Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2253
Peer-review started: January 24, 2024
First decision: January 31, 2024
Revised: February 7, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: May 15, 2024
Undifferentiated pleomorphic sarcoma (UPS) is a rare malignant mesenchymal tumor with a poor prognosis. It mainly occurs in the extremities, trunk, head and neck, and retroperitoneum regions. Owing to the lack of specific clinical manifestations and imaging features, UPS diagnosis mainly depends on pathological and immunohistochemical examinations for exclusive diagnosis. Here we report an extremely rare case of high-grade UPS in the common bile duct (CBD). There are limited available data on such cases.
A 70-year-old woman was admitted to our department with yellow eyes and urine accompanied by upper abdominal distending pain for 2 wk. Her laboratory data suggested significantly elevated hepatorenal function levels. The imaging data revealed calculous cholecystitis, intrahepatic and extrahepatic bile duct dilation with extrahepatic bile duct calculi, and a space-occupying lesion at the distal CBD. After endoscopic biliary stenting and symptomatic support therapy, CBD exploration and biopsy were performed. The frozen section indicated malignant spindle cell tumor of the CBD mass, and further radical pancreaticoduodenectomy was performed. Finally, the neoplasm was diagnosed as a high-grade UPS combined with the light-microscopic morphology and immunohistochemical results.
This extremely rare case highlighted the need for increasing physicians' vigilance, reducing the odds of misdiagnosis, and providing appropriate treatment stra
Core Tip: Undifferentiated pleomorphic sarcoma (UPS) cases are rare. Herein, we report the case of an elderly woman with high-grade UPS, which was detected because of obstructive jaundice and diagnosed by histopathology. As far as we know, this is the first reported case of UPS in the common bile duct (CBD). Recognizing that UPS can occur in the CBD will help increase physicians' vigilance, reduce the odds of misdiagnosis, and provide appropriate treatment strategies.
- Citation: Zheng LP, Shen WY, Hu CD, Wang CH, Chen XJ, Wang J, Shen YY. Undifferentiated high-grade pleomorphic sarcoma of the common bile duct: A case report and review of literature. World J Gastrointest Oncol 2024; 16(5): 2253-2260
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2253.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2253
Undifferentiated pleomorphic sarcoma (UPS), also known as malignant fibrous histiocytoma (MFH)[1], is a rare malignant mesenchymal tumor with a mixed pattern of fibroblast and histiocyte growth. MFH was divided into five histological subtypes, namely, storiform-pleomorphic, myxoid, giant-cell, inflammatory, and angiomatoid MFH[2]. In 2002, the World Health Organization (WHO) classified myxoid and angiomatoid MFH into myofibroblast-like and uncertain differentiated tumors, respectively[3]. The redefined MFH includes three histological subtypes. The first subtype is Storiform-pleomorphic MFH, also known as high-grade UPS, which was the most common subtype. The typical microstructure of the tumor cells is that they have obvious atypia, predominantly characterized by a combination of storiform and pleomorphic areas. Cells within the storiform area have spindle-shaped or circular morphology. The second subtype is the giant-cell MFH, also known as UPS with giant cells. Its typical microstructure includes a large number of multinucleated giant cells, mostly osteoclast-like. The third subtype is the inflammatory MFH, also known as UPS with obvious inflammation. This subtype is rare and its characteristic microstructure includes spindle cells and xanthoma cells accompanied by inflammatory cells infiltration. According to the 2013 WHO classification of soft tissue tumors (STT), the term MFH was removed and substituted with UPS, categorized as undifferentiated/unclassified soft tissue sarcoma (STS)[4].
UPS mainly occurs in elderly individuals, men are slightly dominant, usually involves the extremities and trunk[5], head and neck[6,7], and retroperitoneum regions[8]. Owing to the complexity of its clinical manifestations and histopathological features, the diagnosis of this condition have presented significant challenges. Here we report an extremely rare case of high-grade UPS in the common bile duct (CBD). For all we know, this is the first reported case of UPS in the CBD.
A 70-year-old woman visited our department because of yellow eyes and urine accompanied by upper abdominal distending pain for 2 wk.
Two weeks ago, the patient gradually developed yellow eyes and urine without obvious inducement, accompanied by upper abdominal distending pain. There was neither nausea and vomiting nor chill and fever.
The patient suffered from hypertension and diabetes for 20 years, and had been taking amlodipine besylate tablet 5 mg once a day and metformin hydrochloride tablet 0.5 g twice a day to control the blood pressure and blood sugar levels, both of which were controlled within the normal ranges.
The patient was married and menopausal at age 50. No family history was presented.
The sclera and skin of the patient were obviously yellow, but superficial lymph nodes were not enlarged. Abdominal examination revealed nothing special except mild tenderness in the upper abdomen.
