Published online Mar 26, 2024. doi: 10.4252/wjsc.v16.i3.257
Peer-review started: October 13, 2023
First decision: December 11, 2023
Revised: December 25, 2023
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 26, 2024
Stem cell transplantation is a promising therapeutic option for curing perianal fistula in Crohn’s disease (CD). Anti-tumor necrotic factor (TNF) therapy combined with drainage procedure is effective as well. However, previous studies are limited to proving whether the combination treatment of biologics and stem cell transplantation improves the effect of fistula closure.
This study aimed to evaluate the long-term outcomes of stem cell transplantation and compare Crohn’s perianal fistula (CPF) closure rates after stem cell transplantation with and without anti-TNF therapy, and to identify the factors affecting CPF closure and recurrence.
The patients with CD who underwent stem cell transplantation for treating perianal fistula in our institution between Jun 2014 and December 2022 were enrolled. Clinical data were compared according to anti-TNF therapy and CPF closure.
A total of 65 patients were included. The median age of females was 26 years (range: 21-31) and that of males was 29 (44.6%). The mean follow-up duration was 65.88 ± 32.65 months, and complete closure was observed in 50 (76.9%) patients. The closure rates were similar after stem cell transplantation with and without anti-TNF therapy (66.7% vs 81.6% at 3 year, P = 0.098). The patients with fistula closure had short fistulous tract and infrequent proctitis and anorectal stricture (P = 0.027, 0.002, and 0.008, respectively). Clinical factors such as complexity, number of fistulas, presence of concurrent abscess, and medication were not significant for closure. The cumulative 1-, 2-, and 3-year closure rates were 66.2%, 73.8%, and 75.4%, respectively.
Anti-TNF therapy does not increase CPF closure rates in patients with stem cell transplantation. However, both refractory and non-refractory CPF have similar closure rates after additional anti-TNF therapy. Fistulous tract length, proctitis, and anal stricture are risk factors for non-closure in patients with CPF after stem cell transplantation.
Core Tip: This study examined the closure rates of Crohn’s perianal fistula (CPF) in patients undergoing stem cell transplantation for treatment. The complete closure was observed in 76.9% of cases, with similar closure rates after stem cell transplantation with and without anti-tumor necrotic factor (TNF) therapy. Factors associated with higher closure rates included shorter fistulous tracts and the absence of proctitis and anorectal stricture. Clinical factors such as complexity, number of fistulas, concurrent abscess presence, and medication did not significantly affect closure. The cumulative 1-, 2-, and 3-year closure rates were 66.2%, 73.8%, and 75.4%, respectively, suggesting that anti-TNF therapy did not increase CPF closure rates.
- Citation: Park MY, Yoon YS, Park JH, Lee JL, Yu CS. Long-term outcome of stem cell transplantation with and without anti-tumor necrotic factor therapy in perianal fistula with Crohn’s disease. World J Stem Cells 2024; 16(3): 257-266
- URL: https://www.wjgnet.com/1948-0210/full/v16/i3/257.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i3.257
A perianal fistula is an abnormal connection between the rectal or anal canal and external perianal or ischioanal skin[1] and one of the most common complications in Crohn’s disease (CD), with a 14%-38% lifetime risk[2]. The incidence of perianal complications in East Asia is higher than that in the West, ranging from 30.3% to 58.8%[3]. Currently, the number of patients with CD in Asia is increasing, and perianal fistula has been detected in a high proportion of patients in Korea. Based on the recently reported epidemiological study of patients with CD in the Songpa-Kangdong district of Seoul, Korea, perianal fistula/abscess was present in 43.3% of cases before or at CD diagnosis[4].
Antibiotics are commonly used as a first-line therapy for fistula treatment, but not effective in treating Crohn’s perianal fistula (CPF)[5]. A meta-analysis of five studies found a response in 54% patients treated with azathioprine or 6-mercaptopurine, but this meta-analysis did not review well-designed prospective clinical studies and the response was assessed based on different criteria with complete closure or reduced discharge as secondary endpoints[6]. Currently, biological agents such as anti-tumor necrosis factor (TNF)-α are increasingly being used to treat CPF[7,8]. In the ACCENT II trial, the fistula closure rate at weeks 14 and 54 were 63% and 36%, respectively[8]. Overall, the currently available treatments for CPF are not satisfactory as they do not achieve complete closure and recurrence reduction.
Owing to the problems of CPF and unmet medical needs, studies have focused on stem cell transplantation. Autologous or allogenic adipose tissue-derived stem cells (ASCs) have been studied for CPF treatment and could be considered effective and safe therapeutic tools[9,10]. Several studies in South Korea have showed the favorable results of using autologous ASCs[11,12]. However, few studies have focused on the long-term outcomes of stem cell trans
Infliximab is a well-established agent for treating CD and CPF[13]. Moreover, autologous ASC transplantation is safe and effective for treating CPF[11,14]. Furthermore, according to the previous systematic review article, the combination of medication and surgery was more effective than single therapy, with 52% (37%-79%) and 43% (24%-67%) complete healing rates, respectively[15]. The study reported a closure rate of 18%-80% in the surgical treatment alone group and 45%-80% in the surgical and medical combined treatment group[16]. Another study reported a closure rate of 20%-65% in the surgical treatment alone group and 18%-85% in the surgical and medical combined treatment group[17]. However, there is a limitation in proving the effectiveness of the combined therapy of anti-TNF agents and stem cell transplantation because stem cell transplantation was not included in the studies as a treatment option.
This study aimed to evaluate the long-term outcomes of stem cell transplantation and compare the closure rates associated with stem cell transplantation with and without anti-TNF therapy. Additionally, the risk factors for therapeutic failure and CPF recurrence after stem cell transplantation were identified.
Data of patients with CD who underwent stem cell transplantation for perianal fistula at the Asan Medical Center, Seoul, Korea from June 2014 to December 2022 were reviewed retrospectively. CD was diagnosed by gastroenterologists based on clinical, endoscopic, radiological, and histopathologic criteria according to the diagnostic guidelines for CD in Korea[16]. Patients with insufficient medical records and those lost to follow-up were excluded. Patient characteristics including age, sex, smoking status, and subclass of the Montreal classification[17] were compared. Fistula evaluation included fistula type (simple vs complex, single vs multiple), perioperative CD medication (immunomodulators or steroids) including biologics, the presence of proctitis or stricture, and the presence of perianal abscess. Autologous adipose tissue-derived mesenchymal stem cells (Cupistem®, Anterogen, South Korea) were used in this study. Cupistem® was approved by the Korea Ministry of Food and Drug Safety in 2012 (advanced therapy medicinal product). Cupistem® manufacturing process was validated and standardized during product development and critical process parameters have been established to ensure product quality. Prior to release, Cupistem® has been tested for cell appearance, cell contents, cell viability, cell surface marker, and impurity in addition to adventitious agents including mycoplasma and bacteria, fungi, and endotoxin[20]. Moreover, anti-TNF agents used in this study were infliximab (Remicade®, Janssen Biotech, Inc., Horsham, PA, United States) and adalimumab (Humira®, AbbVie, Inc., North Chicago, IL, United States). The study protocol was approved by the Institutional Review Board of Asan Medical Center (No. 2020-1059).
Fistulas were classified according to Park’s classification criteria[18]. Intersphincteric fistulas are formed when the tract penetrates the internal sphincter and courses through the intersphincteric space to the perianal skin. Transsphincteric fistulas penetrate both the internal and external sphincter. Suprasphincteric fistulas cross the internal sphincter, spread upward in the intersphincteric space, and cross the levator ani muscle downward before reaching the perianal skin. Extra-sphincteric fistulas originate from the rectal wall and course downward through the levator ani muscle lateral to the external sphincter to reach the perianal skin without penetrating the anal sphincter complex.
Autologous ASCs were isolated from the subcutaneous fat tissues of patients using lipo-aspirates. The lipo-aspirates were washed with phosphate buffered saline (PBS) and digested in an equal volume of PBS containing 1% bovine serum albumin and 0.025% collagenase type I for 80 min at 37 °C with intermittent shaking. The stromal vascular fraction isolated from the fat tissue was cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and 1 ng/mL human basic fibroblast growth factor to obtain the required number of ASCs for injection. Residual animal products were removed through a washing process of ASCs. During the development of manufacturing method of Cupistem®, washing validation and risk assessment for residuals were conducted to ensure product quality and safety. The risk of residual substances such as fetal bovine serum was evaluated based on “Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals (1993)” and 21CFR610.15. The cells were harvested via trypsinization, suspended in DMEM, and packaged into single-use vials containing 3 × 107 cells/mL. All manufacturing procedures were carried out according to the Good Manufacturing Practices authorized by the Korean Food and Drug Administration. Stem cells were sufficiently generated from autologous fat cells evacuated from a patient’s buttocks in 4 to 5 wk[19].
Stem cell transplantation: Before stem cell transplantation, all candidates with CPF underwent seton placement to control inflammation around the fistula. Stem cell transplantation was performed in patients without active inflammation after seton drainage. The tract was curetted to remove the inflamed and fibrotic surrounding tissue, which prevents the penetration of transplanted stem cells. The tract was cleaned with isotonic saline and the previous seton was removed. The internal opening of the tract on the rectal or anal canal was closed using direct Vicryl suture ligation. Then, 3 × 107 autologous adipose tissue-derived mesenchymal stem cells/mL (Cupistem®, Anterogen, South Korea) were injected into the submucosa around the internal opening and fistula tract. The opened fistula tract was also filled with fibrin glue-mixed stem cells. The ASC dose was determined based on fistula length and diameter, which were measured using a probe before injection. When the fistula diameter was < 1 cm, approximately 3 × 107 cells were injected per cm. When the fistula diameter was between 1 and 2 cm, approximately 6 × 107 cells were injected per cm. All patients included in this study received only one stem cell transplantation.
Anti-TNF agents: The initiation of anti-TNF treatment was determined by a gastroenterologist according to the patients’ clinical factors such as the extent of intestinal inflammation and the patient’s response to previous therapeutic drugs. The dosage for infliximab was 5 mg/kg per infusion. Infliximab was administered 2 and 6 wk after the first dose, and every 8 wk after the third dose. Adalimumab was administered every 2 wk after the first dose. Adalimumab was administered at 100 mg for the first dose, 80 mg at the second week, and subsequently 40 mg every 2 wk. The adjustment of the dosage was also determined by a gastroenterologist. The medication was administered according to the protocol unless there were absolute contraindications such as the risk of infection.
Postoperative management: The patients were admitted on the day of operation and discharged the next day. Follow-up was done at an outpatient clinic every 1 or 2 mo. Fistula closure was confirmed through physical examination at the outpatient clinic, where the absence of inflammation or discharge was observed.
Patients treated with anti-TNF agents at least once from 3 months before surgery to 3 months after surgery were defined as the “anti-TNF group” regardless of the interval between the initiation of anti-TNF treatment and stem cell transplantation. And the other patient treated without anti-TNF agents from 3 months before surgery to 3 months after surgery were defined as the “without anti-TNF group”.
Primary outcomes of the present study were evaluating the closure rate of patients who underwent stem cell trans
Categorical and continuous variables were compared by χ2 and Student’s t-tests, respectively. Continuous variables are expressed as the median with inter-quadrant range or the mean with standard deviation, and discrete variables are expressed as numbers with percentages. Statistical analyses were performed using SPSS for Windows, ver. 25.0 (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered statistically significant. The cumulative probability of the event was estimated using the life-table method for 3 years at 3-months intervals. The difference in cumulative probability (closure minus recurrence) was calculated for each time period.
Totally 65 patients underwent stem cell transplantation to treat CPF between June 2014 and December 2022. Among them, 26 were treated with preoperative anti-TNF agents. The mean follow-up duration of all patients was 66.09 ± 32.37 months. Complete closure of perianal fistula occurred in 50 (76.9%) patients, of whom 18 (36.0%) were treated with postoperative anti-TNF agents.
The mean age, sex ratio, fistula characteristics, mean CD duration, and mean number of previous operations for CPF were similar between patients treated with and without anti-TNF agents. However, more immunomodulators were used in the patients without anti-TNF treatment (40.7% vs 73.7%, P = 0.008) and the number of patients with ileocolic involvement (L3) was higher in the patients with anti-TNF treatment (88.9% vs 60.5%, P = 0.025) (Table 1).
| Anti-TNF (n = 27) | Without anti-TNF (n = 38) | P value | |
| Age, median (IQR) (yr) | 26 (23.00-32.00) | 26 (20.00-31.25) | 0.378 |
| Sex | 0.596 | ||
| Male | 11 (40.7) | 18 (47.4) | |
| Female | 16 (59.3) | 20 (52.6) | |
| Montreal classification | |||
| Age at onset | 0.772 | ||
| A1 (≤ 16 yr) | 5 (18.5) | 6 (15.8) | |
| A2 (17-40 yr) | 22 (81.5) | 32 (84.2) | |
| A3 (≥ 41 yr) | 0 (0.0) | 0 (0.0) | |
| Location | 0.025 | ||
| L1 (Ileum) | 3 (11.1) | 9 (23.7) | |
| L2 (Colon) | 0 (0.0) | 6 (15.8) | |
| L3 (Ileocolon) | 24 (88.9) | 23 (60.5) | |
| Behavior | 0.850 | ||
| B1 (non-stricturing, non-penetrating) | 17 (63.0) | 26 (68.4) | |
| B2 (stricturing) | 5 (18.5) | 8 (21.1) | |
| B3 (penetrating) | 5 (18.5) | 4 (10.5) | |
| Fistula type | 0.915 | ||
| Simple | 4 (14.8) | 6 (15.8) | |
| Complex | 23 (85.2) | 32 (84.2) | |
| Multiple fistula | 9 (33.3) | 13 (46.4) | 0.941 |
| Fistula length > 7 cm | 8 (29.6) | 5 (13.2) | 0.102 |
| CDAI, (mean ± SD) | 101.87 ± 55.69 | 80.79 ± 47.47 | 0.111 |
| Proctitis | 10 (37.0) | 12 (31.6) | 0.647 |
| Stricture | 3 (11.1) | 3 (7.9) | 0.659 |
| Abscess | 3 (11.1) | 2 (5.3) | 0.383 |
| Recto-vaginal fistula | 3 (11.1) | 2 (5.3) | 0.383 |
| Medical treatment | |||
| Immunomodulators | 11 (40.7) | 28 (73.7) | 0.008 |
| Smoking | 1 (3.7) | 1 (2.6) | 0.217 |
| Previous fistula OP | 27 (100.0) | 38 (100.0) | 1.000 |
| No. previous fistula OP, mean ± SD (times) | 3.30 ± 2.55 | 2.45 ± 3.29 | 0.266 |
| Disease duration, mean ± SD (yr) | 7.15 ± 4.74 | 5.87 ± 5.28 | 0.310 |
| Follow-up, mean ± SD (months) | 70.52 ± 31.74 | 62.58 ± 33.31 | 0.338 |
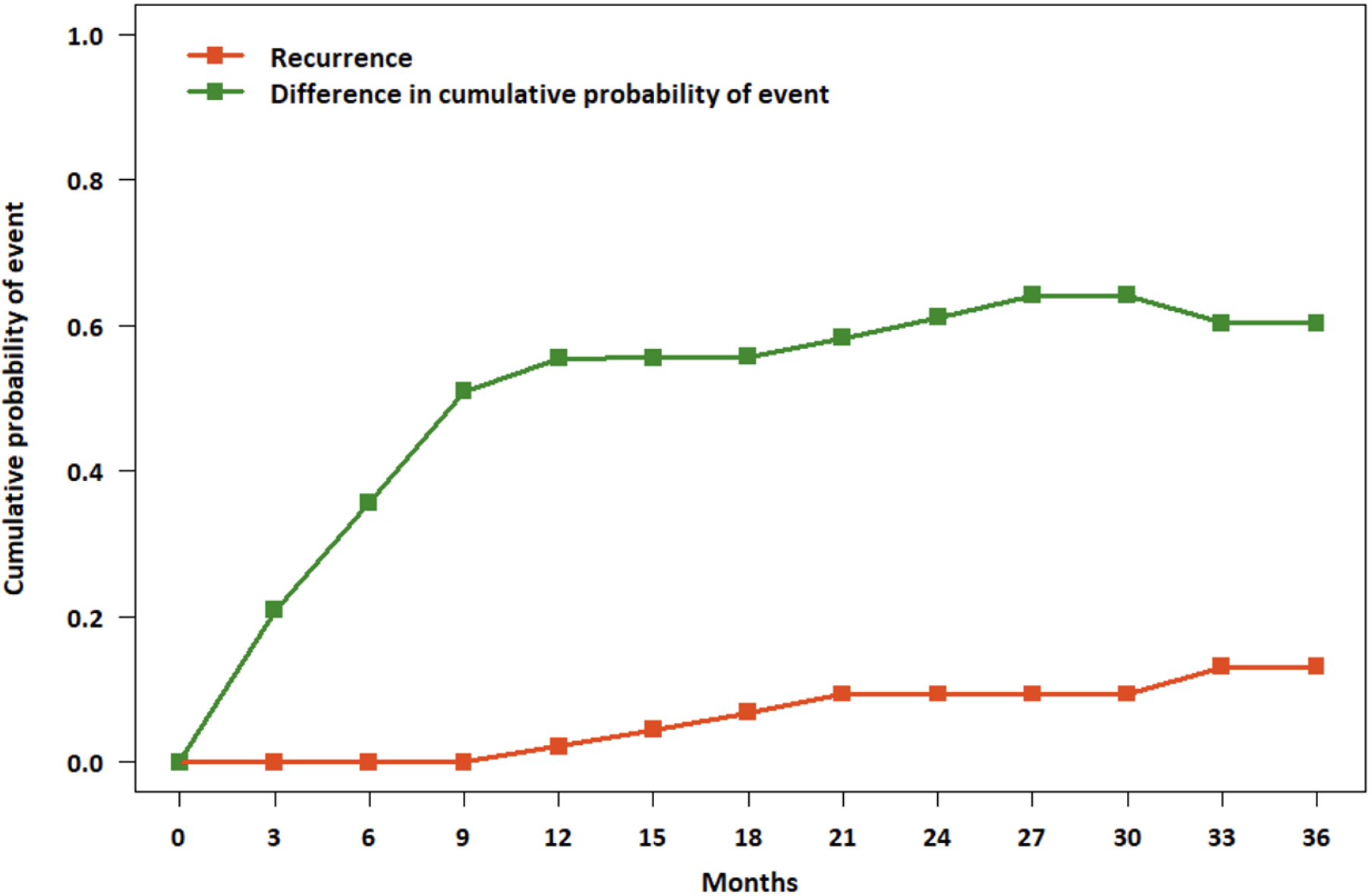
During the follow-up, closure rate after stem cell transplantation was 76.9%. The mean duration from stem cell transplantation to fistula closure was 6.94 ± 9.68 months. Moreover, the recurrence rate in patients experiencing fistula closure was 14.0%, with the mean period from fistula closure to recurrence being 16.57 ± 19.38 months. All recurrences were detected in 3 years. The patients who received anti-TNF treatment experienced fistula closure within 2 years. The closure rates at 1 year and 2 years for the patients who received anti-TNF treatment were 63.0% and 66.7%, respectively. The 1-, 2-, and 3-year closure rates for the patients without anti-TNF treatment were 68.4%, 78.9%, and 81.6%, respectively. Only one patient without anti-TNF treatment experienced fistula closure after 3 years. The closure and recurrence rates were not significantly different according to anti-TNF agent use (66.7% vs 84.2%, P = 0.098; Table 2, Figure 1). The final cumulative closure rate excluding those who had recurrence was about 66.1% (Figure 2).
| Total | Anti-TNF (n = 27) | Without anti-TNF (n = 38) | P value | |
| Cumulative closure rate | 50/65 (76.9%) | 18/27 (66.7%) | 32/38 (84.2%) | 0.098 |
| 1-yr | 43 (66.2) | 17 (63.0) | 26 (68.4) | |
| 2-yr | 48 (73.8) | 18 (66.7) | 30 (78.9) | |
| 3-yr | 49 (75.4) | - | 31 (81.6) | |
| Closure time (months) | 6.94 ± 9.68 | 4.56 ± 4.37 | 8.28 ± 11.52 | 0.195 |
| Cumulative recurrence rate | 7/50 (14.0) | 3/18 (16.7) | 4/32 (12.5) | 0.684 |
| 1-yr | 4 (8.0) | 2 (11.1) | 2 (6.3) | |
| 2-yr | 5 (10.0) | 3 (16.7) | 2 (6.3) | |
| 3-yr | 7 (14.0) | - | 4 (12.5) | |
| Recurrence time (months) | 16.57 ± 19.38 | 7.33 ± 7.77 | 23.50 ± 23.70 | 0.316 |
Mean age, sex ratio, fistula type or number, mean disease duration, or surgical history for CPF were similar between the patients with and without fistula closure. Fistulas longer than 7 cm (40.0% vs 14.0%, P = 0.027), proctitis (66.7% vs 30.0%, P = 0.002), and rectal stricture (26.7% vs 8.0%, P = 0.008) were significantly less in the patients with fistula closure (Table 3). The Crohn’s Disease Activity Index score was higher in the patients with anti-TNF treatment (101.87 ± 55.69 vs 80.79 ± 47.47, P = 0.111) but not significant, and did not affect closure.
| No closure (n = 15) | Closure (n = 50) | P value | |
| Age, median (IQR) (yr) | 28.0 (23.0-33.0) | 26.0 (20.8-31.0) | 0.433 |
| Sex | 0.316 | ||
| Male | 5 (33.3) | 24 (48.0) | |
| Female | 10 (66.7) | 26 (52.0) | |
| Montreal classification | |||
| Age at onset | 0.227 | ||
| A1 (≤ 16 yr) | 1 (6.7) | 10 (20.0) | |
| A2 (17-40 yr) | 14 (93.3) | 40 (80.0) | |
| A3 (≥ 41 yr) | 0 (0.0) | 0 (0.0) | |
| Location | 0.285 | ||
| L1 (Ileum) | 4 (26.7) | 8 (16.0) | |
| L2 (Colon) | 0 (0.0) | 6 (12.0) | |
| L3 (Ileocolon) | 11 (73.3) | 36 (72.0) | |
| Behavior | 0.998 | ||
| B1 (non-stricturing, non-penetrating) | 10 (66.7) | 33 (66.0) | |
| B2 (stricturing) | 3 (20.0) | 10 (20.0) | |
| B3 (penetrating) | 2 (13.3) | 7 (14.0) | |
| Fistula type | 0.572 | ||
| Simple | 3 (20.0) | 7 (14.0) | |
| Complex | 12 (80.0) | 43 (86.0) | |
| Multiple fistula | 8 (53.3) | 14 (28.0) | 0.069 |
| Fistula length > 7 cm | 6 (40.0) | 7 (14.0) | 0.027 |
| CDAI, mean ± SD | 98.84 ± 54.62 | 87.01 ± 51.16 | 0.444 |
| Proctitis | 10 (66.7) | 15 (30.0) | 0.002 |
| Stricture | 4 (26.7) | 2 (8.0) | 0.008 |
| Abscess | 2 (13.3) | 3 (6.0) | 0.350 |
| Recto-vaginal fistula | 1 (6.7) | 4 (8.0) | 0.865 |
| Medical treatment | |||
| Immunomodulators | 8 (53.3) | 31 (62.0) | 0.548 |
| Biologics | 9 (60.0) | 18 (36.0) | 0.098 |
| Smoking | 0 (0.0) | 2 (4.0) | 0.733 |
| Previous fistula OP | 15 (100.0) | 33 (100.0) | 1.000 |
| No. previous fistula OP, mean ± SD (times) | 4.27 ± 5.06 | 2.36 ± 1.91 | 0.173 |
| Disease duration, mean ± SD (yr) | 5.53 ± 4.24 | 6.66 ± 5.29 | 0.454 |
| Follow-up, mean ± SD (months) | 63.87 ± 40.12 | 66.48 ± 30.52 | 0.788 |
Recently, various pharmacological and surgical treatments for CD fistula have been developed and studied[20,21]. Moreover, various studies have proven that stem cell transplantation is effective[10,22]. Biological agents such as anti-TNF-α are increasingly being used as pharmacological treatments to treat CD fistula[7]. Despite extensive research, therapy for CD-derived fistula was inadequate. This study analyzed whether combining these treatment modalities increases the effectiveness and showed that stem cell transplantation in combination with anti-TNF treatment did not increase the fistula closure rate.
The safety and efficacy of autologous MSCs have been studied through multiple phases I, II, and III clinical trials. A phase I trial on five patients with CD fistula showed complete closure of the external opening and an absence of abscess in 75% of patients[22]. A phase II study on complex perianal fistulas reported fistula healing and closure in 71% and 56% of patients receiving autologous ASC treatment, respectively[10]. A phase III trial evaluating ASC efficacy in complex fistulas reported approximately 40% and 50% fistula healing rates at 6 months and 1 year, respectively[23]. Moreover, previous studies using the same ASCs used in this study have shown that the 1- and 2-year closure rates were 88% and 75%, respectively[11,14].
The overall closure rate of patients with stem cell transplantation was 76.9%. Two patients treated with anti-TNF agent only after stem cell transplantation experienced fistula closure. Among them, one patient was treated with anti-TNF due to bowel inflammation 1 months after stem cell transplantation, and the other was treated with anti-TNF for unclosed fistula until 1 year after stem cell transplantation. During the follow-up period of approximately 5 years, 14.0% of patients with fistula closure experienced recurrence. Previous studies have reported the recurrence rate at 1 and 2 years after stem cell transplantation as 11% and 16%, respectively[11,14], which is consistent with the recurrence rate of this study. The closure rate was slightly lower in patients treated with anti-TNF agents, although it was not statistically significant. This result is possibly because ASCs initiate or enhance tissue regeneration by two different mechanisms: Differentiating into skin cells or secreting paracrine factors that initiate the healing process through recruiting endogenous stem cells and endothelial cells or downregulating the inflammatory response. Thus, the anti-inflammatory response of stem cell transplantation, which is a local treatment for CPF, is strong among patients, and prolongs and minimizes the effect of systemic treatment with anti-TNF[24]. Additionally, more refractory CPF was included in the anti-TNF treatment group as the patients treated with anti-TNF agents had a prolonged disease duration and previous surgeries for CPF might affect the result. Furthermore, our study showed that proctitis, stricture, and long fistulous tract reduced the closure rate, and biologics did not affect the closure and recurrence. The actual cumulative fistula closure rate, excluding those with recurrence, was approximately 66.1%.
This study has several limitations. First, its retrospective design may have caused patient selection bias. To minimize the selection bias, we enrolled all patients who underwent stem cell transplantation during the study period. Second, because the surgical procedure, like stem cell transplantation, was determined subjectively by each operator depending on the severity of inflammation in the fistula, it may have affected the high closure rate of stem cell transplantation. Moreover, the actual healing rate after treatment was not evaluated through imaging studies such as magnetic resonance imaging (MRI) because only four patients underwent MRI for other reasons not for fistula, as MRI for diagnosing perianal fistula is not covered by insurance in Korea.
Concludingly, stem cell transplantation is one of the feasible treatment options for CPF. Anti-TNF agents do not increase the fistula closure rate after stem cell transplantation in CPF patients. Furthermore, selecting appropriate patients considering factors such as clinical features like proctitis or stricture, as well as the length of the fistulous tract may influence the prognosis of fistula closure according to this study results. Therefore, if stem cell transplantation is performed in appropriate patients, regardless of the use of anti-TNF agents, favorable outcomes can be expected. However, since most patients enrolled in this study were taking anti-TNF agents for the treatment of bowel inflammation rather than perianal fistula, further research should be considered to determine whether combining stem cell transplantation and anti-TNF solely for perianal fistula treatment is effective. This study was conducted at a single center, and due to the limited number of patients, we believe that further research is needed with a larger cohort from multi-centers to investigate the long-term outcomes and prognostic factors of stem cell transplantation in patients with CPF.
Perianal fistulas are a common complication in Crohn’s disease, with a higher incidence in East Asia. Stem cell transplantation, specifically using autologous adipose tissue-derived stem cells (ASCs), has been explored as a potential effective and safe therapeutic approach, with favorable results reported in South Korean studies.
While the combination of medication and surgery has shown promise, the effectiveness of combining biologics such as anti-tumor necrosis factor (TNF) agents with stem cell transplantation remains an unproven area in improving fistula closure outcomes.
The study focused on assessing the long-term outcomes of stem cell transplantation, comparing closure rates with and without anti-TNF therapy, and evaluating risk factors for therapeutic failure and recurrence in Crohn’s perianal fistula (CPF) after stem cell transplantation.
This retrospective study, conducted at Asan Medical Center in Seoul, Korea, aimed to evaluate the long-term outcomes of stem cell transplantation in patients with CPF. Data from patients who underwent stem cell transplantation from June 2014 to December 2022 were reviewed, considering clinical variables such as age, sex, smoking status, and Montreal classification subclass. Autologous ASCs were used, and the study also included information on surgical procedures, anti-TNF agents (infliximab and adalimumab) used, and postoperative management. The study focused on comparing closure rates between patients who underwent stem cell transplantation with and without biologics, defining closure as the absence of discharge, swelling, or pain, and recurrence as the relapse of symptoms after the closure of the fistulous tract. Statistical analyses were performed to assess differences in outcomes.
Between June 2014 and December 2022, a total of 65 patients underwent stem cell transplantation for the treatment of CPF, with 26 receiving preoperative anti-TNF agents. The mean follow-up duration was 66.09 ± 32.37 months. Among all patients, 76.9% achieved complete closure of perianal fistula, and the recurrence rate after closure was 14.0%. The closure rates at 1 and 2 years for patients with anti-TNF treatment were 63.0% and 66.7%, respectively, while for those without anti-TNF treatment, the rates were 68.4%, 78.9%, and 81.6% at 1, 2, and 3 years, respectively. Fistula closure was less likely in patients with longer fistulas (> 7 cm), proctitis, and rectal stricture. The cumulative closure rate, excluding those with recurrence, was approximately 66.1%. The use of anti-TNF agents did not significantly impact closure and recurrence rates.
In conclusion, stem cell transplantation emerges as a viable treatment option for CPF, with favorable outcomes expected in appropriate patients regardless of the use of anti-TNF agents. This study suggests that selecting patients based on clinical features such as proctitis or stricture, along with considering the length of the fistulous tract, plays an important role in influencing the prognosis of fistula closure.
The study, conducted at a single center with a limited number of patients, underscores the need for further research involving a larger multi-center cohort to thoroughly investigate the long-term outcomes and prognostic factors associated with stem cell transplantation in patients with CPF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Zheng H, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang XD
| 1. | Steinhart AH, Panaccione R, Targownik L, Bressler B, Khanna R, Marshall JK, Afif W, Bernstein CN, Bitton A, Borgaonkar M, Chauhan U, Halloran B, Jones J, Kennedy E, Leontiadis GI, Loftus EV Jr, Meddings J, Moayyedi P, Murthy S, Plamondon S, Rosenfeld G, Schwartz D, Seow CH, Williams C. Clinical Practice Guideline for the Medical Management of Perianal Fistulizing Crohn's Disease: The Toronto Consensus. Inflamm Bowel Dis. 2019;25:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Williams DR, Coller JA, Corman ML, Nugent FW, Veidenheimer MC. Anal complications in Crohn's disease. Dis Colon Rectum. 1981;24:22-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 166] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Im JP. Adalimumab or infliximab: which is better for perianal fistula in Crohn's disease? Intest Res. 2017;15:147-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Park SH, Kim YJ, Rhee KH, Kim YH, Hong SN, Kim KH, Seo SI, Cha JM, Park SY, Jeong SK, Lee JH, Park H, Kim JS, Im JP, Yoon H, Kim SH, Jang J, Kim JH, Suh SO, Kim YK, Ye BD, Yang SK; Songpa-Kangdong Inflammatory Bowel Disease [SK-IBD] Study Group. A 30-year Trend Analysis in the Epidemiology of Inflammatory Bowel Disease in the Songpa-Kangdong District of Seoul, Korea in 1986-2015. J Crohns Colitis. 2019;13:1410-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Present DH. Crohn's fistula: current concepts in management. Gastroenterology. 2003;124:1629-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 702] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 7. | Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, Levesque BG, D'Haens G; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut. 2014;63:1381-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1581] [Cited by in F6Publishing: 1473] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 9. | García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, Montes JA, Pinto FL, Marcos DH, García-Sancho L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 598] [Cited by in F6Publishing: 541] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 11. | Cho YB, Park KJ, Yoon SN, Song KH, Kim DS, Jung SH, Kim M, Jeong HY, Yu CS. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn's fistula. Stem Cells Transl Med. 2015;4:532-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Park KJ, Ryoo SB, Kim JS, Kim TI, Baik SH, Kim HJ, Lee KY, Kim M, Kim WH. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn's disease: a pilot clinical trial. Colorectal Dis. 2016;18:468-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2328] [Cited by in F6Publishing: 2214] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 14. | Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, Jung SH, Kim M, Yoo HW, Kim I, Ha H, Yu CS. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells. 2013;31:2575-2581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 15. | Yassin NA, Askari A, Warusavitarne J, Faiz OD, Athanasiou T, Phillips RK, Hart AL. Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn's disease. Aliment Pharmacol Ther. 2014;40:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1396] [Cited by in F6Publishing: 1420] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 17. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV Jr, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2148] [Cited by in F6Publishing: 2195] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 18. | Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1072] [Cited by in F6Publishing: 867] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 19. | Park MY, Yoon YS, Lee JL, Park SH, Ye BD, Yang SK, Yu CS. Comparative perianal fistula closure rates following autologous adipose tissue-derived stem cell transplantation or treatment with anti-tumor necrosis factor agents after seton placement in patients with Crohn's disease: a retrospective observational study. Stem Cell Res Ther. 2021;12:401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Bouguen G, Siproudhis L, Gizard E, Wallenhorst T, Billioud V, Bretagne JF, Bigard MA, Peyrin-Biroulet L. Long-term outcome of perianal fistulizing Crohn's disease treated with infliximab. Clin Gastroenterol Hepatol. 2013;11:975-81.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Gaertner WB, Decanini A, Mellgren A, Lowry AC, Goldberg SM, Madoff RD, Spencer MP. Does infliximab infusion impact results of operative treatment for Crohn's perianal fistulas? Dis Colon Rectum. 2007;50:1754-1760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 556] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 23. | Garcia-Olmo D, Guadalajara H, Rubio-Perez I, Herreros MD, de-la-Quintana P, Garcia-Arranz M. Recurrent anal fistulae: limited surgery supported by stem cells. World J Gastroenterol. 2015;21:3330-3336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22:313-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |