Published online Apr 27, 2023. doi: 10.4254/wjh.v15.i4.525
Peer-review started: December 17, 2022
First decision: January 22, 2023
Revised: February 1, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 27, 2023
The formation of liver cirrhosis (LC) is an unfavorable event in the natural history of chronic liver diseases and with the development of portal hypertension and/or impaired liver function can cause a fatal outcome. Decompensation of LC is considered the most important stratification variable for the risk of death. It is currently postulated that decompensation of LC occurs through an acute (including acute-on-chronic liver failure) and non-acute pathway. Acute decompensation of LC is accompanied by the development of life-threatening complications, characterized by an unfavorable prognosis and high mortality. Progress in understanding the underlying molecular mechanisms has led to the search for new interventions, drugs, and biological substances that can affect key links in the pathogenesis of acute decompensation in LC, for example the impaired gut-liver axis and associated systemic inflammation. Given that particular alterations in the composition and function of gut microbiota play a crucial role here, the study of the therapeutic possibilities of its modulation has emerged as one of the top concerns in modern hepatology. This review summa
Core Tip: Given that particular alterations in the composition and function of gut microbiota play a crucial role in the pathogenesis of acute decompensation in liver cirrhosis (LC), this review summarized the investigations that describe the theoretical foundations and therapeutic potential of gut microbiota modulation in acute decompensation of LC. Despite the encouraging preliminary data, the majority of the suggested strategies have only been tested in animal models or in preliminary clinical trials. Additional multicenter randomized controlled trials must demonstrate their efficacy in larger patient populations.
- Citation: Garbuzenko DV. Therapeutic possibilities of gut microbiota modulation in acute decompensation of liver cirrhosis. World J Hepatol 2023; 15(4): 525-537
- URL: https://www.wjgnet.com/1948-5182/full/v15/i4/525.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i4.525
The formation of liver cirrhosis (LC) is an unfavorable event in the natural history of chronic liver diseases and with the development of portal hypertension and/or impaired liver function can cause a fatal outcome. During LC, there is a compensated stage, which is usually asymptomatic and characterized by preserved quality of life and a median survival exceeding 12 years, and a decompensated stage associated with the occurrence of life-threatening complications, in which a median survival drops to 2-4 years. Accordingly, the decompensation of LC is considered the most important stratification variable for the risk of death[1]. Decompensation of LC presents as acute decompensation in a portion of patients with the development of one or more major complications and is accompanied by high mortality. In many other patients, its characteristic signs are usually slow-progressing ascites or mild grade 1-2 hepatic encephalopathy or jaundice not requiring hospitalization[2].
The European Association for the Study of the Liver (EASL)-CLIF Consortium CANONIC study established diagnostic criteria for acute-on-chronic liver failure (ACLF) and introduced the concept of acute decompensation as a distinct clinical presentation of decompensation of LC defined by the acute development of more than one major complication: (1) Acute (for less than 2 wk) development of grade 2 or 3 ascites. This may be the first or a new episode of ascites; (2) Acute hepatic encephalopathy, which is manifested by a sudden change in the mental status of a patient with no previous cognitive impairment and no signs of acute neurological disease. This may be the first or a new episode of hepatic encephalopathy; (3) Acute gastrointestinal bleeding from the upper and/or lower gastrointestinal tract of any etiology; and (4) Spontaneous bacterial peritonitis (SBP), spontaneous bacteremia, urinary tract infection, pneumonia, cellulite, as well as any other type of acute bacterial infection[3].
The cause of acute decompensation of LC can be both exogenous factors (e.g., bacterial infections, alcohol abuse, etc) and endogenous factors (e.g., progressive liver disease, translocation of intestinal bacterial immunogenic material to the systemic circulation)[4]. Its most severe form (ACLF) according to the definition of the American College of Gastroenterology is a potentially reversible condition in patients with chronic liver disease with or without LC that is associated with the potential for multiple organ failure and mortality within 3 mo in the absence of treatment of the underlying liver disease, liver support, or liver transplantation. The severity of organ failure may be assessed by the EASL-CLIF sequential organ failure assessment score or North American Consortium for the Study of End-Stage Liver Disease organ failure score[5].
The first investigation derived from the PREDICT study group of the EASL-CLIF Consortium uncovered that acute decompensation of LC without ACLF is a heterogeneous condition with three different clinical courses and two major pathophysiological mechanisms: Systemic inflammation and portal hypertension. The first clinical course includes patients who develop ACLF and have an extremely high short-term mortality rate, termed pre-ACLF. The second clinical course includes patients with unstable decompensated LC who require frequent hospitalizations unrelated to ACLF and is associated with a lower mortality risk than pre-ACLF. The third clinical course includes patients with stable decompensated LC who rarely require hospital admission and have a much lower 1-year mortality risk.
Each clinical course of acute decompensation of LC differs significantly regarding the grade of systemic inflammation and the severity of portal hypertension. A high grade of systemic inflammation at admission with exacerbation during follow-up is observed in pre-ACLF. A low grade of systemic inflammation at admission with subsequent steady course is observed in patients with unstable decompensated LC. A low grade of systemic inflammation at admission with subsequent improvement is observed in patients with stable decompensated LC. A high grade of portal hypertension is observed in patients with unstable decompensated LC. A low grade of portal hypertension is observed in pre-ACLF and stable decompensated LC[6].
The aim of the second investigation derived from the PREDICT study group of the EASL-CLIF Consortium was to analyze and characterize the precipitants leading to acute decompensation of LC without ACLF or with ACLF. Of all the potential precipitants explored, only four (proven bacterial infections, severe acute alcoholic hepatitis, gastrointestinal bleeding associated with shock, and toxic encephalopathy) fulfilled the diagnostic criteria of precipitants. Proven bacterial infections and severe alcoholic hepatitis were present in the absolute majority (> 96%) of the patients. However, no precipitating event could be identified in two-thirds of acute decompensation of LC without ACLF patients and in one-third of acute decompensation of LC with ACLF patients. These data suggest that acute decompensation of LC without ACLF develops more frequently in the context of endogenous mechanisms (e.g., progressive liver disease, bacterial translocation). The prevalence and number of precipitants increased with the severity of the acute decompensation sub-phenotype form stable decompensated LC/unstable decompensated LC to pre-ACLF and ACLF, which were also directly related to clinical course severity and short-term mortality in patients with acute decompensation of LC. These data, therefore, strongly suggest that specific preventive and therapeutic approaches for these precipitants must improve outcomes in decompensated LC[7].
Current therapeutic strategies in acute decompensation of LC provide the removal of the precipitants, the treatment of specific complications, as well as intensive monitoring and support of vital body functions[8]. Liver transplantation may be a successful treatment option for some of the most severe ACLF patients, but its implementation is usually associated with high costs and worse survival compared to “standard” elective surgery[9].
Progress in understanding the underlying molecular mechanisms has led to the search for new interventions, drugs, and biological substances that can affect key links in the pathogenesis of acute decompensation in LC, for example the impaired gut-liver axis and associated systemic inflammation[10]. Given that particular alterations in the composition and function of gut microbiota play a crucial role here, this review summarized the investigations that describe the theoretical foundations and therapeutic potential of gut microbiota modulation in acute decompensation of LC.
This review provided an overview of the current knowledge of the therapeutic possibilities of gut microbiota modulation in acute decompensation of LC. The PubMed and Embase databases, the Web of Science platform, the Google Scholar retrieval system, the Cochrane Database of Systematic Reviews, Reference Citation Analysis (https://www.referencecitationanalysis.com/), and the reference lists from related articles were used to search for relevant publications. Articles corresponding to the aim of the review were selected for 2003–2023 using the keywords: “liver cirrhosis,” “acute decompensation,” “acute-on-chronic liver failure,” “pathogenesis,” “therapy,” “gut microbiota,” and “modulation.” The investigations that described the theoretical foundations and therapeutic potential of gut microbiota modulation in acute decompensation of LC were included.
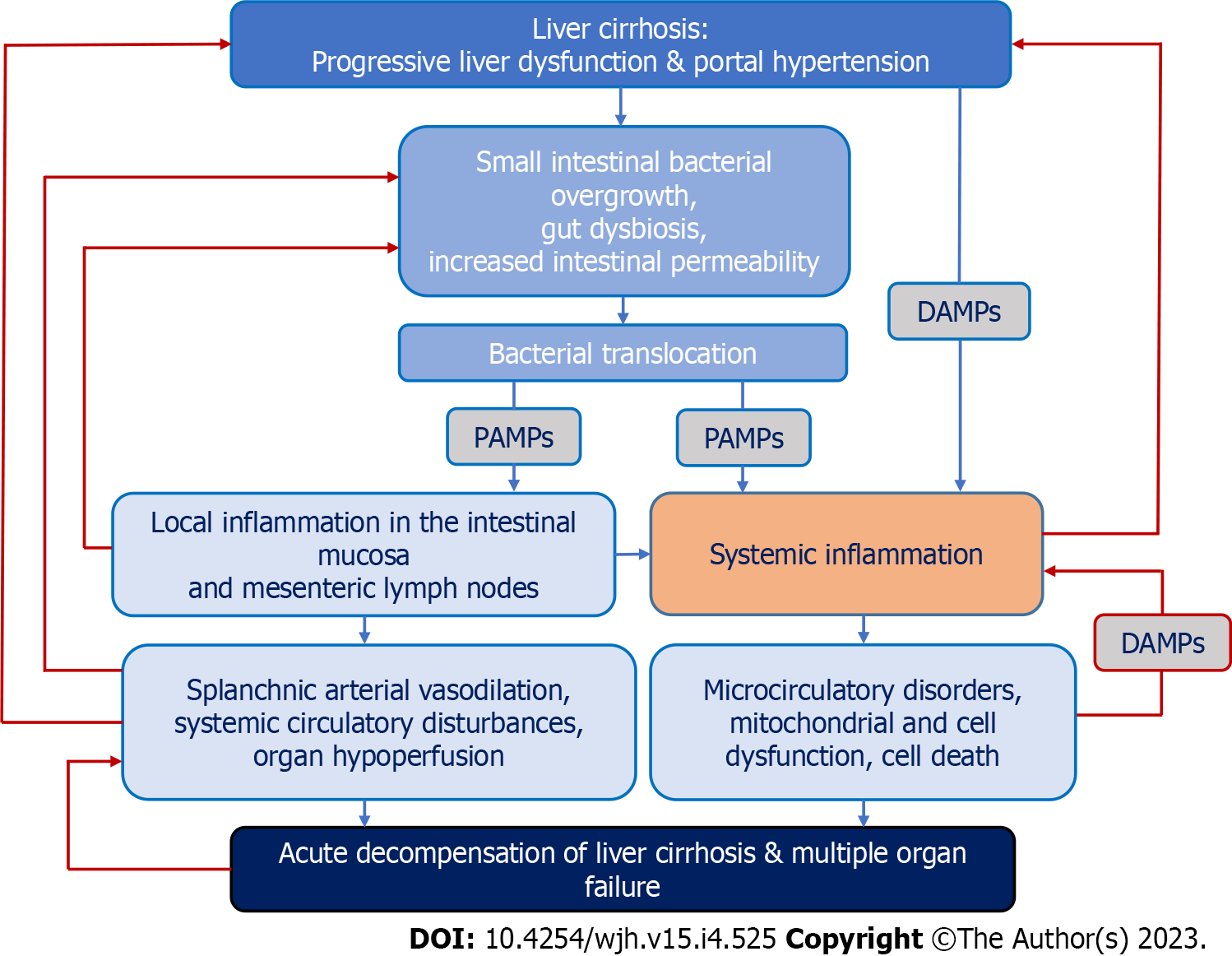
One of the leading hypotheses in recent years suggests that the main driver of acute decompensation and concomitant multiple organ failure in LC is systemic inflammation. Its cause may be the “spill over” of damage-associated molecular patterns, cytokines, and immune regulatory cells from the chronically inflamed liver and potential other inflamed tissue sites to the systemic circulation. Additionally, pathogen-associated molecular patterns (PAMPs), namely bacterial and bacterial products (in particular, the lipopolysaccharides of the cell wall of Gram-negative bacteria), due to pathological translocation from the intestinal lumen through the portal vein, reach the cirrhotic liver where they may be ineffectively cleared by Kupffer cells or shunted through vascularized septae into the systemic circulation, contributing to systemic inflammation (Figure 1). Over time, immune tolerance may develop, which is characterized by accumulation in the systemic circulation of immune cells with immune suppressive or immune regulatory properties, along with high serum levels of proinflammatory and anti-inflammatory cytokines, damage-associated molecular patterns, and PAMPs. Additionally, with further disease progression to ACLF, cells with a reduced capacity to repel microbial challenges appear in the systemic circulation, which increases the risk of infectious complications and sepsis[11].
Once the first episode of acute decompensation of LC develops, systemic inflammation follows a chronic course, with transient episodes of reactivation caused by exogenous proinflammatory factors or to bursts of bacterial translocation. Repeated episodes of acute decompensation during the clinical course of decompensated LC develop in the setting of reactivation of the immune system. The prognosis of patients with acute decompensation of LC associated with moderate, non-progressive systemic inflammation depends on the severity of portal hypertension. Patients with severe portal hypertension frequently develop an unstable clinical course, requiring frequent hospital readmission, and significant short-term and long-term mortality. In contrast, if portal hypertension is moderate, systemic inflammation improves after the episode of acute decompensation of LC, patients develop a benign stable course, and long-term mortality is low[12].
The alteration of gut microbiota composition in acute decompensation of LC creates prerequisites for disruption of the gut-liver axis, and bacterial translocation contributing to systemic inflammation is based on small intestinal bacterial overgrowth (SIBO), gut dysbiosis, and increased permeability of the intestinal epithelial barrier[13].
SIBO, which is characterized by an excessive number of bacteria in the small intestine (≥ 103 colony-forming units/mL) with a predominance of Gram-negative aerobic and anaerobic species, occurs in about half of the patients with LC, but the mechanism of its development has not been definitively established. One of the possible reasons may be the slowing down of orocecal transit[14]. However, the causal relationship between these pathophysiological conditions remains unclear. In some studies, a more pronounced slowing down of orocecal transit was observed in patients with Child-Turcotte-Pugh (CTP) class B and C LC with hepatic encephalopathy, which was explained by the autonomic neuropathy, metabolic derangements due to portosystemic shunting, and SIBO[15].
Because gastric acid is an important barrier that prevents bacterial colonization of the stomach and small intestine, it is assumed that proton pump inhibitor therapy may promote SIBO through chronic acid suppression and subsequent hypochlorhydria. However, a meta-analysis of 19 studies demonstrated that proton pump inhibitor therapy was significantly associated with a moderately increased risk of SIBO (odds ratio: 1.71, 95% confidence interval: 1.20-2.43)[16]. The immune system also plays a role in the genesis of SIBO, as evidenced by the high prevalence of SIBO in patients who have immunodeficiency. Besides, immunoglobulin A content on the duodenum and jejunum mucosa has been shown to be significantly increased in patients with SIBO[17].
Gut dysbiosis in LC is manifested by an unfavorable change in the balance of autochthonous species of microorganisms with a reduction in symbiont bacteria belonging to the Firmicutes phylum (e.g., the Ruminococcaceae and Lachnospiraceae families, etc.) and growth in pathobiont bacteria of the Bacteroidetes and Actinobacteria phyla[18]. These changes largely depend on the etiology of LC and are aggravated in its decompensated stage. For example, they were the most significant in patients with alcoholic LC, who had the highest content of bacteria of the Enterobacteriaceae and Halomonadaceae families and the lowest content of bacteria of the Lachnospiraceae, Ruminococcaceae, and Clostridiales Incertae Sedis XIV families, which was accompanied by an exorbitant level of endotoxemia[19]. Note that the bacteria of the Enterobacteriaceae family express a powerful immunostimulating endotoxin and are the main pathogens involved in the pathogenesis of SBP[20]. In a study by Shu et al[21], in patients with hepatitis B virus-related LC, the most common phyla of bacteria were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia, together constituting 92.1% of the total number of microorganisms studied. Patients with compensated LC had a high relative abundance of bacteria of the genus Faecalibacterium spp. and the Ruminococcaceae family, whereas with decompensated LC, bacteria of the genus Streptococcus spp. and the Enterobacteriaceae family prevailed.
In a prospective study by Solé et al[22], patients with LC had a significant decrease in gene and metagenomic species diversity compared to healthy subjects. This was associated with disease stages and was especially noticeable in patients with ACLF and persisted after antibiotic therapy. ACLF was accompanied by a significant increase in Enterococcus spp. and Peptostreptococcus spp. and a decrease in some autochthonous bacteria. Gut microbiome alterations correlated with the model for end-stage liver disease (MELD) and CTP scores and multiple organ failure and were associated with some complications, especially hepatic encephalopathy and infections. Additionally, the gut microbiome predicted 3-mo survival with good stable predictors. Functional analysis showed that patients with LC had enriched pathways related to ethanol production, γ-aminobutyric acid metabolism, and endotoxin biosynthesis.
According to a study by Philips et al[23] pathogenic genera of bacteria in gut microbiota, in particular, Leptotrichia spp., Neisseria spp., and Erwinia spp., were predominant in patients with decompensated LC with infection, and their survival correlated with the presence of microorganisms with high functional propionate metabolism, for example, bacteria of the genus Megamonas spp.
Bajaj et al[19] developed a quantitative index to describe microbiome alterations accompanying LC based on the ratio of “good vs bad” taxa abundance [cirrhosis dysbiosis ratio (CDR)]. It is designed to predict the course of LC and assess the risk of possible complications. CDR is calculated using the formula:
CDR = [Lachnospiraceae (%) + Ruminococcacceae (%) + Veillonellaceae (%) + Clostridiales Incertae Sedis XIV (%)]/[Bacteroides spp. (%) + Enterobacteriaceae (%)].
The authors found that a low CDR was associated with death and organ failure within 30 d.
In a prospective study by the North American Consortium for the Study of End-Stage Liver Disease, including hospitalized patients with LC across North America, the CDR was lower in subjects who developed ACLF, especially among those with renal failure. Taxa belonging to the Proteobacteria phylum (Enterobacteriaceae, Campylobacteriaceae, and Pasteurellaceae) and Firmicutes phylum (Enterococcaceae and Streptococcaceae) were associated with the development of negative outcomes, whereas other Firmicutes members (Lachnospiraceae and Clostridiales) reduced the risk of negative outcomes. Changes in the microbiota were associated independently on logistic regression analyses with extrahepatic organ failure, transfer to intensive care, ACLF, and death[24].
To study the influence of gut dysbiosis on prognosis in LC, Maslennikov et al[25] modified CDR by placing “bad” bacteria in the numerator and “good” bacteria in the denominator (MDR)[25]:
MDR = [Bacilli (%) + Proteobacteria (%)]/[Clostridia (%) + Bacteroidetes (%)].
Their case-control study included 48 patients with LC and 21 healthy controls. Patients with an MDR greater than the median indicated the group with severe dysbiosis. The other patients were in the non-severe dysbiosis group. The follow-up period was 4 years. The mortality rate of patients with severe dysbiosis was significantly higher than that of patients with non-severe dysbiosis. The abundance of Enterobacteriaceae, Proteobacteria, and Lactobacillaceae was increased and the abundance of Firmicutes and Clostridia was reduced in the deceased patients compared with survivors. The abundance of Bacilli, Enterococcaceae, and Lactobacillaceae was higher and the abundance of Clostridia was lower in those who died during the 1st year of follow-up compared with those who survived the 1st year. The abundance of Enterobacteriaceae and Proteobacteria was higher in those who died in the second to 4th year of follow-up compared with survivors.
The cause of gut dysbiosis in LC is not fully understood. One of the key theories explains its presence by depletion of the pool of bile acids due to a decrease in their synthesis and secretion by hepatocytes. Bile acid synthesis is regulated mainly through the activation of nuclear receptors, in particular the farnesoid X receptor (FXR), which also induces genes affecting intestinal permeability and inflammation, preventing bacterial translocation in experimental LC[26]. Bile acids have both direct and indirect antimicrobial effects through the modulation of FXR activity, which is important for the homeostasis of the epithelial and gut-vascular barrier. Colonic microbial groups are responsible for the deconjugation and 7α-dehydroxylation of bile acids, and it is hypothesized that the presence of microbe toxic bile acids (particularly deoxycholic acid) in the intestine is a factor that keeps undesirable microbial populations under control[27]. The insufficient content of primary bile acids in feces decreased in 7α-dehydroxylating bacteria belonging to the Firmicutes phylum, especially the genera Blautia spp. and Ruminococcus spp. Their deficiency in the small intestine causes overgrowth of proinflammatory pathogenic bacteria belonging to the Proteobacteria phylum, in particular the Enterobacteriaceae family, which induces the release of markers of intestinal inflammation and exacerbates necroinflammatory changes in liver tissues. This triggers a positive feedback mechanism leading to additional inhibition of bile acid synthesis[28]. On the contrary, oral administration of conjugated bile acids to rats with a model of CCl4-induced LC and ascites significantly reduced the bacteria in the ileum (especially Escherichia coli and Enterococcus spp.) to levels comparable to those in healthy rats, decreased the SIBO, bacterial translocation, and endotoxemia[29].
Increased permeability of the intestinal epithelial barrier is associated with both gut dysbiosis[30] and microcirculatory disorders in LC that change the barrier properties of the intestinal mucosa, which include mechanical, biological, immune, and chemical protection factors[31]. Intestinal mucosa and intercellular junctions among epithelial cells form a layer that allows selective passage of the toxins and bacterial products. Intestinal epithelial cells produce mucus, which forms a thick layer on the mucosa and prevents bacterial translocation. Mucous secretions are rich in immunoglobulin A, which neutralizes toxins and microorganisms and prevents their adhesion and colonization. Bile acid secretion also plays a role in intestinal permeability by affecting the intestinal mucosa and by neutralizing endotoxin[32].
In LC, the thickness of the intestinal mucosa is decreased with the loss of mucus-producing goblet cells. The ultrastructural changes of the mucosa, contributing to increased permeability of the intestinal epithelial barrier, are characterized by atrophy, edema, and inflammatory infiltration of the lamina propria, fibromuscular proliferation, expansion of the space between neighboring cells, a reduction in the number of short but thicker microvilli, and a decrease in the villi/crypt ratio. These disorders are associated with a diminution in the expression of the tight junction proteins, including occludin and claudin-1, in the intestinal mucosa. Additionally, irregularities of the glandular epithelia, loss of the normal cylindrical shape, edematous villi, and loosening of the mucous membrane were revealed. High levels of lipid peroxidation markers in enterocytes led to mitochondrial dysfunction and cellular instability[33].
The increased stimulation of gut-associated lymphoid tissue leads to the persistent activation of monocytes, dendritic cells, and T lymphocytes both in the intestinal mucosa and mesenteric lymph nodes and to the consequent production of proinflammatory and anti-inflammatory cytokines. Local inflammatory disorders can be a trigger for systemic inflammation because of immune cells entering the systemic circulation. This is facilitated by a violation of the production of intestinal antimicrobial peptides, in particular α-defensins and Reg3 lectins, a decrease in the ability to bind and neutralize bacterial endotoxin by albumin, lipopolysaccharide-binding protein, high-density lipoproteins, low-density lipoproteins, very low-density lipoproteins, chylomicrons, apolipoproteins, as well as dysfunction of the immune system in patients with LC[34].
The persistence of systemic inflammation leads to a progressive failure of the immune response similar to a condition of immunodeficiency. The immune dysregulation in patients with LC can be defined as a “dysbiotic immune-inflammatory disorder” characterized by abnormal local (gut and liver) and systemic inflammation, triggered by an impaired immune response to gut-derived antigens. The main feature of cirrhotic dysbiotic immune-inflammatory disorder is a perpetual immunologic activation, which involves all the immune cells (neutrophils, monocytes, T and B lymphocytes) that exhibit activation and costimulatory markers.
At the molecular level, the recognition of PAMPs by Toll-like receptors activates MyD88-dependent and MyD88-independent signaling pathways, leading to the activation of nuclear factor kB, production of inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, and interferon-β], chemokines [keratinocyte chemoattractant (CXCL1), MIP-2 (CXCL2), MCP-1 (CCL2), RANTES (CCL5), MIP-1α (CCL3), and MIP-1β (CCL4)], nitric oxide, and reactive oxygen species[35].
The association of Toll-like receptor gene polymorphisms with a decrease in the inflammatory response was established, which further increases the load of circulating bacterial antigens that modulate the immune response and contribute to the development of complications[36]. Cytosolic NOD-like receptors (NLRs) are also involved in this process. The NLRP3 inflammasome formed after the oligomerization of the NLRP3 protein activates caspase 1, which cleaves pro-IL-1β and pro-IL-18, followed by the formation of proinflammatory cytokines IL-1β and IL-18[37].
A cascade of molecular events arising from dysbiotic immuno-inflammatory disorders leads to the enhanced phagocytic activity, vascular endothelial injury, synthesis of acute phase proteins by the liver, chemotaxis of leukocytes to the sites of inflammation (mainly the liver), and activation of leukocytes at the systemic level[38]. This in itself worsens bacterial translocation and contributes to the formation of a vicious circle, which can aggravate the pathological process associated with acute decompensation of LC and predispose to the development of its characteristic complications.
In accordance with the current recommendations of the EASL, one of therapeutic strategy that prevents disease progression in patients with decompensated LC should be aimed to improve the microbiome abnormalities and bacterial translocation to ameliorate the impaired gut-liver axis[39]. In this regard, a potential target for therapy may be the gut microbiota, which is the main regulator of bacterial translocation and systemic inflammation[40].
The use of non-absorbable or poorly absorbable oral antibiotics is an obvious solution aimed at countering bacterial translocation. They affect the gut microbiota with rare side effects and a favorable long-term safety profile and are recommended as primary and secondary prevention of bacterial infections and treatment of hepatic encephalopathy in patients with decompensated LC[41].
Selective decontamination of the intestine with norfloxacin can contribute to a significant reduction in bacterial translocation. In a study by Albillos et al[42], this was manifested by a reduction in the serum levels of lipopolysaccharide-binding protein, soluble CD14, proinflammatory cytokines TNF-α, IL-12 and interferon-γ, as well as the metabolite nitric oxide. In a multicenter, randomized, prospective, double-blind, placebo-controlled trial in parallel groups (NORFLOCIR), including 291 patients with CTP class C LC, the administration of norfloxacin at a dose of 400 mg once daily for 6 mo significantly decreased the incidence of any and Gram-negative bacterial infections without growth infections caused by Clostridium difficile or multiresistant bacteria and an increase in survival in patients with ascites fluid protein concentrations < 15 g/L[43].
At the same time, long-term use of norfloxacin increased gut microbiota resistance to fluo
In an observational study involving 30 patients with decompensated LC after 4 wk of treatment with rifaximin at a dose of 1200 mg/d, there was an improvement in hyperammonemia and cognitive dysfunction, although no significant changes in the serum proinflammatory cytokine levels were observed. Rifaximin reduced the serum levels of ammonia, bacterial endotoxin, soluble CD163, and the D-mannose receptor. At the same time, the serum proinflammatory cytokine levels remained the same. Gut microbial analysis revealed that the richness and complexity of species were unchanged, while the abundance of the genus Streptococcus spp. after treatment with rifaximin was reduced[46].
The current literature data do not provide a clear answer as to which of the antibiotics is more effective in preventing bacterial translocation in patients with decompensated LC. Nevertheless, in a randomized, double-blind, placebo-controlled trial by Kulkarni et al[47], primary prophylaxis with oral norfloxacin (400 mg/d for 30 d) effectively prevented bacterial infections in patients with ACLF. Furthermore, a systematic review and meta-analysis of 17 RCTs showed that rifaximin is useful for both primary and secondary prevention of SBP, whereas norfloxacin daily and alternate norfloxacin and rifaximin are useful for primary prophylaxis[48].
A scientific basis for the use of probiotics in the treatment of liver diseases is their ability to correct gut dysbiosis, elevate the production of short-chain fatty acids, and reduce the increased permeability of the intestinal epithelial barrier[49]. The therapeutic potential of probiotics in LC has been studied in both experimental and clinical studies. For example, oral administration of a combined probiotic VSL#3 containing 8 different strains (Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus bulgaricus, Streptococcus thermophilus) to rats with different models of LC led to stabilization in the intestinal epithelial barrier, reduction of bacterial translocation, and decrease in severity of endotoxemia and systemic inflammation[50,51]. Oral administration of probiotics Bifidobacterium pseudocatenulatum CECT7765 to mice with a model of CCl4-induced LC was accompanied by an improvement in the integrity of the intestinal epithelial barrier and prevented bacterial translocation[52]. It also induced a morphologic, phenotypic, and functional transitional change towards an anti-inflammatory profile in blood-derived and ascitic fluid macrophages from patients with CTP class C LC as well as Kupffer cells from rats with a model of bile duct ligation-induced LC[53]. The combined use of probiotics containing Clostridium butyricum and Bifidobacterium infantis in patients with hepatitis B virus-related LC and minimal hepatic encephalopathy significantly decreased the pathogenic bacteria of the genus Enterococcus spp. and the Enterobacteriaceae family in gut microbiota as well as reduced the circulating levels of bacterial translocation markers and decreased the permeability and damage of the intestinal epithelial barrier[54].
Some RCTs have studied the effect of probiotics on gut microbiota in patients with LC. In one of them, the administration of the probiotic beverage Yakult 400, which contains Lactobacillus casei strain Shirota, twice a day during the first half of the 4-wk study contributed to the normalization of gut microbiota and improved liver function in patients with CTP class A alcoholic LC[55]. This probiotic was safe and effective in patients with cirrhosis (CTP score ≤ 10) who took it three times daily for 6 mo. It significantly reduced the plasma monocyte chemotactic protein-1, plasma IL-1β (alcoholic LC), IL-17a, and macrophage inflammatory protein-1β (non-alcoholic LC) compared to the placebo group. At the same time, no significant differences in intestinal permeability, bacterial translocation, or metabolomic profile were observed[56].
Bajaj et al[57] showed that the administration of the probiotic Lactobacillus rhamnosus GG for 8 wk to patients with LC (mean values of the MELD score of 8.6 ± 2.2) and minimal hepatic encephalopathy was safe and well tolerated and reduced the serum levels of bacterial endotoxin and TNF-α, decreased the relative abundance of Enterobacteriaceae family, and increased the Clostridiales Incertae Sedis XIV and Lachnospiraceae families, without changes in cognitive dysfunction.
Daily intake for 6 mo of a probiotic powder containing eight different bacterial strains (Bifidobacterium bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19, and Lactococcus lactis W58) by patients with cirrhosis (CTP score < 12) had a beneficial effect on immune function, but no effect on the permeability of the intestinal epithelial barrier and bacterial translocation was observed[58]. In addition, it increased the relative abundance of bacteria of the species Faecalibacterium prausnitzii, Syntrophococcus sucromutans, Bacteroides vulgatus, and Alistipes shahii and the genus Prevotella spp. compared to the placebo group. At the same time, the relative abundance of bacteria of the species Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus casei remained unchanged[59].
Thus, although in most studies the probiotic use in LC is associated with an improvement in gut microbiota profile, data concerning their impact on permeability of the intestinal epithelial barrier, bacterial translocation, and systemic inflammation are scarce and contradictory.
In recent years, numerous studies have demonstrated the therapeutic possibilities of fecal microbiota transplantation (FMT) from healthy donors to patients with chronic liver diseases[60]. It is assumed that the effectiveness of FMT is associated with the creation of a competitive environment in the intestine due to non-pathogenic microorganisms and their production of antimicrobial substances, such as bacteriocins. In addition, the positive effect of donor fecal material on the gut virome and microbiota, the metabolism of short-chain fatty acids and some bile acids, as well as various immune mechanisms is not excluded[61]. Much attention has been paid to the use of FMT to treat hepatic encephalopathy in LC. At the same time, the issues of its effectiveness, safety, and tolerability, as well as methods of administration of donor fecal material (using enemas, colonoscopy, or in encapsulated form), the type and number of transplanted microorganisms necessary to obtain a positive result are discussed[62].
In the first open-label RCT involving 10 patients with LC (MELD score < 17) and recurrent hepatic encephalopathy, three frozen-then-thawed FMT units (90 mL total) instilled by enema and retained for 30 min eliminated antibiotic-induced dysbiosis. All patients showed an improvement in cognitive dysfunction, which may have been associated with an increase of the relative abundance of bacteria of the Lactobacillaceae and Bifidobacteriaceae families[63]. With further follow-up (12.9 ± 2.9 mo), no cases of hepatic encephalopathy were detected, and only 1 patient from this cohort required hospitalization. Microbiological analysis of the gut microflora showed an increase of relative abundance of bacteria of the Burkholderiaceae family and a decrease in the relative abundance of bacteria of the Acidaminococcaceae family, while the relative abundance of bacteria of the Lactobacillaceae and Bifidobacteriaceae families did not differ from the placebo group[64].
In a phase I RCT, the administration of 15 capsules with donor fecal microbiota to 10 patients with LC (MELD score < 17) and recurrent hepatic encephalopathy had a positive effect on cognitive dysfunction was safe and well tolerated. After 30 d of monitoring, there was an improvement in duodenal mucosal microbial diversity with higher Ruminococcaceae and Bifidobacteriaceae families and lower Streptococcaceae and Veillonellaceae families (P = 0.01). Reduction in the Veillonellaceae family was seen post-FMT in sigmoid (P = 0.04) and stool (P = 0.05). Duodenal E-cadherin (P = 0.03) and defensin A5 (P = 0.03) increased, while the IL-6 (P = 0.02) and serum levels of lipopolysaccharide-binding protein (P = 0.009) reduced post-FMT[65].
An important problem of FMT is the risk of severe infection transmission, which is especially significant in patients with weakened immunities[66]. For this reason, the United States Food and Drug Administration published a list of minimum requirements for screening and testing of fecal microbiota donors for the presence of multidrug resistant microorganisms in 2019[67].
The coronavirus disease 2019 pandemic has raised concerns about the possible transmission of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in FMT. Although the genetic material of SARS-CoV-2, including the live virus, has been detected in the feces of patients with a new coronavirus infection even after the elimination of respiratory symptoms[68], no actual cases of infection through donor fecal material were reported. Stool testing for SARS-CoV-2 is not currently widely available. Nevertheless, experts advocate screening donors for the presence of symptoms of a new coronavirus infection with quarantine of their fecal material during further monitoring of the disease[69].
Obeticholic acid is a semisynthetic bile acid that, in addition to bacteriostatic activity, is an agonist of FXR and thus can modulate the gut microbiota. For example, oral administration of obeticholic acid to rats with a model of CCl4-induced LC reduced the intestinal content of the genus Enterococcus spp.[70] and decreased the bacterial translocation[71]. Besides, obeticholic acid prevented the increased expression of monocyte chemoattractant protein-1 following stimulation with TNF-α and lipopolysaccharides or TNF-α alone in liver sinusoidal endothelial cells and Kupffer cells in rats with a model of thioacetamide-induced LC[72]. In these studies, obeticholic acid had a beneficial effect on the production of antimicrobial peptides by ileum epithelial cells, the expression of the tight junction proteins, intestinal inflammation, and liver fibrosis.
At present, the therapeutic potential of obeticholic acid in LC has been primarily studied in experimental models, and for safety reasons its use in patients with decompensated LC is still considered premature.
The newly developed carbon-based enterosorbent Carbalive™ (Yaq-001, Yaqrit Limited, United Kingdom) has a high absorption capacity for bacterial toxins and may be a new strategy to counteract changes in gut microbiota and translocation of bacterial products in patients with decompensated LC. It is non-absorbable carbon nanoparticles with a tailored bimodal distribution of porous domains within the macroporous range (> 50 nm) and microporous range (< 2 nm) and a vast surface area.
The biological significance of this is that in addition to binding smaller mediators such as indoles, acetaldehyde, etc carbon granules exhibit rapid adsorption kinetics for larger molecular weight factors, for example bacterial endotoxin, exotoxins, and cytokines. Yaq-001 was found to reduce liver injury, portal pressure, and lipopolysaccharide-induced reactive oxygen species production in an in vivo model of LC and ACLF[73]. Yaq-001 significantly increased the relative abundance of symbiont bacteria belonging to the Firmicutes phylum and decreased the relative abundance of pathobiont bacteria belonging to the Bacteroidetes phylum in fecal samples from rodents with a model of bile duct ligation-induced LC, despite the absence of a direct effect on bacterial growth kinetics[74]. In the first phase II multicenter, randomized, double-blind, placebo-controlled trial (CARBALIVE:634579), 14 patients with decompensated LC with diuretic-responsive ascites and mean MELD scores of 12.4 ± 0.9 received 4 g of Yaq-001 for 12 wk. Yaq-001 was safe, well tolerated, contributed to the restoration of intestinal eubiosis, and by affecting the permeability of the intestinal epithelial barrier weakened the severity of endotoxemia and systemic inflammation[75].
Given that particular alterations in the composition and function of gut microbiota play a crucial role in the pathogenesis of acute decompensation in LC, the study of the therapeutic possibilities of its modulation has emerged as one of the top concerns in modern hepatology. Despite the encouraging preliminary data, the majority of the suggested strategies have only been tested in animal models or in preliminary clinical trials; additional multicenter RCTs must demonstrate their efficacy in larger patient populations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Silva LD, Brazil; Wen XL, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhao S
| 1. | Jalan R, D'Amico G, Trebicka J, Moreau R, Angeli P, Arroyo V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J Hepatol. 2021;75 Suppl 1:S14-S26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | D'Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol. 2022;76:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 3. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1720] [Cited by in F6Publishing: 1860] [Article Influence: 169.1] [Reference Citation Analysis (3)] |
| 4. | Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. 2021;75 Suppl 1:S67-S81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Bajaj JS, O'Leary JG, Lai JC, Wong F, Long MD, Wong RJ, Kamath PS. Acute-on-Chronic Liver Failure Clinical Guidelines. Am J Gastroenterol. 2022;117:225-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 6. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Janicko M, Steib C, Reiberger T, Acevedo J, Gatti P, Bernal W, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Bendtsen F, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solè C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Özdogan OC, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Curto A, Pitarch C, Putignano A, Moreno E, Shawcross D, Aguilar F, Clària J, Ponzo P, Jansen C, Vitalis Z, Zaccherini G, Balogh B, Vargas V, Montagnese S, Alessandria C, Bernardi M, Ginès P, Jalan R, Moreau R, Angeli P, Arroyo V; PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 241] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 7. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 8. | Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2018;15:753-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Queck A, Weiler N, Trebicka J. Transplantation in Acute-on-Chronic Liver Failure: Feasibility and Futility. Clin Liver Dis (Hoboken). 2022;19:191-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Caraceni P, Abraldes JG, Ginès P, Newsome PN, Sarin SK. The search for disease-modifying agents in decompensated cirrhosis: From drug repurposing to drug discovery. J Hepatol. 2021;75 Suppl 1:S118-S134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Van der Merwe S, Chokshi S, Bernsmeier C, Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S82-S100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, Fernández J, Gustot T, Caraceni P, Bernardi M; investigators from the EASL-CLIF Consortium, Grifols Chair and European Foundation for the Study of Chronic Liver Failure (EF-Clif). The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 13. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Gut-liver axis in cirrhosis: Are hemodynamic changes a missing link? World J Clin Cases. 2021;9:9320-9332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (3)] |
| 14. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Kudryavtseva A, Krasnov G. Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis. World J Gastroenterol. 2022;28:1067-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 15. | Lunia MK, Sharma BC, Sachdeva S. Small intestinal bacterial overgrowth and delayed orocecal transit time in patients with cirrhosis and low-grade hepatic encephalopathy. Hepatol Int. 2013;7:268-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53:27-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (2)] |
| 17. | Grace E, Shaw C, Whelan K, Andreyev HJ. Review article: small intestinal bacterial overgrowth--prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38:674-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Acharya C, Bajaj JS. Gut Microbiota and Complications of Liver Disease. Gastroenterol Clin North Am. 2017;46:155-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 715] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 20. | Di Lorenzo F, De Castro C, Silipo A, Molinaro A. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol Rev. 2019;43:257-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Shu W, Shanjian C, Jinpiao L, Qishui O. Gut microbiota dysbiosis in patients with hepatitis B virus-related cirrhosis. Ann Hepatol. 2022;27:100676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Solé C, Guilly S, Da Silva K, Llopis M, Le-Chatelier E, Huelin P, Carol M, Moreira R, Fabrellas N, De Prada G, Napoleone L, Graupera I, Pose E, Juanola A, Borruel N, Berland M, Toapanta D, Casellas F, Guarner F, Doré J, Solà E, Ehrlich SD, Ginès P. Alterations in Gut Microbiome in Cirrhosis as Assessed by Quantitative Metagenomics: Relationship With Acute-on-Chronic Liver Failure and Prognosis. Gastroenterology. 2021;160:206-218.e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 23. | Philips CA, Ahamed R, Abduljaleel JKP, Rajesh S, Augustine P. Identification and Analysis of Gut Microbiota and Functional Metabolism in Decompensated Cirrhosis with Infection. J Clin Transl Hepatol. 2023;11:15-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 24. | Bajaj JS, Vargas HE, Reddy KR, Lai JC, O'Leary JG, Tandon P, Wong F, Mitrani R, White MB, Kelly M, Fagan A, Patil R, Sait S, Sikaroodi M, Thacker LR, Gillevet PM. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:756-765.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Maslennikov R, Ivashkin V, Efremova I, Alieva A, Kashuh E, Tsvetaeva E, Poluektova E, Shirokova E, Ivashkin K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J Hepatol. 2021;13:557-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, de Gottardi A, Moghadamrad S, Hassan M, Albillos A, Francés R, Juanola O, Spadoni I, Rescigno M, Wiest R. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71:1126-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 27. | Farooqui N, Elhence A, Shalimar. A Current Understanding of Bile Acids in Chronic Liver Disease. J Clin Exp Hepatol. 2022;12:155-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Shao JW, Ge TT, Chen SZ, Wang G, Yang Q, Huang CH, Xu LC, Chen Z. Role of bile acids in liver diseases mediated by the gut microbiome. World J Gastroenterol. 2021;27:3010-3021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V, Gassull MA. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 30. | Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 31. | Garbuzenko DV. [The role of intestinal microflora in the development of complications of hepatic cirrhosis-associated portal hypertension]. Klin Med (Mosk). 2007;85:15-19. [PubMed] [Cited in This Article: ] |
| 32. | Alexopoulou A, Agiasotelli D, Vasilieva LE, Dourakis SP. Bacterial translocation markers in liver cirrhosis. Ann Gastroenterol. 2017;30:486-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Philips CA, Augustine P. Gut Barrier and Microbiota in Cirrhosis. J Clin Exp Hepatol. 2022;12:625-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018;12:641-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 36. | Piñero P, Juanola O, Caparrós E, Zapater P, Giménez P, González-Navajas JM, Such J, Francés R. Toll-like receptor polymorphisms compromise the inflammatory response against bacterial antigen translocation in cirrhosis. Sci Rep. 2017;7:46425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1426] [Cited by in F6Publishing: 2327] [Article Influence: 581.8] [Reference Citation Analysis (0)] |
| 38. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 726] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 39. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1177] [Cited by in F6Publishing: 1448] [Article Influence: 241.3] [Reference Citation Analysis (1)] |
| 40. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 812] [Article Influence: 203.0] [Reference Citation Analysis (0)] |
| 41. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: Varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol. 2020;26:83-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 42. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 43. | Moreau R, Elkrief L, Bureau C, Perarnau JM, Thévenot T, Saliba F, Louvet A, Nahon P, Lannes A, Anty R, Hillaire S, Pasquet B, Ozenne V, Rudler M, Ollivier-Hourmand I, Robic MA, d'Alteroche L, Di Martino V, Ripault MP, Pauwels A, Grangé JD, Carbonell N, Bronowicki JP, Payancé A, Rautou PE, Valla D, Gault N, Lebrec D; NORFLOCIR Trial Investigators. Effects of Long-term Norfloxacin Therapy in Patients With Advanced Cirrhosis. Gastroenterology. 2018;155:1816-1827.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 44. | de Lastours V, Fantin B. Impact of fluoroquinolones on human microbiota. Focus on the emergence of antibiotic resistance. Future Microbiol. 2015;10:1241-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, Krag A, Al-Soud WA, Mortensen MS, Sørensen SJ, Møller S, Bendtsen F; members of the CoRif study group. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: A randomized trial. J Gastroenterol Hepatol. 2018;33:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics (Basel). 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Kulkarni AV, Tirumalle S, Premkumar M, Kumar K, Fatima S, Rapole B, Simhadri V, Gora BA, Sasikala M, Gujjarlapudi D, Yelamanchili S, Sharma M, Gupta R, Rao PN, Reddy DN. Primary Norfloxacin Prophylaxis for APASL-Defined Acute-on-Chronic Liver Failure: A Placebo-Controlled Double-Blind Randomized Trial. Am J Gastroenterol. 2022;117:607-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Soni H, Kumar-M P, Sharma V, Bellam BL, Mishra S, Mahendru D, Mandavdhare HS, Medhi B, Dutta U, Singh V. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: systematic review & Bayesian network meta-analysis. Hepatol Int. 2020;14:399-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Probiotics in hepatology: An update. World J Hepatol. 2021;13:1154-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 50. | Rashid SK, Idris-Khodja N, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, Schini-Kerth VB. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS One. 2014;9:e97458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Sánchez E, Nieto JC, Boullosa A, Vidal S, Sancho FJ, Rossi G, Sancho-Bru P, Oms R, Mirelis B, Juárez C, Guarner C, Soriano G. VSL#3 probiotic treatment decreases bacterial translocation in rats with carbon tetrachloride-induced cirrhosis. Liver Int. 2015;35:735-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Moratalla A, Gómez-Hurtado I, Santacruz A, Moya Á, Peiró G, Zapater P, González-Navajas JM, Giménez P, Such J, Sanz Y, Francés R. Protective effect of Bifidobacterium pseudocatenulatum CECT7765 against induced bacterial antigen translocation in experimental cirrhosis. Liver Int. 2014;34:850-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Moratalla A, Caparrós E, Juanola O, Portune K, Puig-Kröger A, Estrada-Capetillo L, Bellot P, Gómez-Hurtado I, Piñero P, Zapater P, González-Navajas JM, Such J, Sanz Y, Francés R. Bifidobacterium pseudocatenulatum CECT7765 induces an M2 anti-inflammatory transition in macrophages from patients with cirrhosis. J Hepatol. 2016;64:135-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596-3604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Koga H, Tamiya Y, Mitsuyama K, Ishibashi M, Matsumoto S, Imaoka A, Hara T, Nakano M, Ooeda K, Umezaki Y, Sata M. Probiotics promote rapid-turnover protein production by restoring gut flora in patients with alcoholic liver cirrhosis. Hepatol Int. 2013;7:767-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Macnaughtan J, Figorilli F, García-López E, Lu H, Jones H, Sawhney R, Suzuki K, Fairclough S, Marsden J, Moratella A, Cox IJ, Thomas L, Davies N, Williams R, Mookerjee R, Wright G, Jalan R. A Double-Blind, Randomized Placebo-Controlled Trial of Probiotic Lactobacillus casei Shirota in Stable Cirrhotic Patients. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 58. | Horvath A, Leber B, Schmerboeck B, Tawdrous M, Zettel G, Hartl A, Madl T, Stryeck S, Fuchs D, Lemesch S, Douschan P, Krones E, Spindelboeck W, Durchschein F, Rainer F, Zollner G, Stauber RE, Fickert P, Stiegler P, Stadlbauer V. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther. 2016;44:926-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 59. | Horvath A, Durdevic M, Leber B, di Vora K, Rainer F, Krones E, Douschan P, Spindelboeck W, Durchschein F, Zollner G, Stauber RE, Fickert P, Stiegler P, Stadlbauer V. Changes in the Intestinal Microbiome during a Multispecies Probiotic Intervention in Compensated Cirrhosis. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 2020;72:1003-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 61. | Segal JP, Mullish BH, Quraishi MN, Iqbal T, Marchesi JR, Sokol H. Mechanisms underpinning the efficacy of faecal microbiota transplantation in treating gastrointestinal disease. Therap Adv Gastroenterol. 2020;13:1756284820946904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Hassouneh R, Bajaj JS. Gut Microbiota Modulation and Fecal Transplantation: An Overview on Innovative Strategies for Hepatic Encephalopathy Treatment. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 376] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 64. | Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes of Fecal Microbiota Transplantation in Patients With Cirrhosis. Gastroenterology. 2019;156:1921-1923.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 65. | Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 66. | Gupta S, Mullish BH, Allegretti JR. Fecal Microbiota Transplantation: The Evolving Risk Landscape. Am J Gastroenterol. 2021;116:647-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 67. | US Food and Drug Administration. Information pertaining to additional safety protections regarding use of fecal microbiota for transplantation–screening and testing of stool donors for multi-drug resistant organisms [internet] (2019). [Accessed 30 June 2020]. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-pertaining-additional-safety-protections-regarding-use-fecal-microbiota-transplantation. [Cited in This Article: ] |
| 68. | Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W, Zhang L, Lin R, Liu J, Ding Z, Hou X. Digestive Symptoms in COVID-19 Patients With Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol. 2020;115:916-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 363] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 69. | Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, Iqbal TH, Allegretti JR, Bibbò S, Sokol H, Zhang F, Fischer M, Costello SP, Keller JJ, Masucci L, van Prehn J, Quaranta G, Quraishi MN, Segal J, Kao D, Satokari R, Sanguinetti M, Tilg H, Gasbarrini A, Cammarota G. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020;69:1555-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 70. | Yan K, Hung A, Parmer C, Yang H, Jain D, Lim B, Goodman AL, Garcia-Tsao G. Obeticholic Acid Decreases Intestinal Content of Enterococcus in Rats With Cirrhosis and Ascites. Hepatol Commun. 2021;5:1507-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Úbeda M, Lario M, Muñoz L, Borrero MJ, Rodríguez-Serrano M, Sánchez-Díaz AM, Del Campo R, Lledó L, Pastor Ó, García-Bermejo L, Díaz D, Álvarez-Mon M, Albillos A. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol. 2016;64:1049-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Verbeke L, Mannaerts I, Schierwagen R, Govaere O, Klein S, Vander Elst I, Windmolders P, Farre R, Wenes M, Mazzone M, Nevens F, van Grunsven LA, Trebicka J, Laleman W. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 73. | Tripisciano C, Kozynchenko OP, Linsberger I, Phillips GJ, Howell CA, Sandeman SR, Tennison SR, Mikhalovsky SV, Weber V, Falkenhagen D. Activation-dependent adsorption of cytokines and toxins related to liver failure to carbon beads. Biomacromolecules. 2011;12:3733-3740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Macnaughtan J, Ranchal I, Soeda J, Sawhney R, Oben J, Davies N, Mookerjee R, Marchesi J, Cox J, Jalan R. Oral therapy with non-absorbable carbons of controlled porosity (YAQ-001) selectively modulates stool microbiome and its function and this is associated with restoration of immune function and inflammasome activation [abstract]. J Hepatol. 2015;62:S240. [DOI] [Cited in This Article: ] |