Routine blood test, coagulation function, and tumor markers, such as carcinoma embryonic antigen (3.02 ng/mL; normal range: 0-5 ng/mL), carbohydrate antigen 19-9 (10.83 U/mL; normal range 0-37 U/mL), and alpha-fetoprotein (1.71 ng/mL; normal range 0-20 ng/mL), were all within normal ranges. Biochemical results showed that the total bilirubin (140 µmol/L; normal range 0-23 µmol/L), direct bilirubin (99 µmol/L; normal range 0-4 µmol/L), total bile acid (>180 µmol/L; normal range 0-6.71 µmol/L), alanine aminotransferase (415 U/L; normal range 7-40 U/L), gamma-glutamyl transpeptidase (1053 U/L; normal range 7-45 U/L), alkaline phosphatase (309 U/L; normal range 50-135 U/L), lactic dehydrogenase (271 U/L; normal range 120-250 U/L), urea (12.95 mmol/L; normal range 3.1-8.8 mmol/L), creatinine (175 µmol/L; normal range 53-97 µmol/L), and beta 2-microglobulin (4.03 mg/L, normal range 1-3 mg/L) levels were significantly elevated.
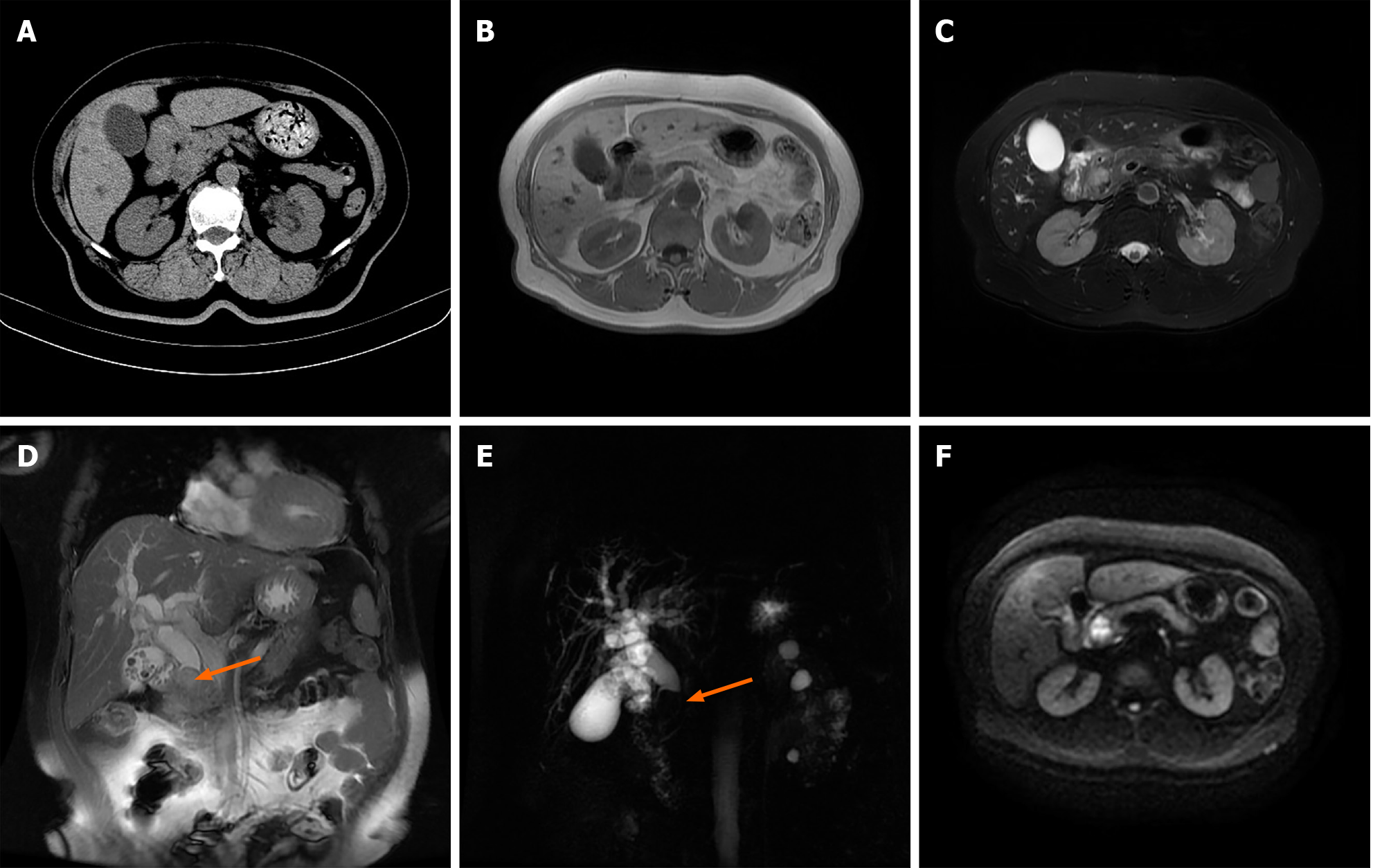
The patient's chest computed tomography (CT) findings showed no significant abnormalities. Given her renal function, enhanced CT or magnetic resonance imaging (MRI) was not performed. Abdominal plain CT revealed calculous cholecystitis, suspicious soft tissue density shadow at the distal CBD with intrahepatic and extrahepatic bile duct dilatation (Figure 1A). To further clarify the diagnosis, we performed an epigastric MRI examination, which showed calculous cholecystitis, intrahepatic and extrahepatic bile duct dilation with extrahepatic bile duct calculi, and a hypointense shadow on T1-weighed images and an uneven hyperintense shadow on T2-weighed images and a diffusion-restricted hyperintense shadow on diffusion-weighted imaging at the distal CBD (Figure 1B-F).
According to these clinical manifestations and radiographic findings, the patient's primary diagnosis was STT of the distal CBD, with minor diagnoses of intrahepatic and extrahepatic bile duct dilation with extrahepatic bile duct calculi, obstructive jaundice, calculous cholecystitis, hepatorenal dysfunction, hypertension, and diabetes.
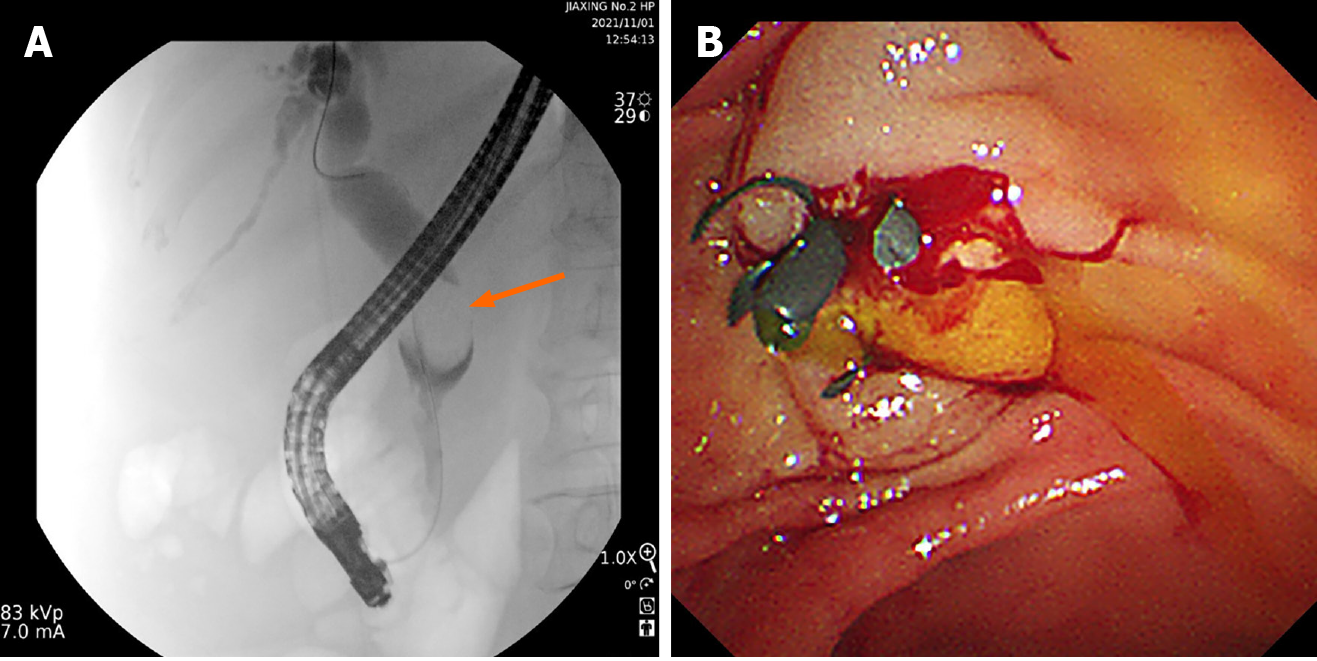
Owing to hepatorenal insufficiency, the initial treatment for the patient involved endoscopic biliary stent implantation to alleviate jaundice (Figure 2), followed by adjunctive hepatorenal protection treatment. After the hepatorenal functions were significantly improved, the patient underwent an exploration and biopsy of the CBD, and the frozen section indicated that the neoplasm at the distal CBD was spindle malignant cell tumor. Therefore, further radical pancreaticoduodenectomy was performed.
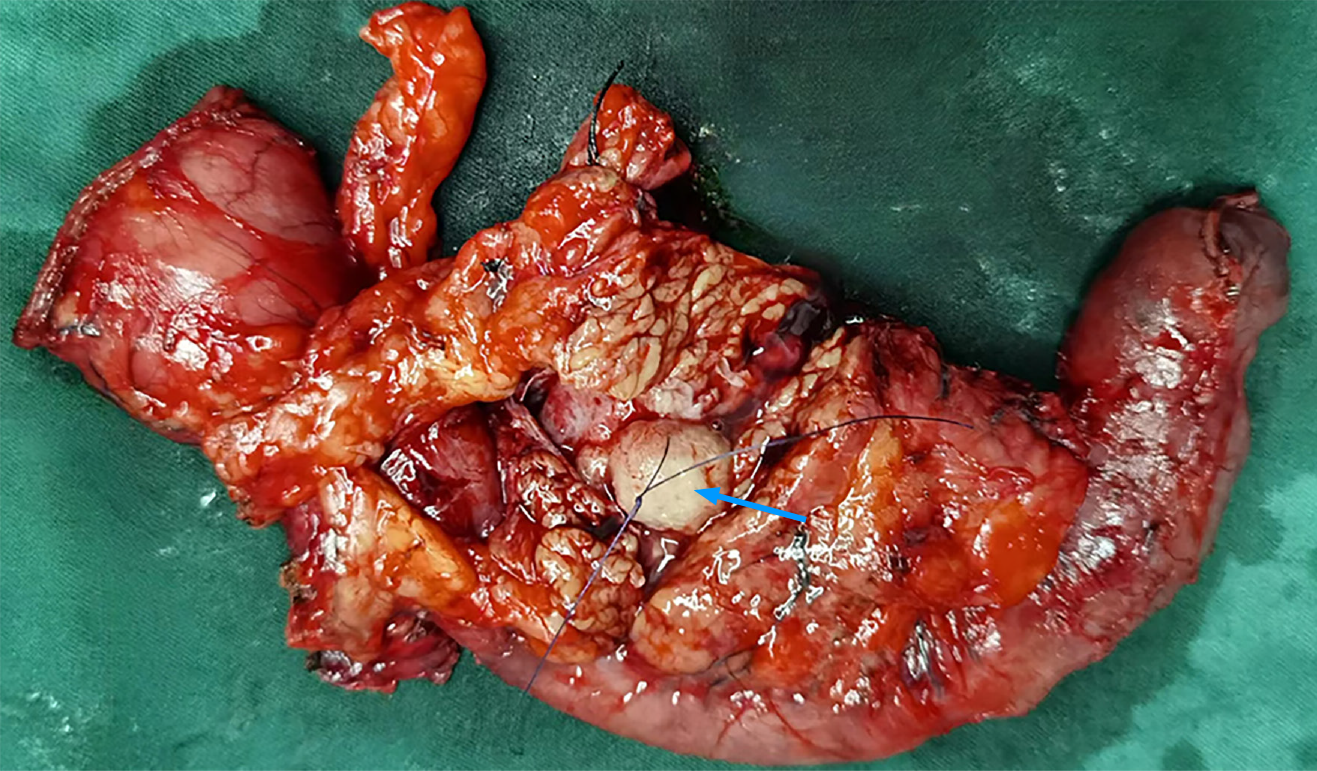
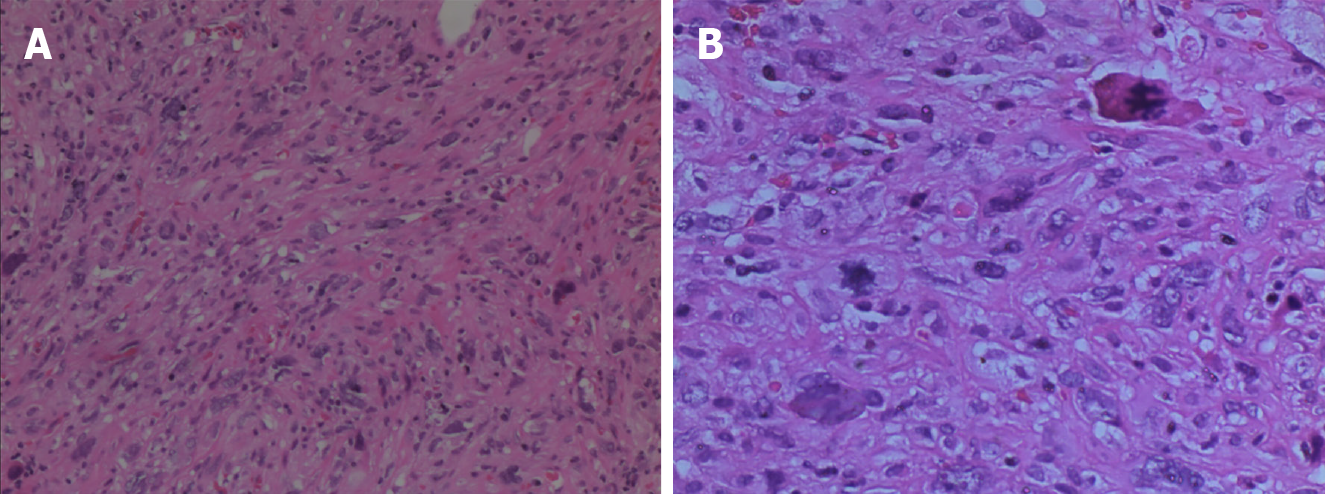
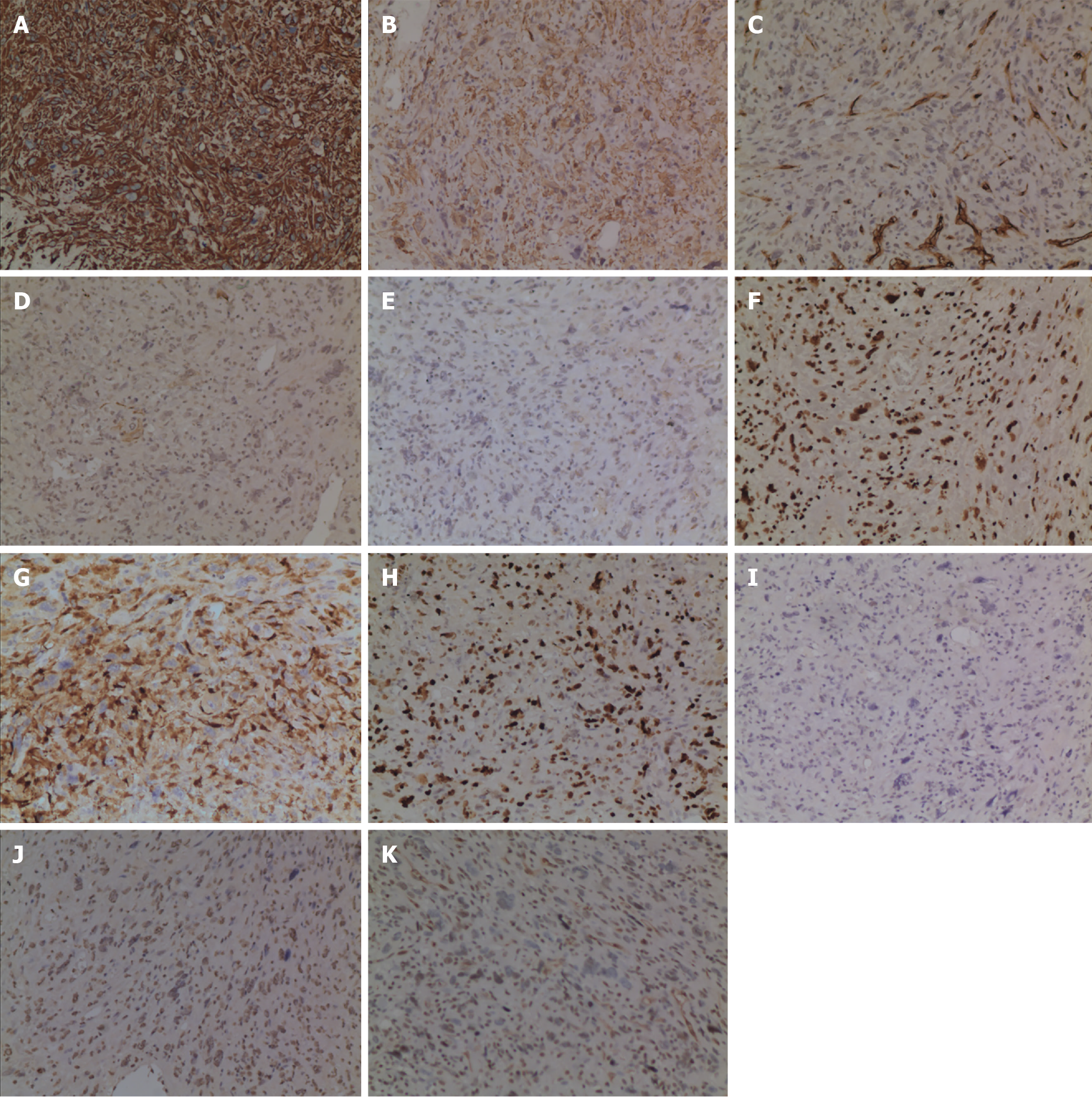
The patient experienced a smooth recovery, with the exception of mild pancreatic leakage and an incisional infection. In situ, the neoplasm was located in the distal CBD, measuring approximately 2.2 cm × 1.8 cm in size, and exhibited clear boundaries (Figure 3). Microscopically, the neoplastic cells presented a spindle-shaped or pleomorphic morphology, arranged in sarciniform structure. They exhibited deeply stained nuclei, singular nuclei with evidence of nuclear division, abundant cytoplasm, and scattered infiltration of inflammatory cells (Figure 4). Immunohistochemically, the cytoplasm of the neoplasm cells was diffuse positive for Vimentin (Figure 5A), partially positive for SMA and CD34 (Figure 5B and C), and scattered positive for CK (AE1/AE3) and EMA (Figure 5D and E). The nuclei of the neoplastic cells were positive for H3K27Me3 (Figure 5F). The cytoplasm and nuclei of the tumor cells were positive for S-100 (Figure 5G). The proportion of Ki-67-positive cells was 60% (Figure 5H). However, the neoplastic cells were negative for CK7, Desmin, and CD117 (Figure 5I-K). Based on these histopathologic features, the neoplasm was eventually diagnosed as a high-grade UPS. The patient refused to undergo follow-up treatment and died 22 months later due to tumor recurrence and metastasis.
The etiology and pathogenesis of UPS remain unclear, but it may be secondary to ionizing radiation, inflammation, surgical trauma, smoking or other cancers[9-12]. Recently, with the advancement of molecular gene technology, some studies have suggested that TP53, RB1, PTEN, and ATRX may play an important role in UPS tumorigenesis[13,14]. In addition to common sites, UPS has also been reported in the liver, pancreas, small intestine, spleen and even the gallbladder in a small amount[15-19]. However, it occurring in the CBD is extremely rare.
UPS lacks special clinical manifestations and imaging characteristics. The diagnosis depends on the differentiation of cell morphology under the microscope and the application of immunohistochemistry. Microscopically, UPS presents as a mixture of spindle-shaped and pleomorphic cells, which can be arranged in a storiform or sarciniform pattern[20,21]. Nuclear division is easily observable, and the presentation may be accompanied by various histiocytic and inflammatory cell infiltrations[21,22]. Immunohistochemically, UPS showed no characteristic markers, most of which expressed Vimentin and CD86[21,23], but not HMB45[18]. P53 Overexpression or Rb1 deficiency was common in UPS[24]. In our case, the tumor cells were spindle-shaped or pleomorphic, arranged in sarciniform structure, with deeply stained nuclei, singular nuclei and nuclear division, abundant cytoplasm, and scattered inflammatory cells infiltration. Vimentin exhibited diffuse positivity, while SMA, CD34, H3K27Me3, and S-100 showed partial positivity. CK (AE1/AE3) and EMA showed scattered positivity, whereas CK7, Desmin, and CD117 were negative. The Ki-67 proliferation index 60%.
UPS should to be distinguished from other well-classified STS. For example, myxiod liposarcoma is composed of vacuolar lipoblast cells with myxiod mesenchyme, slender plexiform capillary structure, and S-100 is often expressed[25]. Additionally, pleomorphic liposarcoma is characterized by a bizarre giant lipoblast and often expresses S-100[26]. Moreover, pleomorphic rhabdomyosarcoma is composed of large pleomorphic rhabdomyoblast, cytoplasmic stripes may be observed, and Desmin exhibited diffuse positive, MyoD1 exhibited positive[27]. Furthermore, in pleomorphic leiomyosarcoma cases, classic leiomyosarcoma areas are commonly observed, and the tumor cells express at least one antigen that symbolizes smooth muscle differentiation, such as SMA, Desmin, and h-caldesmon[28]. Finally, in cases of dedifferentiated liposarcoma morphologically indistinguishable from UPS, we need to look for highly differentiated liposarcoma components. If such components are not identified, the inclusion of the MDM2 fluorescence in situ hybridization (FISH) test is warranted. If the FISH tests show MDM2 gene amplification, dedifferentiated liposarcoma should be considered[29]. Therefore, based on microscopic cytological morphology and immunohistochemical analysis, our case was diagnosed as a high-grade UPS. In recent years, Zhou et al[30] have showed that neurofibromin 1 and its related miRNAs, including hsa-miRNA-34a-5p and hsa-miRNA-199a-3p, may be novel biomarkers for the diagnosis of UPS[30].
The UPS treatment mainly consists of early surgical complete resection and postoperative adjuvant radiotherapy. Doxorubicin and/or ifosfamide first-line chemotherapy can be used for patients with UPS who cannot undergo resection or present metastasis[31]. However, the overall efficacy of this treatment approach is not satisfactory. Although the anti-angiogenic small-molecule targeted drug pazopanib was approved by the United States Food and Drug Administration in April 2012 for second-line treatment in patients with advanced STS (excluding liposarcoma and gastrointestinal stromal tumor) and has shown some efficacy and tolerability, there are no a standard guideline[32,33]. Recent immune checkpoint inhibitors (ICIs) have been identified as a treatment for many solid cancers. However, there is limited evidence on the efficacy of ICIs in UPS, mainly comprising small studies[34,35]. Currently, no ICIs are approved for STS treatment. In our case, the patient underwent radical surgical resection, but refused to undergo subsequent treatment, such as radiotherapy and chemotherapy, and eventually died of tumor recurrence and metastasis, with an overall survival period of 22 months.
UPS in CBD is extremely rare. Recognition of this type of neoplasm in the CBD is of great significance for increasing physicians' vigilance, reducing the odds of misdiagnosis, and providing appropriate treatment strategies to improve the prognosis of patients.
We wish to acknowledge Quan Zhou (Department of Pathology, The Second Affiliated Hospital of Jiaxing University, Jiaxing) for his support in pathology of this case, and Xin Wang (Department of Radiology, The Second Affiliated Hospital of Jiaxing University, Jiaxing) for her interpretations in radiology of this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lamichane SR, Nepal S-Editor: Li L L-Editor: A P-Editor: Li X
| 1. | Matushansky I, Charytonowicz E, Mills J, Siddiqi S, Hricik T, Cordon-Cardo C. MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev Anticancer Ther. 2009;9:1135-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250-2266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 3. | Mangham DC. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. J Bone Joint Surg Br. 2004;86-B:466-466. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 4. | Miller DD, Gupta A. Histopathology of vascular anomalies: update based on the revised 2014 ISSVA classification. Semin Cutan Med Surg. 2016;35:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Zhang S, Zhang X, Zhao Z, Xu L, Xu S, Liu T, Yu S. Undifferentiated pleomorphic sarcoma of the extremity and trunk: a retrospective cohort study of 166 cases in a large institution. Transl Cancer Res. 2022;11:678-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Sharma A, Vyas P, Agarwal D. Undifferentiated pleomorphic sarcoma of the floor of mouth: A rare case. J Oral Maxillofac Pathol. 2023;27:S33-S37. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 7. | Shahrokh S, Shahin M, Malboosbaf R, Shayanfar N. Primary Undifferentiated Pleomorphic Thyroid Sarcoma Presenting as Superior Vena Cava Syndrome: A Case Report. Cureus. 2021;13:e20104. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 8. | Fuu T, Yano A, Urakami S. Undifferentiated pleomorphic sarcoma of the retroperitoneum mimicking a cortisol- and catecholamine-secreting adrenal tumor. IJU Case Rep. 2022;5:195-198. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Inchaustegui ML, Kon-Liao K, Ruiz-Arellanos K, Silva GAE, Gonzalez MR, Pretell-Mazzini J. Treatment and Outcomes of Radiation-Induced Soft Tissue Sarcomas of the Extremities and Trunk-A Systematic Review of the Literature. Cancers (Basel). 2023;15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Tepper SC, Lee L, Fice MP, Jones CM, Klein ED, Vijayakumar G, Batus M, Colman MW, Gitelis S, Blank AT. Association between neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and survival in undifferentiated pleomorphic sarcoma (NLR, PLR, and overall survival in UPS). Surg Oncol. 2023;49:101949. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Kinoshita H, Tsukanishi T, Ishii T, Kamoda H, Hagiwara Y, Orita S, Inage K, Hirosawa N, Ohtori S, Yonemoto T. Giant Protruding High-Grade Undifferentiated Pleomorphic Sarcoma Arising in a Keloid Scar on the Abdominal Wall. Case Rep Dermatol Med. 2020;2020:4898965. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 12. | Araki Y, Yamamoto N, Tanzawa Y, Higashi T, Kuchiba A, Hayashi K, Takeuchi A, Miwa S, Igarashi K, Endo M, Kobayashi E, Tsuchiya H, Kawai A. Family cancer history and smoking habit associated with sarcoma in a Japanese population study. Sci Rep. 2022;12:17129. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Li GZ, Okada T, Kim YM, Agaram NP, Sanchez-Vega F, Shen Y, Tsubokawa N, Rios J, Martin AS, Dickson MA, Qin LX, Socci ND, Singer S. Rb and p53-Deficient Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma Require Skp2 for Survival. Cancer Res. 2020;80:2461-2471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Steele CD, Tarabichi M, Oukrif D, Webster AP, Ye H, Fittall M, Lombard P, Martincorena I, Tarpey PS, Collord G, Haase K, Strauss SJ, Berisha F, Vaikkinen H, Dhami P, Jansen M, Behjati S, Amary MF, Tirabosco R, Feber A, Campbell PJ, Alexandrov LB, Van Loo P, Flanagan AM, Pillay N. Undifferentiated Sarcomas Develop through Distinct Evolutionary Pathways. Cancer Cell. 2019;35:441-456.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Wang Z, Xiao Y, Zhang B, Xiaosong L, Shao J, Liao J. Ultrasonographic diagnosis of primary hepatic undifferentiated pleomorphic sarcoma. J Clin Ultrasound. 2023;51:169-176. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 16. | Patel MH, Guerrero Vinsard D, Agrawal U, Kendziora RW, Siontis BL, Swaroop Vege S, Sweetser S. Primary Pancreatic Undifferentiated Pleomorphic Sarcoma. ACG Case Rep J. 2023;10:e01011. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Song SO, Kim MS, Lee KH, Choi SJ. [Undifferentiated Pleomorphic Sarcoma of the Small Intestine with Distant Endobronchial Metastasis Presenting as Intussusception: A Case Report]. Taehan Yongsang Uihakhoe Chi. 2021;82:1304-1309. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | Gatt R, Casingena L, Pisani D, Agius R, Cassar N. Undifferentiated pleomorphic sarcoma of the spleen: a case report and literature review. Surg Case Rep. 2023;9:166. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 19. | Badmos KB, Salah Seada L, Fahad Al Rashid F, Oreiby HA. Undifferentiated spindle-cell carcinoma of the gallbladder: a report of a case, an immunohistochemistry profile, and a review of the literature. Case Rep Pathol. 2013;2013:267194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Almalki W, Alzahrani M, Alssaqqaf I, Baker B. A case of undifferentiated pleomorphic sarcoma of a retro-gastric origin, case report and review of literature. Int J Surg Case Rep. 2021;89:106555. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 21. | Guo H, Xiong Y, Nong L, Zhang S, Li T. [Reassessment of the pathological diagnosis in 33 cases of malignant fibrous histiocytoma]. Beijing Da Xue Xue Bao Yi Xue Ban. 2008;40:374-379. [PubMed] [Cited in This Article: ] |
| 22. | Yoshimoto M, Yamada Y, Ishihara S, Kohashi K, Toda Y, Ito Y, Yamamoto H, Furue M, Nakashima Y, Oda Y. Comparative Study of Myxofibrosarcoma With Undifferentiated Pleomorphic Sarcoma: Histopathologic and Clinicopathologic Review. Am J Surg Pathol. 2020;44:87-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Sarode SC, Sarode GS, Ingale Y, Ingale M, Raj AT, Patil S. Malignant fibrous histiocytoma of the mandible - A case report and review of published case reports. J Oral Biol Craniofac Res. 2019;9:221-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Cui Y, Han L, Shang J, Fang W, Zhao M, Chen D, Liu H. Primary cardiac undifferentiated pleomorphic sarcoma is associated with TP53 mutation during lack of MDM2 amplification, and targeted sequencing analysis reveals potentially actionable targets. Hum Pathol. 2022;123:113-122. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 25. | Kojima N, Kubo T, Mori T, Satomi K, Matsushita Y, Iwata S, Yatabe Y, Ichimura K, Kawai A, Ichikawa H, Yoshida A. Myxoid liposarcoma with nuclear pleomorphism: a clinicopathological and molecular study. Virchows Arch. 2024;484:71-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Farah S, Haythem M, Ameni A, Samia H, Slim H, Mahmoud S. Primary pleomorphic liposarcoma of bone: A case report with literature review. Int J Surg Case Rep. 2023;109:108584. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 27. | Deb PQ, Chokshi RJ, Li S, Suster DI. Pleomorphic Rhabdomyosarcoma: A Systematic Review with Outcome Analysis and Report of a Rare Abdominal Wall Lesion. Int J Surg Pathol. 2023;31:548-556. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 28. | Shimada Y, Naito T, Hayashi T, Saito T, Suehara Y, Kakinuma C, Nozaki Y, Takagi H, Yao T. Establishment of a patient-derived xenograft mouse model of pleomorphic leiomyosarcoma. J Toxicol Pathol. 2021;34:89-93. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Zhou S, Zhang C, Zhang Z, Hu Y, Zhao L, Hu W, Chen S, Li B, Xiao S. A novel HMGA2::KITLG fusion in a dedifferentiated liposarcoma with amplification of MDM2 and HMGA2. Genes Chromosomes Cancer. 2024;63:e23200. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 30. | Zhang P, Huang L, Ma P, Niu X. Altered Expressions of NF1 and NF1-Related microRNAs as Biomarkers in the Diagnosis of Undifferentiated Pleomorphic Sarcoma. Front Genet. 2022;13:870191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Goy BW, Syed S, Padmanabhan A, Burchette RJ, Helmstedter CS. The role of Ifosfamide-doxorubicin chemotherapy in histology-specific, high grade, locally advanced soft tissue sarcoma, a 14-year experience. Radiother Oncol. 2021;165:174-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 32. | Bilici A, Koca S, Karaagac M, Aydin SG, Eraslan E, Kaplan MA, Ocak B, Goksu SS, Paydas S, Akgul F, Derin S, Ergun Y, Yekeduz E, Erol C, Ozyukseler DT, Demiray AG, Karaca M, Guc ZG, Menekse S, Cinkir HY, Gumusay O, Sakin A, Ozkul O, Demir H, Erdem D, Besiroglu M, Unal OU, Acar R, Koral L, Sahin S, Sakalar T, Bahceci A, Ozveren A, Gunaydin UM, Seker MM, Sunar V, Dal P, Artac M, Turhal S. Real-world outcomes of pazopanib in metastatic soft tissue sarcoma: a retrospective Turkish oncology group (TOG) study. J Cancer Res Clin Oncol. 2023;149:8243-8253. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 33. | Oh CR, Hong JY, Kim JH, Lee JS, Kim HS, Kim TW, Ahn JH, Kim JE. Real-World Outcomes of Pazopanib Treatment in Korean Patients with Advanced Soft Tissue Sarcoma: A Multicenter Retrospective Cohort Study. Target Oncol. 2020;15:485-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | You Y, Guo X, Zhuang R, Zhang C, Wang Z, Shen F, Wang Y, Liu W, Zhang Y, Lu W, Hou Y, Wang J, Zhang X, Lu M, Zhou Y. Activity of PD-1 Inhibitor Combined With Anti-Angiogenic Therapy in Advanced Sarcoma: A Single-Center Retrospective Analysis. Front Mol Biosci. 2021;8:747650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Liu J, Fan Z, Bai C, Li S, Xue R, Gao T, Zhang L, Tan Z, Fang Z. Real-world experience with pembrolizumab in patients with advanced soft tissue sarcoma. Ann Transl Med. 2021;9:339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |