Published online Dec 15, 2010. doi: 10.4251/wjgo.v2.i12.421
Revised: December 6, 2010
Accepted: December 13, 2010
Published online: December 15, 2010
Ever since its discovery two decades ago, the erythropoietin-producing hepatoma (EPH)-EPHRIN system has been shown to play multifaceted roles in human gastroenterological cancer as well as neurodevelopment. Over-expression, amplification and point mutations have been found in many human cancers and many investigators have shown correlations between these up-regulations and tumor angiogenesis. Thus, the genes in this family are considered to be potential targets of cancer therapy. On the other hand, the down-regulation of some members as a result of epigenetic changes has also been reported in some cancers. Furthermore, the correlation between altered expressions and clinical prognosis seems to be inconclusive. A huge amount of protein-protein interaction studies on the EPH-EPHRIN system have provided a basic scheme for signal transductions, especially bi-directional signaling involving EPH-ERPHRIN molecules at the cell membrane. This information also provides a manipulative strategy for harnessing the actions of these molecules. In this review, we summarize the known alterations of EPH-EPHRIN genes in human tumors of the esophagus, stomach, colorectum, liver and pancreas and present the perspective that the EPH-EPHRIN system could be a potential target of cancer therapy.
- Citation: Sugimura H, Wang JD, Mori H, Tsuboi M, Nagura K, Igarashi H, Tao H, Nakamura R, Natsume H, Kahyo T, Shinmura K, Konno H, Hamaya Y, Kanaoka S, Kataoka H, Zhou XJ. EPH-EPHRIN in human gastrointestinal cancers. World J Gastrointest Oncol 2010; 2(12): 421-428
- URL: https://www.wjgnet.com/1948-5204/full/v2/i12/421.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v2.i12.421
Erythropoietin-producing hepatoma (EPH) amplified sequence is an acronym for erythropoietin-producing hepatocellular carcinoma[1] from which the first member of the EPH family was isolated. The involvement of one gene in this family in human gastric cancer was reported in 1994[2] prior to the designation of this gene as EPHB2 according to a unified nomenclature system[3]. EPH and EPHRIN, receptor kinases EPH and their ligands EPHRIN (EFN), were classified according to the structures of the ligands, the EPHRINs. GPI-anchored-type ligands were called EPHRIN-As and transmembrane-type ligands were called EPHRIN-Bs. The corresponding receptors recognizing each ligand were called EPH-As and EPH-Bs. The relationships are mostly exclusive except for EPHA4-EPHRINB2 and EPHB4-EPHRINA2. Thus, we can say that the EPH-EPHRIN (or EPH-EFN) system has been recognized as a major player in human gastrointestinal carcinogenesis for more than 20 years[4]. In this paper, we review the accumulated data on alterations in EPH receptors in human gastrointestinal tract cancers by each category.
The mutations and a summary of the up-regulation and down-regulation of the EPH receptors are shown in Tables 1 and 2. Readers can access a database containing updates on alterations of genes of interest in specific organs[5] at the web site http://www.sanger.ac.uk/genetics/CGP/Studies/.
| Amino acid substitutions | Organs | ||
| EPHA2 | 777G>S | Stomach | |
| EPHA3 | 792S>P | 806D>N | Colon |
| EPHA6 | 649R>S | 813K>N | Stomach |
| EPHA7 | 768S>I | Colon | |
| EPHA8 | 179R>C | 873D>N | Colon, Stomach |
| EPHB1 | 719I>V | 743R>Q | Stomach |
| EPHB4 | 889R>W | 1030I>M | Colon,Stomach |
| Up-regulation | Down-regulation | ||||
| Overexpression | Amplification | Promoter methylation | Loss of heterozygosity | Others or unknown | |
| EPHA1 | Stomach | Colon | Colon | Colon | |
| EPHA2 | Stomach, colon, esophagus | Colon | (Melanoma) | ||
| EPHA3 | (Lung) | ||||
| EPHA4 | Colon | ||||
| EPHA5 | |||||
| EPHA6 | |||||
| EPHA7 | Stomach, colon | Stomach, colon | |||
| EPHA8 | Stomach, colon | ||||
| EPHB1 | |||||
| EPHB2 | Stomach, colon | Stomach | Colon | Colon | (Prostate) |
| EPHB3 | Colon | ||||
| EPHB4 | Colon | Colon | Colon | ||
| EPHB5 | |||||
| EPHB6 | (Neuroblastoma) | ||||
The original isolation paper described the over-expression and not the amplification of EPHA1 in human colorectal cancer[1]. However, the significance of EPHA1 in human cancers is far from being solved. Although EPHA1 was first suspected to be an oncogene (growth factor receptor-like epidermal growth factor receptor), many investigators have recently focused on its down-regulation in human tumors and its possible clinical significance[6,7]. On the other hand, from the standpoint of the pro-angiogenic activity of EPHAs, Chen et al[8] reported that the silencing of EPHA1 induces an anti-angiogenic effect in human hepatocellular carcinoma. Recently, down-regulation by epigenetic silencing was shown to be correlated with a poor survival outcome in patients with colorectal cancer[7,9]. Furthermore, Wang et al[10] extended their observation on colorectal cancer to gastric cancer; that is, they reported the correlation between EPHA1 expression and gastric cancer metastasis and survival. Contrary to the situation reported by Dong et al[6] in colorectal cancer, EPHA1 up-regulation was related to a poor survival outcome and the metastasis of gastric cancer.
EPHA1 expression is possibly regulated by environmental factors. Doleman et al[11] reported that EPHA1 expression and EPHB4 are influenced by n-3 fatty acid eicosapentaenoic acid (EPA). This observation may imply the important involvement of EPH pathways in the mechanism responsible for the presumed health benefits of polyunsaturated fatty acids (PUFA).
Most research published so far about the relationship between EPHA2 expression and human gastrointestinal cancers has indicated that EPHA2 up-regulation in tumor cells results in a more aggressive nature[12-14]. In addition, EPHA2 has been extensively investigated from the standpoint of cell and vascular biology. The ligand for this receptor is EPHRINA1 (EFNA1), isolated as an acute phase reactant induced by TNF in endothelial cells[15]. This observation has tempted many investigators to study the expressions of EFNA1 and its receptor EPHA2 in tumor cells and their relation with tumor angiogenesis. In human cancers, Kataoka[12] demonstrated an increased microvessel density in EPHA2 over-expressing colorectal cancers. The mechanisms by which the overexpression of EPHA2 contributes to the aggressive behavior of cancer cells have been widely debated. Fang et al[16] discussed the importance of receptor phosphorylation and the kinase activity of EPHA2 toward the aggressive and migratory nature of tumor cells. Miao et al[17] on the other hand, reported that the activation of EPHA2 inhibits the Ras/MAPK pathway, that is, the activation of EPHA2 may reduce the aggressive nature of tumor cells. The degradation of EPHA2 is dependent on ligand inducible phosphorylation[18]; thus, the clinico-pathological effects of EPHA2 activation should be assessed, including the complex situation of the genetic profile of the tumor cells themselves and their microenvironment.
EPHA2 and its major ligand EFNA1 are perturbed by various metabolites including deoxycholic acid (DCA) and its derivative. Li et al[19] showed the up-regulation of EPHA2 by DCA in colorectal cancer cells. This may be another example of the involvement of EPH pathways and endogenous metabolites in addition to EPHA1 and PUFA.
There have been few reports on the alteration of EPHA3 in human tumors until a recent high throughput sequencing project identified a high prevalence of a somatic mutation in EPHA3 in human cancers[20,21]. The somatic mutation in EPHA3 resides in D806 where the residue is evolutionally conserved (Table 1). The prevalence does not seem to be high in any population; actually, no mutations of EPHA3 were observed in follow-up studies of 46 Japanese patients with colorectal cancer reported by Shao et al[22]. Cell signaling studies using a culture system disclosed a role of EPHA3 in the formation of a cell’s shape[23]. Thus, changes in EPHA3 are likely to produce particular morphological and biological characteristics in the tumor cells carrying these changes, although no correlation between the EPHA3 status and the clinico-pathological features of gastrointestinal cancers has yet been described. Although the clinical relevance is unknown, there is a report investigating the LINE-1 methylation pattern in the introns of EPHA3 in tumor cells[24].
The over-expression of EPHA4 has been reported in gastric and colorectal cancers[25,26]. In both cancers, the over-expression of EPHA4 is an ominous sign with a shorter survival period and frequent liver metastasis respectively. EPHA4 is the only type A receptor that binds a B family ligand, EPHRIN(EFN)B2, in addition to a type A ligand, EPHRIN(EFN)A2 (Table 3). A structural study has been conducted to reveal the stereoscopic interactions between several members of EPH receptors and EPHRIN(EFN)s[27]. The potential significance of EPHA4 over-expression in clinical oncology and the possibility of its use as a therapeutic target remain unknown.
| EFNA1 | EFNA2 | EFNA3 | EFNA4 | EFNA5 | EFNB1 | EFNB2 | EFNB3 | |
| EPHA1 | x | |||||||
| EPHA2 | x | x | x | x | x | |||
| EPHA3 | x | x | x | x | x | |||
| EPHA4 | x | x | x | x | x | x | x | |
| EPHA5 | x | x | x | x | x | |||
| EPHA6 | x | |||||||
| EPHA7 | x | x | ||||||
| EPHA8 | x | x | x | x | x | |||
| EPHB1 | x | x | x | |||||
| EPHB2 | x | x | x | |||||
| EPHB3 | x | x | x | |||||
| EPHB4 | x | x | x | |||||
| EPHB5 | ||||||||
| EPHB6 | x | x | x |
There is no information regarding alterations in EPHA5 in human gastrointestinal cancers. EPHA5 is not expressed in the intestine at any age, as reported by Islam et al[28].
Research on EPHA6 in the gastrointestinal tract is sparse. EPHA6 is commonly expressed in the testis and brain[29].
Since the first description of the down-regulation of EPHA7 in colorectal cancer[30], several papers have assessed the expression of EPHA7 in human gastrointestinal cancers[31], human lung cancer[32] and prostate cancer[33]. The biological basis for these clinicopathological observations and their significance in oncology remain to be investigated. The promoter methylation of EPHA7 was the first example of down-regulation by methylation in EPH receptors but a subsequent survey of other EPH receptors, including EPHB receptors in colon cancer, produced negative results[34]. Another topic concerning EPHA7 is its secretory form. The secretory form of EPHA7 contains only the extracellular part of the molecule and does not anchor at the cell membrane. Its biological and clinical significance remain unknown. A secretory form of EPHA7 is known to exist in malignant lymphoma[35] and lung cancer[32] but no study has been conducted on the presence of the secretory form of EPHA7 in clinical gastrointestinal cancer.
Although the clinical significance is still unclear, Kim et al[36] reported a single nucleotide polymorphism (SNP) at the EPHA7 locus, rs2278107; this SNP was related to the chemoresponsiveness to fluoropyrimidine-based adjuvant chemotherapy for colorectal cancer[36].
EPHA8 was screened for mutation in Japanese colorectal cancer but no mutations were found[22], similar to other EPHA receptors such as EPHA3 and EPHA7. The EPHA8 receptor induces the sustained up-regulation of MAP kinase; thus, it is supposed to play a role in tumor cell growth and proliferation[37]. EPHA8 is expressed during the fetal period of intestinal morphogenesis[28] and missense mutations in stomach cancer and colon cancer are known (Table 1).
EPHB1 has been investigated in terms of signal transduction involved in the biological behavior of tumor cells[38,39], but little information is available on its status in human clinical cancer. An EPHB1 mutation was recently identified in ovarian cancer and missense mutations have also been found in gastric cancer[40] (Table 1).
EPHB2 is the most extensively studied member of EPH receptors in the field of oncology. Kiyokawa et al[2] reported the overexpression of EPHB2 in human gastric cancer and assigned it to the chromosomal locus at 1p36 which many investigators have assumed to be a tumor suppressor locus of human colon cancer because of the frequent loss of heterozygosity that has been documented[41]. Subsequently, Oba et al[42] demonstrated the loss of heterozygosity of the EPHB2 locus in human colorectal cancer. Furthermore, Batlle et al[43] argued that EPHB receptor activity could suppress the progression of colorectal cancer and EPHB2 is now viewed, at least in some contexts, as a tumor suppressor or a suppressor against tumor progression[26,44-48], although different aspects have also been discussed[49]. A group led by Hans Clevers put forward the comprehensive idea of EPHB2-EPHRINB1 interplay at the bottom of human colon crypts[50,51]. They showed the clear territory of EPHB2 and EPHRINB1 in a human colorectal crypt, its important role in cell positioning and the ordered developmental migration of intestinal cells using EphB2/EphB3 knockout mice[51]. This view is now prevalent[52] and they have further refined the concept of a stem cell unit in human gastrointestinal crypts[53,54]. Based on the mutually exclusive localization of EPHB2 and EPHRINB1, Cortina suggested that tumor compartmentalization arising from the repulsive action of cells expressing EPHB2 and EPHRINB1 is a possible mechanistic basis for tumor suppression by the EPHB2-EPHRINB1 system[55].
Then, what happened to the previous interpretation for the over-expression of EPHB2 in human cancer[2,56,57]? Mao[58] reported EPHB2 as a therapeutic antibody drug target for EPHB2 over-expressing tumors. Mutation analyses in kinase genes have been very popular and somatic mutations of EPHB2 have also been reported in many cancers[59,60], including GI tract cancers[61,62].
However, these mutations occur mostly in the microsatellite repeats of tumors with microsatellite instability or nonsense mutations causing RNA decay. No naturally occurring missense mutation that may positively or negatively influence the kinase activity of EPHB2 has ever been reported. At this moment, we can only say that individual tumors may have an individual EPHB2 status in an individual environment. The prevalence of methylation in the EPHB2 promoter, on the other hand, is low compared with RASSF2 and O-6-methylguanine-DNA methyl transferase (MGMT) in early colorectal tumors[63].
There are reports investigating the possible contribution of germline EPHB2 variants to rare polyposis syndrome[64,65]. The detailed mechanistic basis controlling the EPHB2-EPHRIN (EFN) B1 system has also been investigated. Tanaka et al[66] reported that C-terminal EFNB1 regulates matrix metalloproteinase secretion and that the phosphorylation of EFNB1 regulates the dissemination of gastric cancer cells in an animal model[66]. He also showed the successful suppression of peritoneal dissemination in an animal model using an EFNB1-derived peptide[67]. The translational approaches using this method (use of EFNB1 peptide to suppress human cancer dissemination) have not yet been shown.
The localization and function of EPHB3 partially overlaps with EPHB2 in a Paneth cell compartment. EPHB3 also has EFNB1 as a ligand. Both are controlled by the beta-catenin/Tcf4 pathway[51].
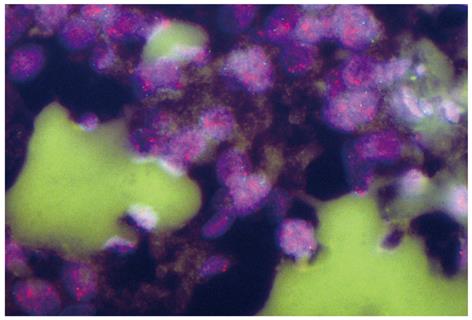
Chiu reported that the over-expression of EPHB3 enhanced cell-cell contact and suppressed tumor growth in HT-29 human colon cancer cells[68]. The defect in the positioning of Paneth cells is thought to arise from the disruption of the EPHB2-EPHB3 system[69]. Clinicopathological information on EPHB3 alone (not accompanied with EPHB2) in human gastrointestinal tract cancers remains limited. A clinical interpretation of the over-expression and/or amplification of EPHB3 (Figure 1) in gastrointestinal cancer[70,71] awaits further investigations.
Kumar reported that EPHB4 over-expression is more prevalent than EPHB2 over-expression and the cyclic AMP-responsive element binding protein-binding protein (CBP) complex reciprocally regulates EPHB2 and EPHB4 (CBP complex suppresses EPHB2 and induces EPHB4 expression)[48]. EPHB4 is thought to act in an EPHB4-EPHB6 system[72] to regulate cancer cell invasiveness. The structure and dynamism on EPHB4-EFNB2 was investigated[73,74] and the translational application of this basic knowledge awaits further investigation.
EPHB6 is the oldest EPH family member to attract enthusiastic interest from cancer researchers, especially neuroblastoma researchers. EPHB6 is unique in that there is no kinase activity. It is one of the major genes involved in the clinico-biological behaviors of neuroblastomas[75-77]. Unlike other EPHBs, a suppressor role of EPHB6 has been pointed out from an early stage of research[78-80], although its over-expression has been identified in leukemic cells[81]. A functional enigma of EPHB6, a kinase defective receptor affecting tumor invasiveness, has been gradually clarified in the fields of lung cancer research[82] but the role of EPHB6 in carcinogenesis in the human digestive tract is not clear, although its alteration such as promoter methylation in lung adenocarcinoma, has been recently reported[83]. Recently, some missense variants have been reported in familial colorectal cancer[84]. Somatic changes in colorectal cancers according to ethnic stratification have revealed EPHB6 to be one of the most frequently deleted genes in African Americans[85].
Choi et al[86] reported the discovery of EPHB2 receptor kinase inhibitors. They also performed crystallographic analyses of EPHA3 and EPHA7 in complex with their inhibitors and discussed the possibility of generating new inhibitors using a structure-based design[86]. This discovery and other structural studies[27,73,87] should pave the way for the development of drugs that specifically inhibit tumor cells over-expressing these receptors.
EPHA2 has been considered as a target for anti-angiogenesis therapy for a long time[8,88-92]. The EPHA2-Fc receptor was used to inhibit an EFNA1-EPHA2 forward signal and to reduce neovascularization in rodent retina[91].
Although the EPH family is well known to be involved in the development of neural and vascular systems, their pivotal contributions to cancer biology, especially in clinical settings, remain to be elucidated. Enthusiasm regarding the use of EPHs as cancer therapy targets remains less than that of expectations for other groups of kinase receptors such as EGFR, HER2, MET and RAF[40,93]. The unique biological nature of EPHs such as bidirectional signaling and the presence of a secreted form, however, may provide a possible clue to manipulating the regulation of EPH-EPHRIN systems for human gastrointestinal cancer therapy. Gastrointestinal cancers have a special niche in Asian diseases in terms of their heterogeneity and uniqueness in etiology, both genetic and environmental[94]. An extensive search of EPH-EPHRIN systems in Asian gastrointestinal cancer patients will provide an important tool for the clinical management of Asian gastrointestinal cancer patients.
The real scale of the involvement of those genes in carcinogenesis in the human gastrointestinal tract still remains unclear and several research groups including Asians continue the search for molecular alterations of the EPH-EPHRIN system that may be relevant to detection and treatment of gastrointestinal cancers. The information stated here will be updated every year in future.
Peer reviewer: Barbara W Chwirot, Professor, Department of Medical Biology, Institute of General and Molecular Biology, Nicolaus Copernicus University, Gagarina 9, Torun 87-100, Poland
S- Editor Wang JL L- Editor Roemmele A E- Editor Ma WH
| 1. | Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717-1720. [Cited in This Article: ] |
| 2. | Kiyokawa E, Takai S, Tanaka M, Iwase T, Suzuki M, Xiang YY, Naito Y, Yamada K, Sugimura H, Kino I. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994;54:3645-3650. [Cited in This Article: ] |
| 3. | Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403-404. [Cited in This Article: ] |
| 4. | Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165-180. [Cited in This Article: ] |
| 5. | Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR. The Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum Genet. 2008;Chapter 10:Unit 10.11. [Cited in This Article: ] |
| 6. | Dong Y, Wang J, Sheng Z, Li G, Ma H, Wang X, Zhang R, Lu G, Hu Q, Sugimura H. Downregulation of EphA1 in colorectal carcinomas correlates with invasion and metastasis. Mod Pathol. 2009;22:151-160. [Cited in This Article: ] |
| 7. | Herath NI, Doecke J, Spanevello MD, Leggett BA, Boyd AW. Epigenetic silencing of EphA1 expression in colorectal cancer is correlated with poor survival. Br J Cancer. 2009;100:1095-1102. [Cited in This Article: ] |
| 8. | Chen G, Wang Y, Zhou M, Shi H, Yu Z, Zhu Y, Yu F. EphA1 receptor silencing by small interfering RNA has antiangiogenic and antitumor efficacy in hepatocellular carcinoma. Oncol Rep. 2010;23:563-570. [Cited in This Article: ] |
| 9. | Herath NI, Boyd AW. The role of Eph receptors and ephrin ligands in colorectal cancer. Int J Cancer. 2010;126:2003-2011. [Cited in This Article: ] |
| 10. | Wang J, Dong Y, Wang X, Ma H, Sheng Z, Li G, Lu G, Sugimura H, Zhou X. Expression of EphA1 in gastric carcinomas is associated with metastasis and survival. Oncol Rep. 2010;24:1577-1584. [Cited in This Article: ] |
| 11. | Doleman JF, Eady JJ, Elliott RM, Foxall RJ, Seers J, Johnson IT, Lund EK. Identification of the Eph receptor pathway as a novel target for eicosapentaenoic acid (EPA) modification of gene expression in human colon adenocarcinoma cells (HT-29). Nutr Metab (Lond). 2010;7:56. [Cited in This Article: ] |
| 12. | Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Maruyama K. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136-141. [Cited in This Article: ] |
| 13. | Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657-663. [Cited in This Article: ] |
| 14. | Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42-47. [Cited in This Article: ] |
| 15. | Cheng N, Chen J. Tumor necrosis factor-alpha induction of endothelial ephrin A1 expression is mediated by a p38 MAPK- and SAPK/JNK-dependent but nuclear factor-kappa B-independent mechanism. J Biol Chem. 2001;276:13771-13777. [Cited in This Article: ] |
| 16. | Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859-7868. [Cited in This Article: ] |
| 17. | Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527-530. [Cited in This Article: ] |
| 18. | Wang Y, Ota S, Kataoka H, Kanamori M, Li Z, Band H, Tanaka M, Sugimura H. Negative regulation of EphA2 receptor by Cbl. Biochem Biophys Res Commun. 2002;296:214-220. [Cited in This Article: ] |
| 19. | Li Z, Tanaka M, Kataoka H, Nakamura R, Sanjar R, Shinmura K, Sugimura H. EphA2 up-regulation induced by deoxycholic acid in human colon carcinoma cells, an involvement of extracellular signal-regulated kinase and p53-independence. J Cancer Res Clin Oncol. 2003;129:703-708. [Cited in This Article: ] |
| 20. | Wood LD, Calhoun ES, Silliman N, Ptak J, Szabo S, Powell SM, Riggins GJ, Wang TL, Yan H, Gazdar A. Somatic mutations of GUCY2F, EPHA3, and NTRK3 in human cancers. Hum Mutat. 2006;27:1060-1061. [Cited in This Article: ] |
| 21. | Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, Markowitz S, Willson JK, Parmigiani G, Kinzler KW. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. [Cited in This Article: ] |
| 22. | Shao RX, Kato N, Lin LJ, Muroyama R, Moriyama M, Ikenoue T, Watabe H, Otsuka M, Guleng B, Ohta M. Absence of tyrosine kinase mutations in Japanese colorectal cancer patients. Oncogene. 2007;26:2133-2135. [Cited in This Article: ] |
| 23. | Lawrenson ID, Wimmer-Kleikamp SH, Lock P, Schoenwaelder SM, Down M, Boyd AW, Alewood PF, Lackmann M. Ephrin-A5 induces rounding, blebbing and de-adhesion of EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated signalling. J Cell Sci. 2002;115:1059-1072. [Cited in This Article: ] |
| 24. | Phokaew C, Kowudtitham S, Subbalekha K, Shuangshoti S, Mutirangura A. LINE-1 methylation patterns of different loci in normal and cancerous cells. Nucleic Acids Res. 2008;36:5704-5712. [Cited in This Article: ] |
| 25. | Oki M, Yamamoto H, Taniguchi H, Adachi Y, Imai K, Shinomura Y. Overexpression of the receptor tyrosine kinase EphA4 in human gastric cancers. World J Gastroenterol. 2008;14:5650-5656. [Cited in This Article: ] |
| 26. | Oshima T, Akaike M, Yoshihara K, Shiozawa M, Yamamoto N, Sato T, Akihito N, Nagano Y, Fujii S, Kunisaki C. Overexpression of EphA4 gene and reduced expression of EphB2 gene correlates with liver metastasis in colorectal cancer. Int J Oncol. 2008;33:573-577. [Cited in This Article: ] |
| 27. | Bowden TA, Aricescu AR, Nettleship JE, Siebold C, Rahman-Huq N, Owens RJ, Stuart DI, Jones EY. Structural plasticity of eph receptor A4 facilitates cross-class ephrin signaling. Structure. 2009;17:1386-1397. [Cited in This Article: ] |
| 28. | Islam S, Loizides AM, Fialkovich JJ, Grand RJ, Montgomery RK. Developmental expression of Eph and ephrin family genes in mammalian small intestine. Dig Dis Sci. 2010;55:2478-2488. [Cited in This Article: ] |
| 29. | Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490-499. [Cited in This Article: ] |
| 30. | Wang J, Kataoka H, Suzuki M, Sato N, Nakamura R, Tao H, Maruyama K, Isogaki J, Kanaoka S, Ihara M. Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene. 2005;24:5637-5647. [Cited in This Article: ] |
| 31. | Wang J, Li G, Ma H, Bao Y, Wang X, Zhou H, Sheng Z, Sugimura H, Jin J, Zhou X. Differential expression of EphA7 receptor tyrosine kinase in gastric carcinoma. Hum Pathol. 2007;38:1649-1656. [Cited in This Article: ] |
| 32. | Tsuboi M, Mori H, Bunai T, Kageyama S, Suzuki M, Okudela K, Takamochi K, Ogawa H, Niwa H, Shinmura K. Secreted form of EphA7 in lung cancer. Int J Oncol. 2010;36:635-640. [Cited in This Article: ] |
| 33. | Guan M, Xu C, Zhang F, Ye C. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int J Cancer. 2009;124:88-94. [Cited in This Article: ] |
| 34. | Wu Q, Lind GE, Aasheim HC, Micci F, Silins I, Tropé CG, Nesland JM, Lothe RA, Suo Z. The EPH receptor Bs (EPHBs) promoters are unmethylated in colon and ovarian cancers. Epigenetics. 2007;2:237-243. [Cited in This Article: ] |
| 35. | Dawson DW, Hong JS, Shen RR, French SW, Troke JJ, Wu YZ, Chen SS, Gui D, Regelson M, Marahrens Y. Global DNA methylation profiling reveals silencing of a secreted form of Epha7 in mouse and human germinal center B-cell lymphomas. Oncogene. 2007;26:4243-4252. [Cited in This Article: ] |
| 36. | Kim JC, Kim SY, Cho DH, Roh SA, Choi EY, Jo YK, Jung SH, Na YS, Kim TW, Kim YS. Genome-wide identification of chemosensitive single nucleotide polymorphism markers in colorectal cancers. Cancer Sci. 2010;101:1007-1013. [Cited in This Article: ] |
| 37. | Gu C, Shim S, Shin J, Kim J, Park J, Han K, Park S. The EphA8 receptor induces sustained MAP kinase activation to promote neurite outgrowth in neuronal cells. Oncogene. 2005;24:4243-4256. [Cited in This Article: ] |
| 38. | Han DC, Shen TL, Miao H, Wang B, Guan JL. EphB1 associates with Grb7 and regulates cell migration. J Biol Chem. 2002;277:45655-45661. [Cited in This Article: ] |
| 39. | Vindis C, Teli T, Cerretti DP, Turner CE, Huynh-Do U. EphB1-mediated cell migration requires the phosphorylation of paxillin at Tyr-31/Tyr-118. J Biol Chem. 2004;279:27965-27970. [Cited in This Article: ] |
| 40. | Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869-873. [Cited in This Article: ] |
| 41. | Rau D, Köster A, Tittelbach H, Sachse R, Landgraf S, Neubauer S, Giedl J, Dingermann T, Gebhart E. Chromosome and oncogene studies in human rectal and colon carcinomas. Anticancer Res. 1991;11:1477-1484. [Cited in This Article: ] |
| 42. | Oba SM, Wang YJ, Song JP, Li ZY, Kobayashi K, Tsugane S, Hamada GS, Tanaka M, Sugimura H. Genomic structure and loss of heterozygosity of EPHB2 in colorectal cancer. Cancer Lett. 2001;164:97-104. [Cited in This Article: ] |
| 43. | Batlle E, Bacani J, Begthel H, Jonkheer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126-1130. [Cited in This Article: ] |
| 44. | Jubb AM, Zhong F, Bheddah S, Grabsch HI, Frantz GD, Mueller W, Kavi V, Quirke P, Polakis P, Koeppen H. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res. 2005;11:5181-5187. [Cited in This Article: ] |
| 45. | Guo DL, Zhang J, Yuen ST, Tsui WY, Chan AS, Ho C, Ji J, Leung SY, Chen X. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours. Carcinogenesis. 2006;27:454-464. [Cited in This Article: ] |
| 46. | Holmberg J, Genander M, Halford MM, Annerén C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisén J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151-1163. [Cited in This Article: ] |
| 47. | Senior PV, Zhang BX, Chan ST. Loss of cell-surface receptor EphB2 is important for the growth, migration, and invasiveness of a colon cancer cell line. Int J Colorectal Dis. 2010;25:687-694. [Cited in This Article: ] |
| 48. | Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736-3745. [Cited in This Article: ] |
| 49. | Song JH, Kim CJ, Cho YG, Kwak HJ, Nam SW, Yoo NJ, Lee JY, Park WS. Genetic and epigenetic analysis of the EPHB2 gene in gastric cancers. APMIS. 2007;115:164-168. [Cited in This Article: ] |
| 50. | Clevers H, Batlle E. EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res. 2006;66:2-5. [Cited in This Article: ] |
| 51. | Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251-263. [Cited in This Article: ] |
| 52. | Booth C, Brady G, Potten CS. Crowd control in the crypt. Nat Med. 2002;8:1360-1361. [Cited in This Article: ] |
| 53. | Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681-1696. [Cited in This Article: ] |
| 54. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [Cited in This Article: ] |
| 55. | Cortina C, Palomo-Ponce S, Iglesias M, Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N, Gallego L, Jonkheer S. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376-1383. [Cited in This Article: ] |
| 56. | Kataoka H, Tanaka M, Kanamori M, Yoshii S, Ihara M, Wang YJ, Song JP, Li ZY, Arai H, Otsuki Y. Expression profile of EFNB1, EFNB2, two ligands of EPHB2 in human gastric cancer. J Cancer Res Clin Oncol. 2002;128:343-348. [Cited in This Article: ] |
| 57. | Lugli A, Spichtin H, Maurer R, Mirlacher M, Kiefer J, Huusko P, Azorsa D, Terracciano L, Sauter G, Kallioniemi OP. EphB2 expression across 138 human tumor types in a tissue microarray: high levels of expression in gastrointestinal cancers. Clin Cancer Res. 2005;11:6450-6458. [Cited in This Article: ] |
| 58. | Mao W, Luis E, Ross S, Silva J, Tan C, Crowley C, Chui C, Franz G, Senter P, Koeppen H. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004;64:781-788. [Cited in This Article: ] |
| 59. | Huusko P, Ponciano-Jackson D, Wolf M, Kiefer JA, Azorsa DO, Tuzmen S, Weaver D, Robbins C, Moses T, Allinen M. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet. 2004;36:979-983. [Cited in This Article: ] |
| 60. | Kittles RA, Baffoe-Bonnie AB, Moses TY, Robbins CM, Ahaghotu C, Huusko P, Pettaway C, Vijayakumar S, Bennett J, Hoke G. A common nonsense mutation in EphB2 is associated with prostate cancer risk in African American men with a positive family history. J Med Genet. 2006;43:507-511. [Cited in This Article: ] |
| 61. | Alazzouzi H, Davalos V, Kokko A, Domingo E, Woerner SM, Wilson AJ, Konrad L, Laiho P, Espín E, Armengol M. Mechanisms of inactivation of the receptor tyrosine kinase EPHB2 in colorectal tumors. Cancer Res. 2005;65:10170-10173. [Cited in This Article: ] |
| 62. | Davalos V, Dopeso H, Velho S, Ferreira AM, Cirnes L, Díaz-Chico N, Bilbao C, Ramírez R, Rodríguez G, Falcón O. High EPHB2 mutation rate in gastric but not endometrial tumors with microsatellite instability. Oncogene. 2007;26:308-311. [Cited in This Article: ] |
| 63. | Nosho K, Yamamoto H, Takahashi T, Mikami M, Taniguchi H, Miyamoto N, Adachi Y, Arimura Y, Itoh F, Imai K. Genetic and epigenetic profiling in early colorectal tumors and prediction of invasive potential in pT1 (early invasive) colorectal cancers. Carcinogenesis. 2007;28:1364-1370. [Cited in This Article: ] |
| 64. | Kokko A, Laiho P, Lehtonen R, Korja S, Carvajal-Carmona LG, Järvinen H, Mecklin JP, Eng C, Schleutker J, Tomlinson IP. EPHB2 germline variants in patients with colorectal cancer or hyperplastic polyposis. BMC Cancer. 2006;6:145. [Cited in This Article: ] |
| 65. | Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131-150. [Cited in This Article: ] |
| 66. | Tanaka M, Sasaki K, Kamata R, Sakai R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J Cell Sci. 2007;120:2179-2189. [Cited in This Article: ] |
| 67. | Tanaka M, Kamata R, Yanagihara K, Sakai R. Suppression of gastric cancer dissemination by ephrin-B1-derived peptide. Cancer Sci. 2010;101:87-93. [Cited in This Article: ] |
| 68. | Chiu ST, Chang KJ, Ting CH, Shen HC, Li H, Hsieh FJ. Over-expression of EphB3 enhances cell-cell contacts and suppresses tumor growth in HT-29 human colon cancer cells. Carcinogenesis. 2009;30:1475-1486. [Cited in This Article: ] |
| 69. | Preston SL, Leedham SJ, Oukrif D, Deheregoda M, Goodlad RA, Poulsom R, Alison MR, Wright NA, Novelli M. The development of duodenal microadenomas in FAP patients: the human correlate of the Min mouse. J Pathol. 2008;214:294-301. [Cited in This Article: ] |
| 70. | Sugimura H, Mori H, Nagura K, Kiyose S, Tao H, Isozaki M, Igarashi H, Shinmura K, Hasegawa A, Kitayama Y. Fluorescence in situ hybridization analysis with a tissue microarray: 'FISH and chips' analysis of pathology archives. Pathol Int. 2010;60:543-550. [Cited in This Article: ] |
| 71. | Sugimura H. Detection of chromosome changes in pathology archives: an application of microwave-assisted fluorescence in situ hybridization to human carcinogenesis studies. Carcinogenesis. 2008;29:681-687. [Cited in This Article: ] |
| 72. | Truitt L, Freywald T, DeCoteau J, Sharfe N, Freywald A. The EphB6 receptor cooperates with c-Cbl to regulate the behavior of breast cancer cells. Cancer Res. 2010;70:1141-1153. [Cited in This Article: ] |
| 73. | Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321-330. [Cited in This Article: ] |
| 74. | Chrencik JE, Brooun A, Kraus ML, Recht MI, Kolatkar AR, Han GW, Seifert JM, Widmer H, Auer M, Kuhn P. Structural and biophysical characterization of the EphB4*ephrinB2 protein-protein interaction and receptor specificity. J Biol Chem. 2006;281:28185-28192. [Cited in This Article: ] |
| 75. | Tang XX, Evans AE, Zhao H, Cnaan A, London W, Cohn SL, Brodeur GM, Ikegaki N. High-level expression of EPHB6, EFNB2, and EFNB3 is associated with low tumor stage and high TrkA expression in human neuroblastomas. Clin Cancer Res. 1999;5:1491-1496. [Cited in This Article: ] |
| 76. | Tang XX, Zhao H, Robinson ME, Cnaan A, London W, Cohn SL, Cheung NK, Brodeur GM, Evans AE, Ikegaki N. Prognostic significance of EPHB6, EFNB2, and EFNB3 expressions in neuroblastoma. Med Pediatr Oncol. 2000;35:656-658. [Cited in This Article: ] |
| 77. | Tang XX, Zhao H, Robinson ME, Cohen B, Cnaan A, London W, Cohn SL, Cheung NK, Brodeur GM, Evans AE. Implications of EPHB6, EFNB2, and EFNB3 expressions in human neuroblastoma. Proc Natl Acad Sci USA. 2000;97:10936-10941. [Cited in This Article: ] |
| 78. | Hafner C, Bataille F, Meyer S, Becker B, Roesch A, Landthaler M, Vogt T. Loss of EphB6 expression in metastatic melanoma. Int J Oncol. 2003;23:1553-1559. [Cited in This Article: ] |
| 79. | Tang XX, Evans AE, Zhao H, Cnaan A, Brodeur GM, Ikegaki N. Association among EPHB2, TrkA, and MYCN expression in low-stage neuroblastomas. Med Pediatr Oncol. 2001;36:80-82. [Cited in This Article: ] |
| 80. | Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882-892. [Cited in This Article: ] |
| 81. | Müller-Tidow C, Schwäble J, Steffen B, Tidow N, Brandt B, Becker K, Schulze-Bahr E, Halfter H, Vogt U, Metzger R. High-throughput analysis of genome-wide receptor tyrosine kinase expression in human cancers identifies potential novel drug targets. Clin Cancer Res. 2004;10:1241-1249. [Cited in This Article: ] |
| 82. | Yu J, Bulk E, Ji P, Hascher A, Koschmieder S, Berdel WE, Müller-Tidow C. The kinase defective EPHB6 receptor tyrosine kinase activates MAP kinase signaling in lung adenocarcinoma. Int J Oncol. 2009;35:175-179. [Cited in This Article: ] |
| 83. | Yu J, Bulk E, Ji P, Hascher A, Tang M, Metzger R, Marra A, Serve H, Berdel WE, Wiewroth R. The EPHB6 receptor tyrosine kinase is a metastasis suppressor that is frequently silenced by promoter DNA hypermethylation in non-small cell lung cancer. Clin Cancer Res. 2010;2275-2283. [Cited in This Article: ] |
| 84. | Gylfe AE, Sirkiä J, Ahlsten M, Järvinen H, Mecklin JP, Karhu A, Aaltonen LA. Somatic mutations and germline sequence variants in patients with familial colorectal cancer. Int J Cancer. 2010;Epub ahead of print. [Cited in This Article: ] |
| 85. | Ashktorab H, Schäffer AA, Daremipouran M, Smoot DT, Lee E, Brim H. Distinct genetic alterations in colorectal cancer. PLoS One. 2010;5:e8879. [Cited in This Article: ] |
| 86. | Choi Y, Syeda F, Walker JR, Finerty PJ Jr, Cuerrier D, Wojciechowski A, Liu Q, Dhe-Paganon S, Gray NS. Discovery and structural analysis of Eph receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009;19:4467-4470. [Cited in This Article: ] |
| 87. | Davis TL, Walker JR, Loppnau P, Butler-Cole C, Allali-Hassani A, Dhe-Paganon S. Autoregulation by the juxtamembrane region of the human ephrin receptor tyrosine kinase A3 (EphA3). Structure. 2008;16:873-884. [Cited in This Article: ] |
| 88. | Dobrzanski P, Hunter K, Jones-Bolin S, Chang H, Robinson C, Pritchard S, Zhao H, Ruggeri B. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910-919. [Cited in This Article: ] |
| 89. | Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J. Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression. FASEB J. 2005;19:1884-1886. [Cited in This Article: ] |
| 90. | Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037-2049. [Cited in This Article: ] |
| 91. | Chen J, Hicks D, Brantley-Sieders D, Cheng N, McCollum GW, Qi-Werdich X, Penn J. Inhibition of retinal neovascularization by soluble EphA2 receptor. Exp Eye Res. 2006;82:664-673. [Cited in This Article: ] |
| 92. | Cheng N, Brantley D, Fang WB, Liu H, Fanslow W, Cerretti DP, Bussell KN, Reith A, Jackson D, Chen J. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445-456. [Cited in This Article: ] |
| 93. | Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, Han B, Cao Q, Cao X, Suleman K. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793-798. [Cited in This Article: ] |
| 94. | Kawahara N. Perspectives on strategies for establishing cancer on the global health agenda: possibilities of creating infrastructure for cancer prevention information using school health classes. Asian Pac J Cancer Prev. 2009;10:1101-1106. [Cited in This Article: ] |