outcomes of a randomised controlled trial of patients with recurrent low back pain. Clin Biomech (Bristol, Avon). 2005;20:474-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Crossman K, Mahon M, Watson PJ, Oldham JA, Cooper RG. Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally determined "adverse" fiber-type composition. Spine (Phila Pa 1976). 2004;29:628-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Sung W PT, PhD, Wong A BS, MS, Pourshogi A PhD, Pourrezaei K PhD, Silfies S PT. Near infrared spectroscopy confirms recruitment of specific lumbar extensors through neuromuscular electrical stimulation. Physiother Theory Pract. 2020;36:516-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Biedermann HJ, DeFoa JL, Forrest WJ. Muscle fibre directions of iliocostalis and multifidus: male-female differences. J Anat. 1991;179:163-167. [PubMed] [Cited in This Article: ] |
| 31. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9979] [Cited by in F6Publishing: 11918] [Article Influence: 1489.8] [Reference Citation Analysis (0)] |
| 32. | Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19:231-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 1191] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 33. | Roebroeck ME, Harlaar J, Lankhorst GJ. The application of generalizability theory to reliability assessment: an illustration using isometric force measurements. Phys Ther. 1993;73:386-95; discussion 396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 159] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Larivière C, Arsenault AB, Gravel D, Gagnon D, Loisel P. Evaluation of measurement strategies to increase the reliability of EMG indices to assess back muscle fatigue and recovery. J Electromyogr Kinesiol. 2002;12:91-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Goubert D, Meeus M, Willems T, De Pauw R, Coppieters I, Crombez G, Danneels L. The association between back muscle characteristics and pressure pain sensitivity in low back pain patients. Scand J Pain. 2018;18:281-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Dallaway A, Kite C, Griffen C, Duncan M, Tallis J, Renshaw D, Hattersley J. Age-related degeneration of the lumbar paravertebral muscles: Systematic review and three-level meta-regression. Exp Gerontol. 2020;133:110856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Kankaanpää M, Laaksonen D, Taimela S, Kokko SM, Airaksinen O, Hänninen O. Age, sex, and body mass index as determinants of back and hip extensor fatigue in the isometric Sørensen back endurance test. Arch Phys Med Rehabil. 1998;79:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Sung PS, Lammers AR, Danial P. Different parts of erector spinae muscle fatigability in subjects with and without low back pain. Spine J. 2009;9:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd. Harlow, Essex: Pearson Education Ltd., 2014. [Cited in This Article: ] |
| 40. | Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, van Tulder M, Koes BW. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27:2791-2803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 673] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 41. | Hodges PW, Danneels L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J Orthop Sports Phys Ther. 2019;49:464-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 42. | Roy SH, Oddsson LI. Classification of paraspinal muscle impairments by surface electromyography. Phys Ther. 1998;78:838-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Harding AT, Weeks BK, Horan SA, Little A, Watson SL, Beck BR. Validity and test-retest reliability of a novel simple back extensor muscle strength test. SAGE Open Med. 2017;5:2050312116688842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Arnall FA, Koumantakis GA, Oldham JA, Cooper RG. Between-days reliability of electromyographic measures of paraspinal muscle fatigue at 40, 50 and 60% levels of maximal voluntary contractile force. Clin Rehabil. 2002;16:761-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Mooney V, Gulick J, Perlman M, Levy D, Pozos R, Leggett S, Resnick D. Relationships between myoelectric activity, strength, and MRI of lumbar extensor muscles in back pain patients and normal subjects. J Spinal Disord. 1997;10:348-356. [PubMed] [Cited in This Article: ] |
| 46. | da Silva RA, Vieira ER, Cabrera M, Altimari LR, Aguiar AF, Nowotny AH, Carvalho AF, Oliveira MR. Back muscle fatigue of younger and older adults with and without chronic low back pain using two protocols: A case-control study. J Electromyogr Kinesiol. 2015;25:928-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Kankaanpää M, Taimela S, Laaksonen D, Hänninen O, Airaksinen O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil. 1998;79:412-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 222] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Biedermann HJ, Shanks GL, Forrest WJ, Inglis J. Power spectrum analyses of electromyographic activity. Discriminators in the differential assessment of patients with chronic low-back pain. Spine (Phila Pa 1976). 1991;16:1179-1184. [PubMed] [Cited in This Article: ] |
| 49. | Elfving B, Dedering A. Task dependency in back muscle fatigue--correlations between two test methods. Clin Biomech (Bristol, Avon). 2007;22:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Käser L, Mannion AF, Rhyner A, Weber E, Dvorak J, Müntener M. Active therapy for chronic low back pain: part 2. Effects on paraspinal muscle cross-sectional area, fiber type size, and distribution. Spine (Phila Pa 1976). 2001;26:909-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Mannion AF, Taimela S, Müntener M, Dvorak J. Active therapy for chronic low back pain part 1. Effects on back muscle activation, fatigability, and strength. Spine (Phila Pa 1976). 2001;26:897-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Nijs J, Clark J, Malfliet A, Ickmans K, Voogt L, Don S, den Bandt H, Goubert D, Kregel J, Coppieters I, Dankaerts W. In the spine or in the brain? Clin Exp Rheumatol. 2017;35 Suppl 107:108-115. [PubMed] [Cited in This Article: ] |
| 53. | Muceli S, Falla D, Farina D. Reorganization of muscle synergies during multidirectional reaching in the horizontal plane with experimental muscle pain. J Neurophysiol. 2014;111:1615-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Larivière C, Arsenault AB, Gravel D, Gagnon D, Loisel P. Surface electromyography assessment of back muscle intrinsic properties. J Electromyogr Kinesiol. 2003;13:305-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Elfving B, Dedering A, Németh G. Lumbar muscle fatigue and recovery in patients with long-term low-back trouble--electromyography and health-related factors. Clin Biomech (Bristol, Avon). 2003;18:619-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Falla D, Gizzi L, Tschapek M, Erlenwein J, Petzke F. Reduced task-induced variations in the distribution of activity across back muscle regions in individuals with low back pain. Pain. 2014;155:944-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |