Liver Detox Product for Treatment and Prevention of Liver Diseases: A Case Report
Shridhar J Pandya* and Chetan H Savliya
Ph.D., Director, Gplife Healthcare Pvt. Ltd, India
Received Date: 13/07/2023; Published Date: 07/12/2023
*Corresponding author: Dr. Shridhar J. Pandya, Gplife Healthcare Pvt. Ltd, 705-706, Orbit-1 Building, Punagum-Saroli Road, Near RRTM Market, Surat, 395010, India. Email ID: gplifehealthcare@gmail.com
Abstract
A liver disease is characterized by the progressive deterioration of the liver's functions. Several causes of liver disease can be attributed to its development, including toxins, alcohol abuse, infection, autoimmune diseases, genetic defects, and metabolic disorders. Due to the lack of a perfect treatment for liver disease management, Gplife has developed a product that has proven effective. After 60 days of evaluation of cases, it has been observed that the SGPT levels have been reduced by 90.27%, the SGOT levels have been reduced by 88.93%, the GGT levels have been reduced by 86.81%, the Total Bilirubin levels have been reduced by 63.49%, the Creatinine levels have been reduced by 43.09%, and the GFR has been increased by 26.65%. An overview of the current understanding of liver disease and the efficacy of Liver Detox product is presented in this case study. Furthermore, the product helps to reduce the need for conventional liver treatments. The liver detox product is recommended for further use as a monotherapy or adjunctive therapy to manage and improve the prognosis of various liver diseases.
Keywords: Liver disease; Liver detox; Cirrhosis; Liver health
Introduction
The liver plays a vital role in the organ system in the body. It interacts with the endocrine and gastrointestinal systems by aiding digestion and metabolism. The liver is located in the upper right-hand portion of the abdominal cavity, beneath the diaphragm, and on top of the stomach. It detoxifies various metabolites. It also breaks down red blood cells and substances, synthesizes bile and proteins, stores glycogen, and serves as a blood reservoir [1,2]. Bile is an essential fluid as it aids in the absorption and digestion of lipids via the secretion of bile salts and acids. When bile is secreted into the duodenum, it is circulated enterohepatically and transported back to the liver after absorption in the ileum [3]. Liver diseases are characterized by elevations in alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Alkaline Phosphatase (ALP), Gamma-Glutamyl Transferase (GGT). The results of these tests can be used to estimate liver damage extent and to organize a differential diagnosis [4]. Several factors contribute to the development of liver disease. Viral infections, including hepatitis B and C, are responsible for the majority of cases. Other causes include excessive alcohol consumption, obesity, type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), and autoimmune diseases. Moreover, certain medications, toxins, and genetic conditions can also damage the liver, leading to disease progression [5].
Liver disease accounts for approximately 2 million deaths per year worldwide, 1 million due to cirrhosis complications, and 1 million due to viral hepatitis and hepatocellular carcinoma. About 2 billion people consume alcohol worldwide; over 75 million are diagnosed with alcohol-use disorders and at risk of alcohol-associated liver disease. Approximately 2 billion adults are obese or overweight and over 400 million have diabetes; both risk factors for non-alcoholic fatty liver disease [6]. Liver disease is a growing concern globally, affecting people. Managing liver disease requires a multifaceted approach that includes dietary modifications, regular exercise, medication adherence, and avoiding hepatotoxic substances. Additionally, vaccinations, stress management, and regular liver function testing play important roles in maintaining liver health. While conventional treatments are available, the potential side effects associated with pharmaceutical drugs have led many individuals to seek alternative solutions. In recent years, natural products have gained popularity as a safe and effective approach to managing liver disease [7,8].
By assessing the need for strong alternatives in the management of liver diseases, Gplife Healthcare P. Ltd. has developed liver detox products, which have been comprised of Milk thistle, Kalmegh, Punarnava, Himsara, Shallaki, Bhuiamla, Chicory, and Sea buckthorn like herbal ingredients. We have used all standardized and potential extracts of herbal ingredients to develop a liver detox product. A proprietary technology called "Synergistic Optimized Blend Technology" is used to develop and manufacture our Liver Detox product. An attempt has been made in this case-report of different cases to demonstrate the product’s efficacy in managing Liver diseases.
Case Presentation and Methodology
2 tablets of Liver detox product two times a day were given to the subjects involved in the case studies.
Statistical Analysis
Statistical analysis was done according to the intention-to-treat principles. Changes in SGPT and SGOT parameters from baseline and at the end of the study were analysed using Wilcoxon Signed-rank test. Changes in GGT, Total Bilirubin, Creatinine, and GFR parameters from baseline,15 days, 30 days, and at the end of the study (day 60) were analysed using a student t-test. Values are expressed as mean ± SD or as incidences of patients with or without symptoms. P-value < 0.05 was considered significant. Statistical analysis was performed using SPSS version 10.0.
Results and Discussion
The effect of the Liver detox product on various laboratory parameters in patients with various liver diseases was evaluated. A total of 10 cases were evaluated who have taken treatment of liver detox product of Gplife Healthcare for 60 days. Only those case are considered who has not missed the doses in the treatment duration. Out of 10 cases, 5 were male and 5 were female. The average age of 10 cases was found to be 46±11.89 males and 41.2±17.18 for females (Table 1).
Table 1: Demographic data.

SGPT was evaluated in 10 cases who were treated with liver detox products for a period of 60 days. The initial mean SGPT significantly reduced from 338.3±672.9 to 79.3±25.84 after 15 days, to 52.05±16.25 after 30 days, and to 32.9±6.704 after 60 days, where p<0.00512 was found to be significant. In inference to the evaluation of the above parameter after 60 days, it has been found that the SGPT levels were reduced by 76.56 % after 15 days, 84.61% after 30 days, 90.27% after 60 days (Table 2, Figure 1).
Table 2: Effect of Liver Detox product on SGPT levels.
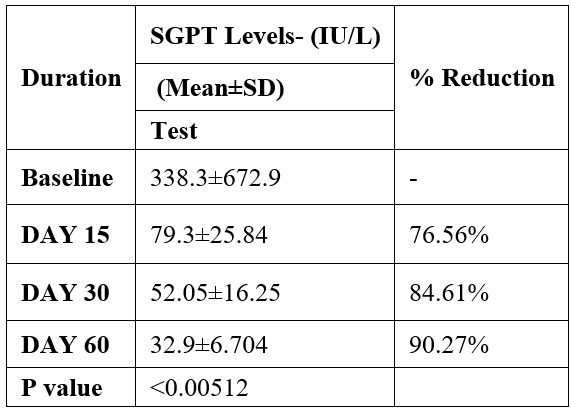
Data was analyzed by Wilcoxon test. Significant at p< 0.05
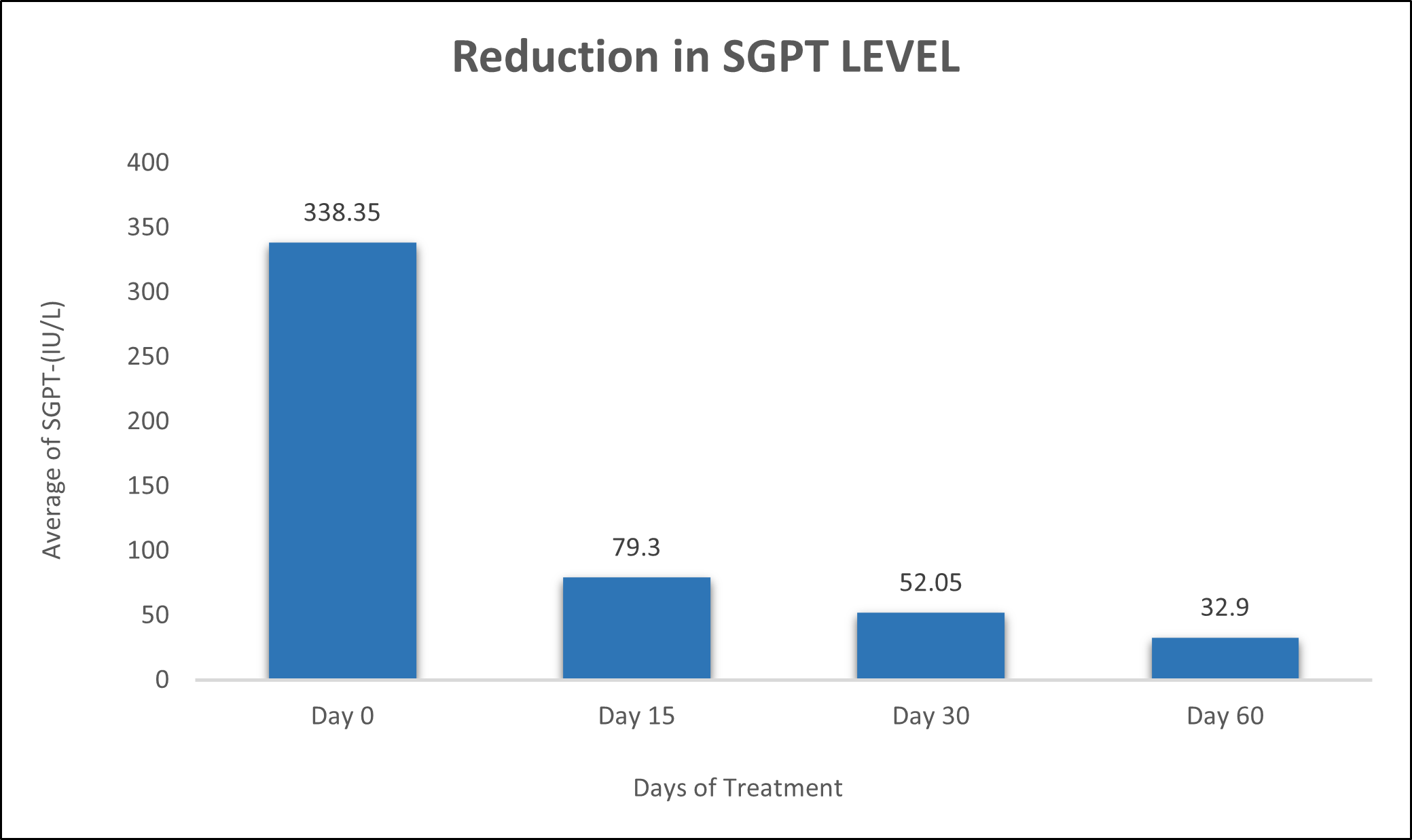
Figure 1: Effect of Liver Detox product on SGPT levels.
SGOT was evaluated in 10 cases who were treated with liver detox products for a period of 60 days. The initial mean SGOT significantly reduced from 271.4±494.6to 55.2±12.40 after 15 days, to 46.15±10.02after 30 days, and to 30.04±8.97after 60 days, where p<0.00512 was found to be significant. In inference to the evaluation of the above parameter after 60 days, it has been found that the SGPT levels were reduced by 79.66 % after 15 days, 82.99% after 30 days, 88.93% after 60 days (Table 3, Figure 2).
Table 3: Effect of Liver Detox product on SGOT levels.
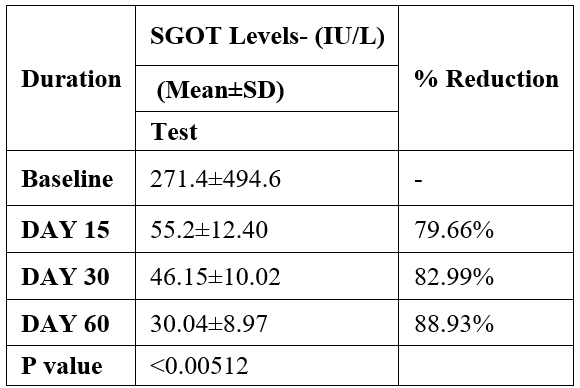
Data was analyzed by Wilcoxon test. Significant at p< 0.05
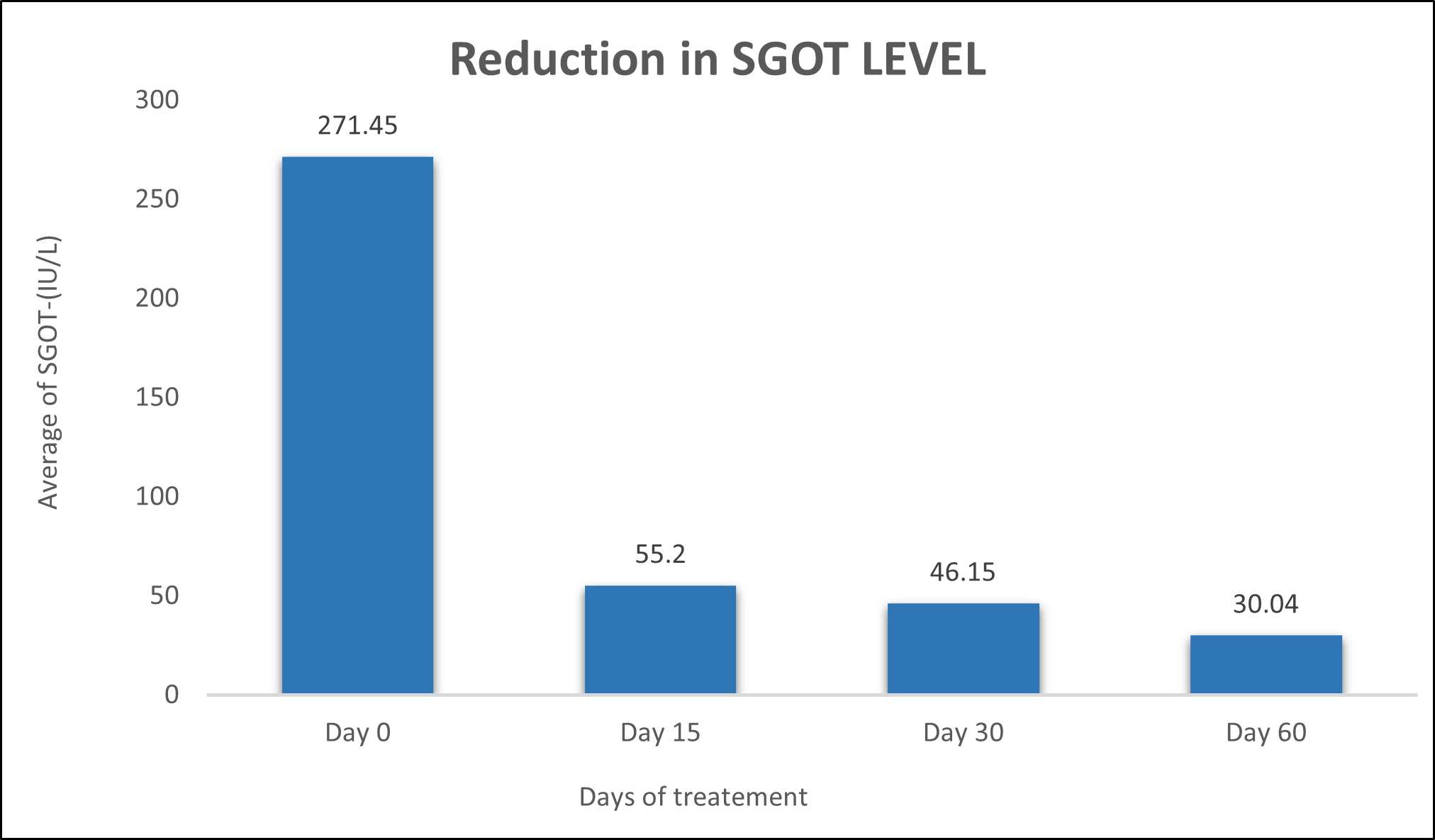
Figure 2: Effect of Liver Detox product on SGOT levels.
GGT was evaluated in 10 cases who were treated with liver detox products for a period of 60 days. The initial mean GGT significantly reduced from 368.18±301.88 to 197.4±85.04 after 15 days, to 105.58±39.52 after 30 days, and to 48.54±7.002 after 60 days, where p<0.05 was found to be significant. In inference to the evaluation of the above parameter after 60 days, it has been found that the GGT levels were reduced by 46.38%% after 15 days, 71.32% after 30 days, 86.81% after 60 days (Table 4, Figure 3).
Table 4: Effect of Liver Detox product on GGT level.
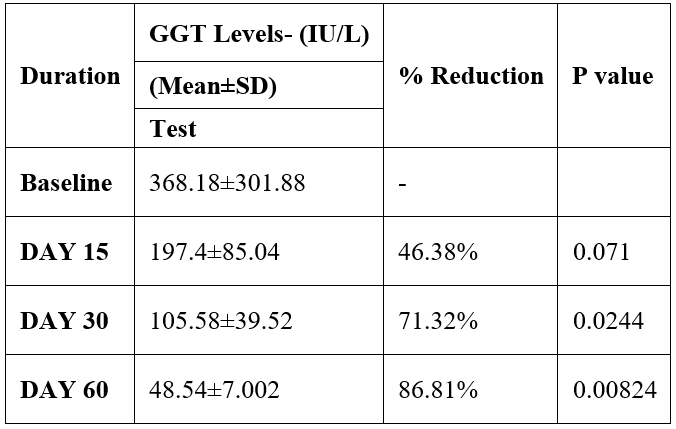
Data was analysed by Student t test. Significant at p< 0.05
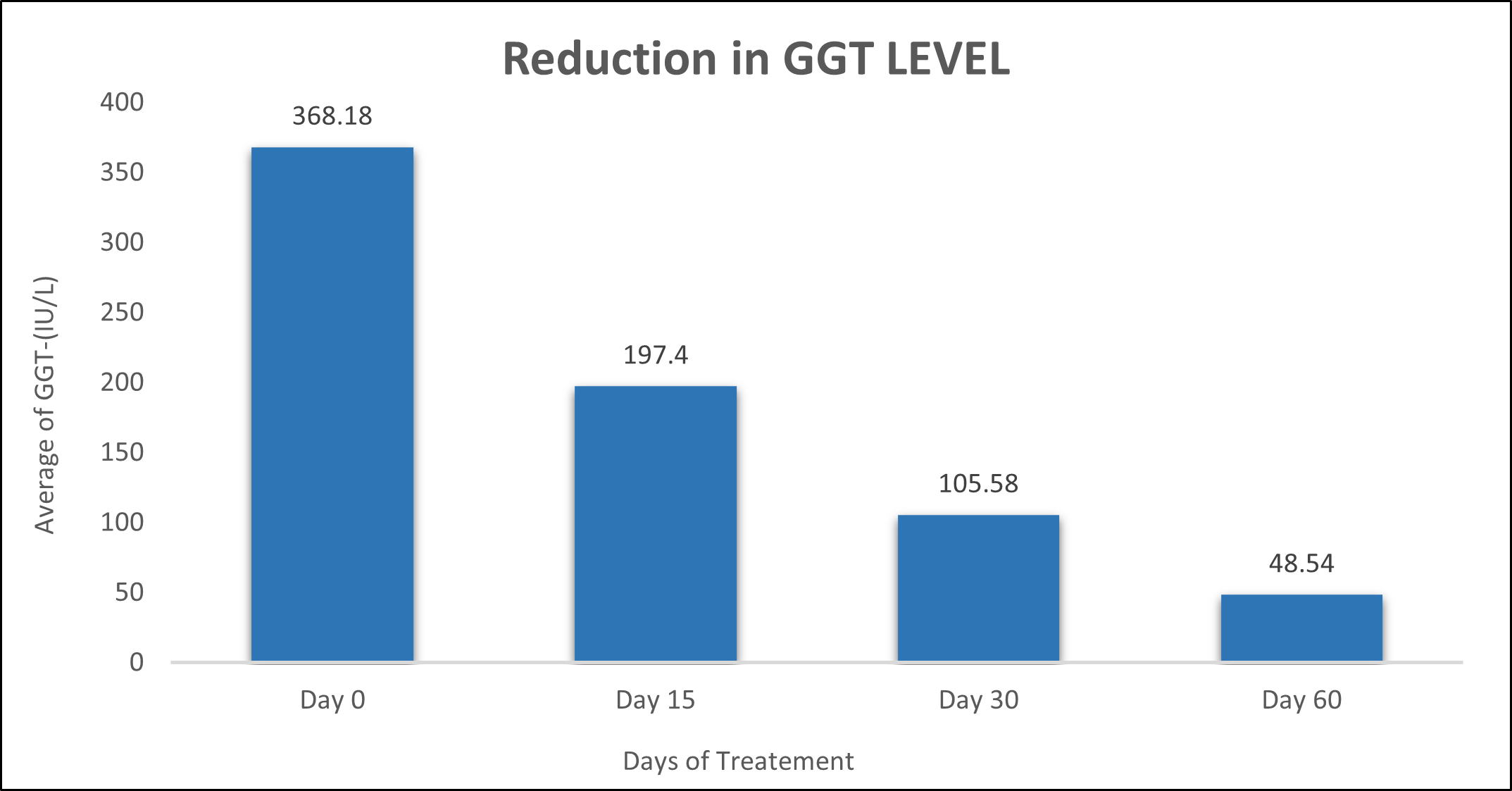
Figure 3: Effect of Liver Detox product on GGT level.
Total Bilirubin was evaluated in 10 cases who were treated with liver detox products for a period of 60 days. The initial mean Total Bilirubin significantly reduced from 1.63±0.834 to 1.41±0.519 after 15 days, to 0.991±0.330 after 30 days, and to 0.595±0.238 after 60 days, where p<0.05 was found to be significant. In inference to the evaluation of the above parameter after 60 days, it has been found that the Total Bilirubin levels were reduced by 13.49% after 15 days, 39.20% after 30 days, 63.49% after 60 days (Table 5, Figure 4).
Table 5: Effect of Liver Detox product on Total Bilirubin level.

Data was analysed by Student t test. Significant at p< 0.05
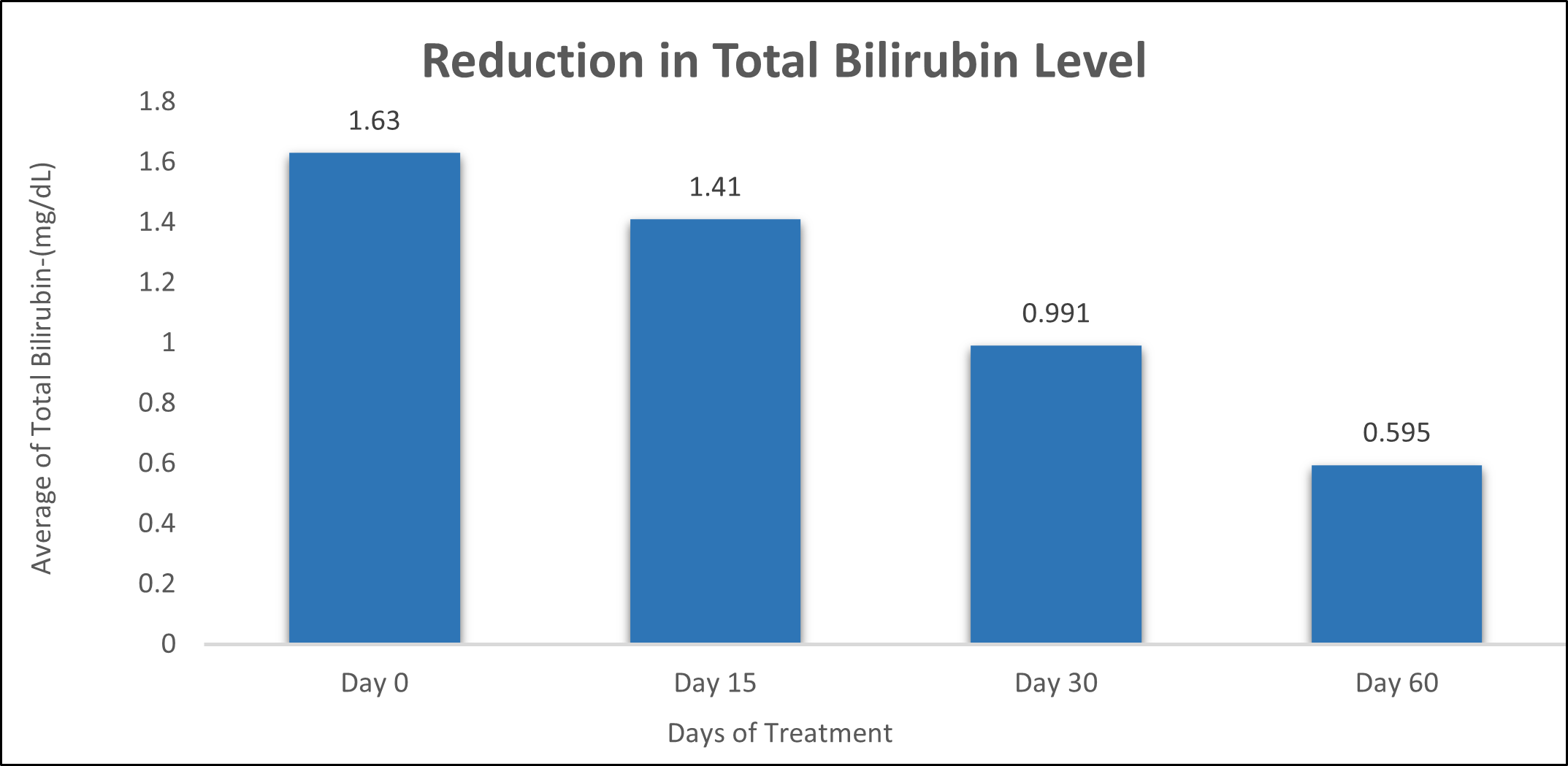
Figure 4: Effect of Liver Detox product on Total Bilirubin Level.
Creatinine was evaluated in 10 cases who were treated with liver detox products for a period of 60 days. The initial mean Creatinine significantly reduced from 1.81±0.624 to 1.549±0.678 after 15 days, to 1.35±0.469 after 30 days, and to 1.03±0.309 after 60 days, where p<0.05 was found to be significant. In inference to the evaluation of the above parameter after 60 days, it has been found that the Creatinine levels were reduced by 14.41% after 15 days, 25.41% after 30 days, 43.09% after 60 days (Table 6, Figure 5).
Table 6: Effect of Liver Detox product on Creatinine level.

Data was analysed by Student t test. Significant at p< 0.05

Figure 5: Effect of Liver Detox product on Creatinine level.
GFR was evaluated in 10 cases who were treated with liver detox products for a period of 60 days. The initial mean GFR significantly improved from 80.3±11.09 to 85.7±8.857 after 15 days, to 93.9±6.154 after 30 days, and to 101.7±4.137 after 60 days, where p<0.05 was found to be significant. In inference to the evaluation of the above parameter after 60 days, it has been found that the GFR was improved by 6.72% after 15 days, 16.93% after 30 days, 26.65% after 60 days (Table 7, Figure 6).
Table 7: Effect of Liver Detox product on GFR.
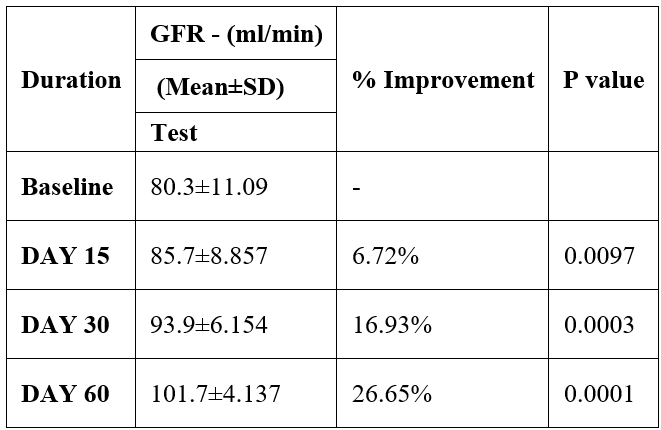
Data was analyzed by Student t test. Significant at p< 0.05
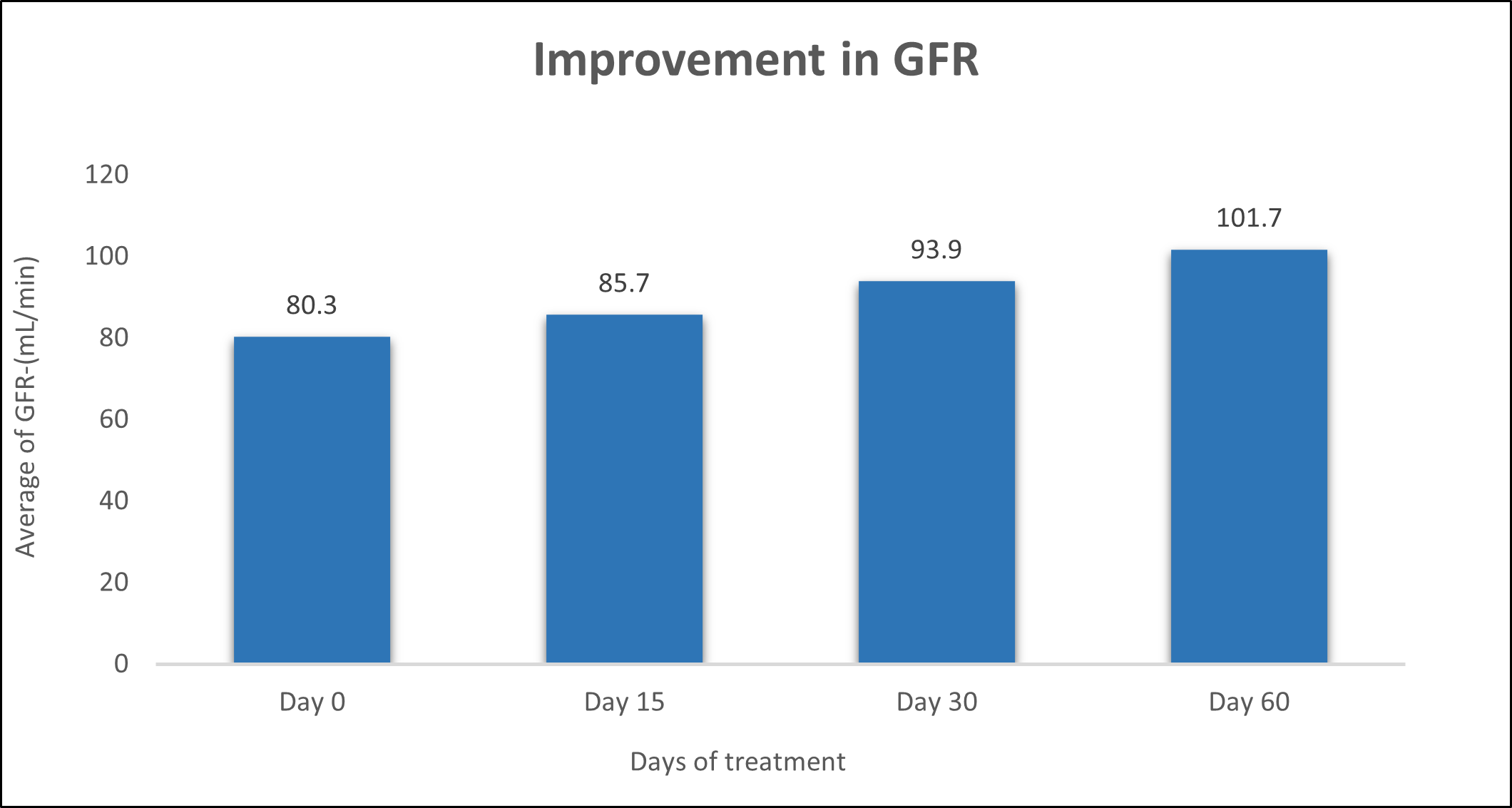
Figure 6: Effect of Liver Detox product on GFR.
Conclusion
It is concluded that liver disease is a growing global health concern that affects millions of people worldwide. Where Gplife Healthcare Pvt. Ltd. Liver detox product is complementary and supportive of liver health. Indeed, for the development this product, Gplife Healthcare P Ltd. used both standardized and potential extracts of herbs such as Milk thistle, Shallaki, Himsara, Punarnava, and Bhuiamla, etc. which have shown promising results in liver disease management. Out of 10 cases, one patient with non-alcoholic fatty liver grade-3 and another one with hepatitis and non-alcoholic fatty liver grade-2 got completely resolved with their conditions in 60 days. While, two patients exhibited notable improvements in their conditions, leading to downgrading from grade-3 to grade-1 following 60 days of liver detox treatment. After 60 days of evaluation of cases, it has been observed that the SGPT levels have been reduced by 90.27%, the SGOT levels have been reduced by 88.93%, the GGT levels have been reduced by 86.81%, the Total Bilirubin levels have been reduced by 63.49%, the Creatinine levels have been reduced by 43.09%, and the GFR has been increased by 26.65%. No adverse events were reported during the course of the study to any of the cases owing to the liver detox product. Based on the findings of the study as discussed above, the product has the potential to be a safe alternative to conventional medications in the treatment of a various of liver diseases. It is suggested that the Liver Detox product should be further extensively used as a monotherapy or adjunctive therapy for the management of liver disease.
Authorship Criteria: Study conception and design, data collection, analysis and interpretation of results, manuscript preparation: Dr. Shridhar Pandya and Dr. Chetan Savaliya.
Conflicts of Interest: Dr. Shridhar Pandya and Dr. Chetan Savaliya are directors in Gplife Healthcare Pvt. Ltd. Authors received funding from Gplife Healthcare Pvt. Ltd.
Acknowledgment: The authors would like to acknowledge the research team and the back-office team involved in the research work. We would like to acknowledge the support provided by back office, Gplife Healthcare Pvt. Ltd.
References
- Glauert HP, Calfee-Mason K, Stemm DN, Tharappel JC, Spear BT. Dietary antioxidants in the prevention of hepatocarcinogenesis: a review. Nutr Food Res, 2010; 54(7): 875–896. doi: 10.1002/mnfr.200900482
- Glauert HP. Role of NF-κB in hepatocarcinogenesis and its potential inhibition by dietary antioxidants. Curr Cancer Drug Targets, 2012; 12(9): 1160–1172.
- O'Brien L, Hosick PA, John K, Stec DE, Hinds TD. Biliverdin reductase isozymes in metabolism. Trends Endocrinol Metab, 2015; 26(4): 212-220.
- Ribeiro AJS, Yang X, Patel V, Madabushi R, Strauss DG. Liver Microphysiological Systems for Predicting and Evaluating Drug Effects. Clin Pharmacol Ther, 2019; 106(1): 139-147.
- Björnsson E, Olsson R. "Outcome and prognostic markers in severe drug-induced liver disease". Hepatology, 2005; 42(2): 481–489. doi:1002/hep.20800
- Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol, 2023; S0168-8278(23)00194-0. doi: 10.1016/j.jhep.2023.03.017.
- Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci, 2005; 50(10): 1807-1812. doi: 10.1007/s10620-005-2942-9. PMID: 16187178.

