Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.2181
Peer-review started: November 24, 2023
First decision: January 19, 2024
Revised: February 11, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: May 15, 2024
Recent reviews have outlined the main nanomaterials used in relation to gastrointestinal tumors and described the basic properties of these materials. However, the research hotspots and trends in the application of nanomaterials in gastric cancer (GC) remain obscure.
To demonstrate the knowledge structure and evolutionary trends of research into the application of nanomaterials in GC.
Publications related to the application of nanomaterials in GC were retrieved from the Web of Science Core Collection for this systematic review and bibliometric study. VOSviewer and CiteSpace were used for bibliometric and visualization analyses.
From 2000 to 2022, the application of nanomaterials in GC developed rapidly. The keyword co-occurrence analysis showed that the related research topics were divided into three clusters: (1) The application of nanomaterials in GC treatment; (2) The application and toxicity of nanomaterials in GC diagnosis; and (3) The effects of nanomaterials on the biological behavior of GC cells. Complexes, silver nanoparticles, and green synthesis are the latest high-frequency keywords that represent promising future research directions.
The application of nanomaterials in GC diagnosis and treatment and the mechanisms of their effects on GC cells have been major themes in this field over the past 23 years.
Core Tip: From 2000 to 2022, the application of nanomaterials in gastric cancer (GC) developed rapidly. The keywords co-occurrence analysis showed that the related research topics were divided into three clusters: (1) The application of nanomaterials in GC treatment; (2) The application and toxicity of nanomaterials in GC diagnosis; and (3) The effect of nanomaterials on the biological behavior of GC cells. Complexes, silver nanoparticles, and green synthesis were the latest high-frequency keywords that represent promising future research directions. Thus, the application of nanomaterials in GC diagnosis and treatment; and the mechanisms of their effects on GC cells have been major themes in this field over the past 23 years.
- Citation: Liu BN, Gao XL, Piao Y. Mapping the intellectual structure and emerging trends for the application of nanomaterials in gastric cancer: A bibliometric study. World J Gastrointest Oncol 2024; 16(5): 2181-2199
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/2181.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.2181
In recent years, accompanied by the development of nanotechnology, great strides have been made in nanomedicine. Nanomaterials have special optical, physicochemical, biological, and pharmacological properties. Compared with other materials, nanomaterials have smaller sizes and larger surface areas, enabling them to better retain or control the release of drugs and prevent their early degradation or metabolism[1]. In addition, the combination of nanomaterials with various types of biomaterials may reduce cytotoxicity and lead to enhanced biocompatibility. Applications of nanomaterials in cancer include use in drug delivery systems, photothermal therapy, targeted imaging, and cancer detection[2-4]. Various nanomaterials have been developed with good potential for cancer diagnosis and treatment.
Gastric cancer (GC) is the fifth most common cancer and ranks as the third leading cause of cancer-related death worldwide[5,6]. Due to the highly heterogeneous nature of GC and the strongly acidic environment in the stomach, the diagnosis and treatment of GC face immense challenges, and the unique properties of nanomaterials allow them to function effectively and play multiple roles in the harsh stomach environment. Indeed, numerous studies have shown that nanomaterials hold tremendous promise for several applications in GC, such as the direct use of nanomaterials as therapeutic agents, adoption of nanomaterials as diagnostic imaging materials, and development of nanomaterial-based drug delivery systems to enhance drug targeting, improve drug efficacy, and reduce toxic adverse effects. Recent reviews have outlined the main nanomaterials used for gastrointestinal tumors and described the basic properties of these materials. However, the research hotspots and trends in the application of nanomaterials in GC remain obscure. In the present study, we use different bibliometric tools to demonstrate the knowledge structure and evolutionary trends of research into the application of nanomaterials in GC and comprehensively summarize the application forms and related mechanisms. We also identify the classic nanomaterials that used to be the research focus, as well as the novel nanomaterials that have recently become the hotspots in the diagnosis and treatment of GC. This study comprises a review with a comprehensive and systematic analysis and also includes a bibliometric and visualization study. Our results will help practitioners and researchers to develop a comprehensive understanding of the research hotspots, determine new topics, and identify research directions in this area.
According to previous research[7], the Web of Science Core Collection (WoSCC) is one of the most authoritative and suitable academic databases for bibliometric analysis; therefore, we chose WoSCC as the data source. All publications and relevant information were downloaded in “plain text” format on a single day (December 31, 2022).
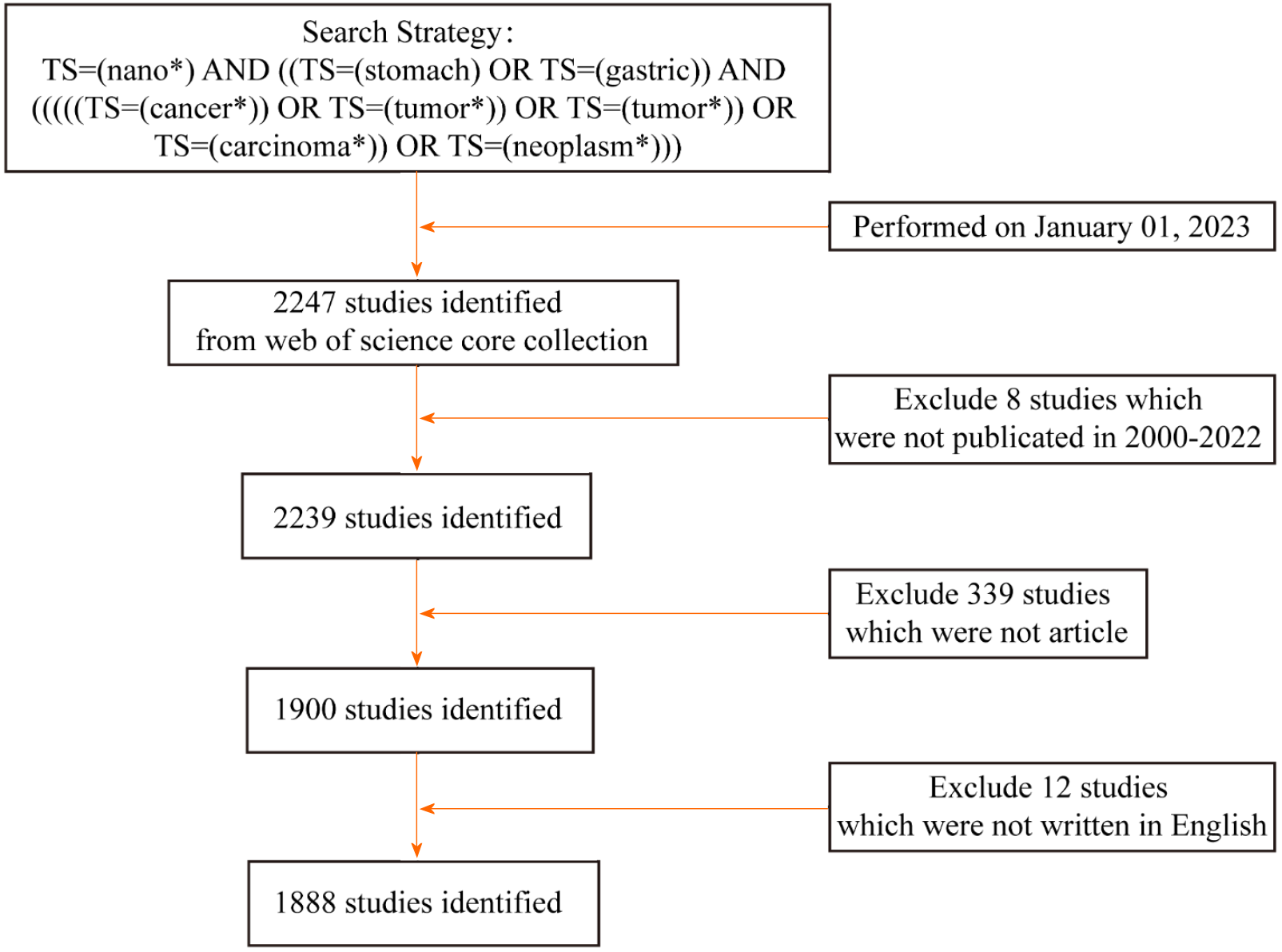
A flowchart of the retrieval strategy and articles selected for bibliometric analysis is depicted in Figure 1. Concretely, the following criteria had to be fulfilled: (1) The retrieval strategy was performed as topic search (TS)=(nano*) AND ((TS=(stomach) OR TS=(gastric)) AND (((((TS=(cancer*)) OR TS=(tumor*)) OR TS=(tumor*)) OR TS=(carcinoma*)) OR TS=(neoplasm*))); (2) The publication type was limited to "article"; (3) The publication dates were from January 1, 2000 to December 31, 2022; and (4) The publication language was set to English only.
VOSviewer, CiteSpace, R Programming Language, and GraphPad Prism were used for bibliometric analysis and visualization of publications retrieved from WoSCC. VOSviewer is literature knowledge visualization software based on similarity visualization techniques that produces better structured mappings than other commonly used bibliometric software[8]. In this study, we used VOSviewer to perform the co-occurrence analysis of the application of nanomaterials in GC to create collaborative network maps among countries, institutions, and authors, as well as a keyword network visualization map and overlay visualization map.
CiteSpace is another characteristic and influential bibliometric analysis visualization software that can reveal the research hotspots and development processes and detect recent emerging trends[9]. In this study, we analyzed and visualized the application of nanomaterials in GC using CiteSpace software to reveal the knowledge structure of the field and to discover the latest research trends.
The Impact Index Per Article values for the 10 most cited papers were obtained from Reference Citation Analysis (RCA, https://www.referencecitationanalysis.com). RCA is an open citation analysis database that spans diverse fields and is owned by Baishideng Publishing Group Inc (Pleasanton, CA)[10-12].
In addition, R Programming Language (R version 4.2.0) and GraphPad Prism (GraphPad Software 9.0) were used for statistical analysis, such as for analysis of the distribution of publications in time and space.
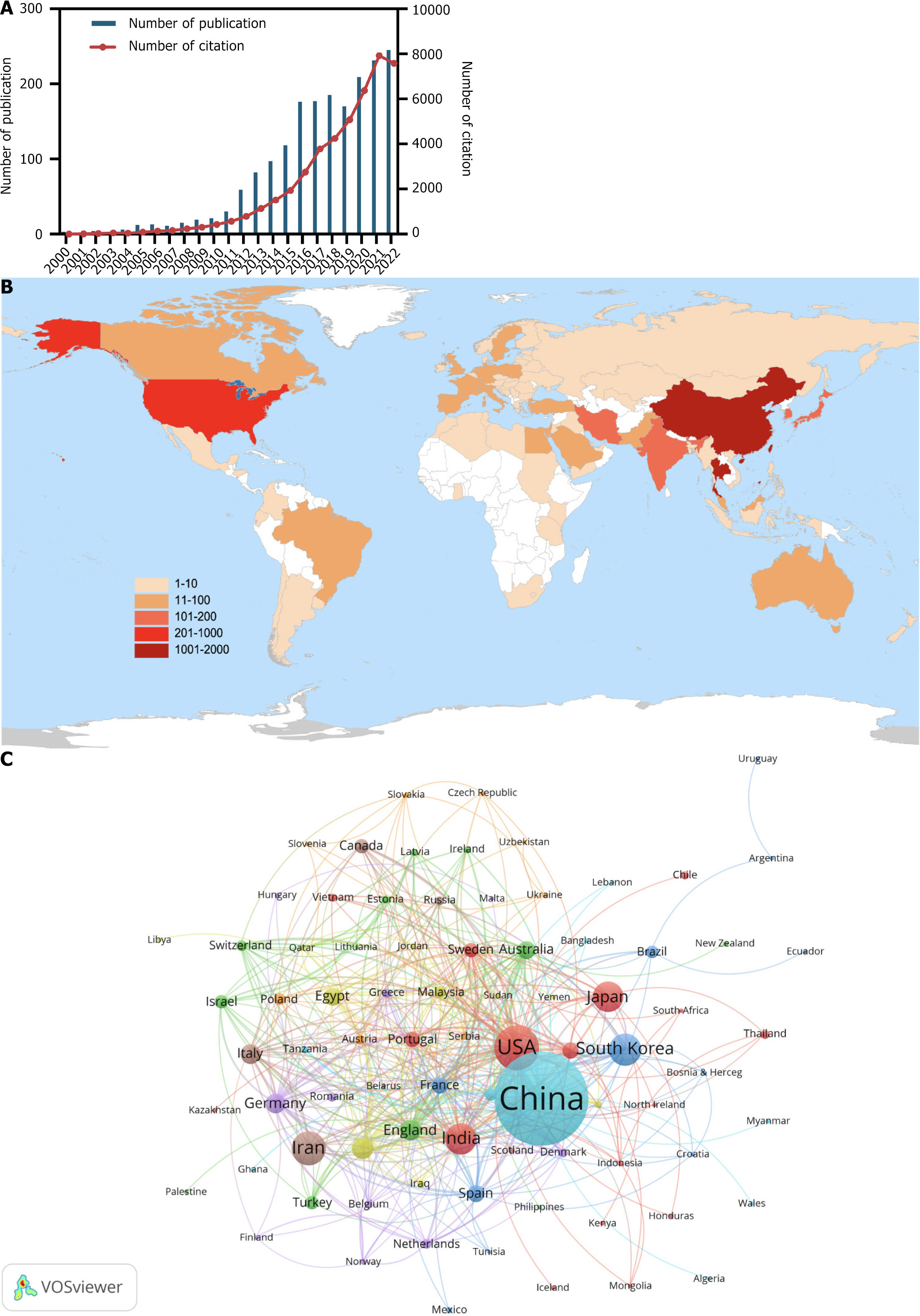
In total, 1888 articles on the application of nanomaterials in GC from 2000 to 2022 were collected from WoSCC. The annual publication trend is shown in Figure 2A. Over the past 23 years, interest in research into nanomaterial applications in GC has skyrocketed. Global annual publications have increased from 3 in 2000 to 245 in 2022. Specifically, the annual publication volume of related studies was less than 50 from 2000 to 2011 and then steadily increased from 59 to 118 between 2012 and 2015. From 2016 to 2022, the annual publication volume peaked at more than 160. In addition, annual citations have been on an increasing trend over the years.
Both GC and nanomaterials are subjects of worldwide interest. A total of 86 countries/regions have made contributions to research into the application of nanomaterials in GC (Figure 2B). The 10 most productive countries are listed in Table 1. In terms of the countries involved in the research area, China (1007 papers, 25279 citations) had the largest number of publications, followed by the United States (232 papers, 7621 citations) and Iran (135 papers, 1740 citations). In this study, a co-authorship analysis between countries was conducted using VOSviewer to visualize the international collaboration relationship (Figure 2C). The collaborative network, which included 83 of 86 countries, was split into eight clusters symbolized as various colors. The United States (n = 46), China (n = 41), and England (n = 36) were the three countries with the most partners.
| Rank | Country | Publications | Citations |
| 1 | China | 1007 | 25279 |
| 2 | USA | 232 | 7621 |
| 3 | Iran | 135 | 1740 |
| 4 | South Korea | 121 | 2733 |
| 5 | India | 113 | 2072 |
| 6 | Japan | 106 | 3605 |
| 7 | Saudi Arabia | 53 | 627 |
| 8 | England | 50 | 1753 |
| 9 | Germany | 47 | 1501 |
| 10 | Italy | 46 | 1200 |
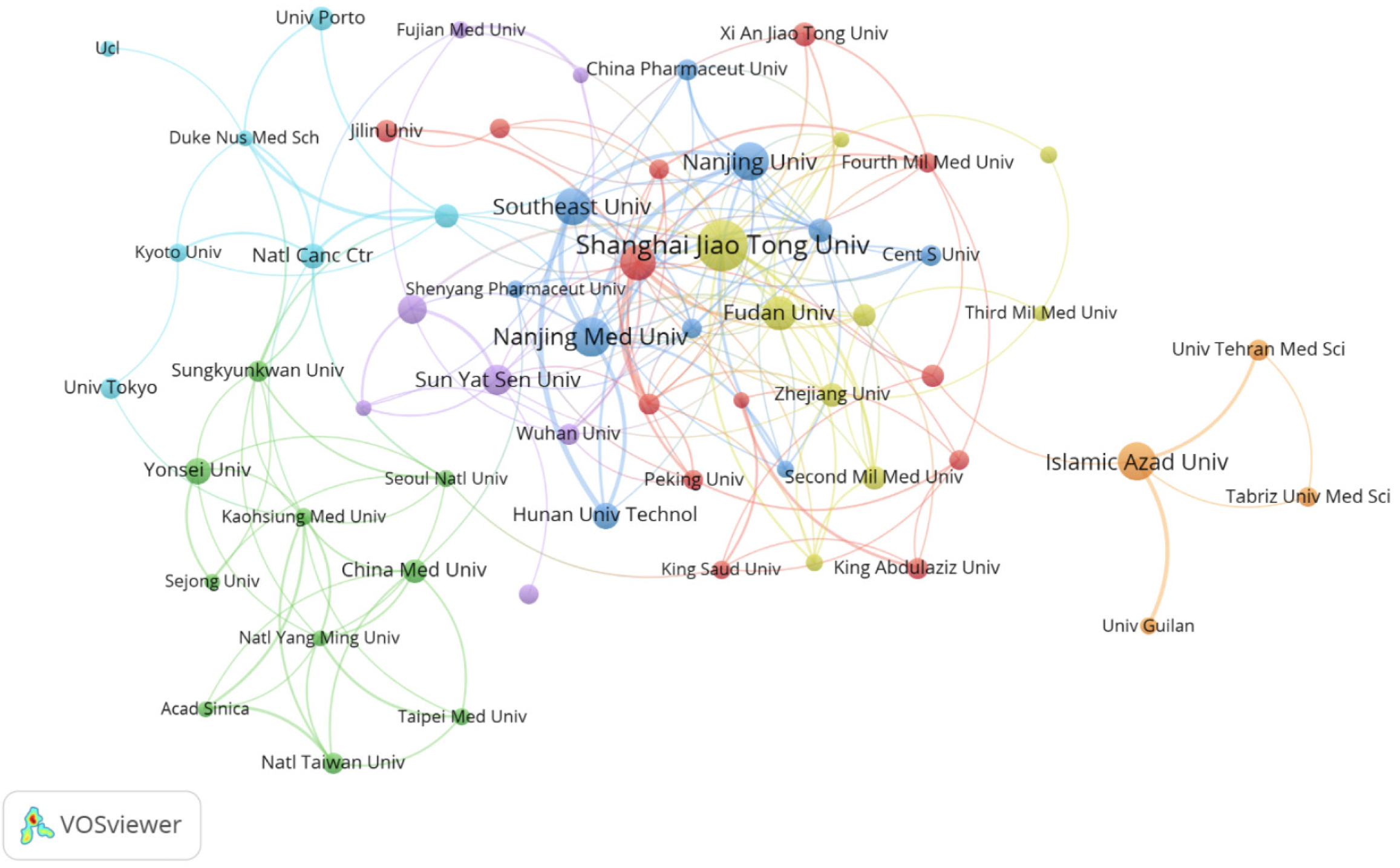
Globally, 2353 institutions have been involved in research into the application of nanomaterials in GC. The 10 most productive institutions are listed in Table 2. Shanghai Jiao Tong University (94 publications, 3981 citations) had the highest productivity, followed by Nanjing Medical University (59 publications, 1273 citations) and Nanjing University (55 publications, 1589 citations). When the minimum number of papers published by an institution was set at 10, 60 institutions met the criteria. These 60 institutions were then analyzed for co-authorship using VOSviewer, and the results are shown in Figure 3. The collaboration network comprised seven clusters, indicated by different colors, with Shanghai Jiao Tong University (n = 21), Chinese Academy of Sciences (n = 17), and Fudan University (n = 15) located at the center and having the largest number of partners.
| Rank | Institution | Country | Publications | Citations |
| 1 | Shanghai Jiao Tong University | China | 94 | 3981 |
| 2 | Nanjing Medical University | China | 59 | 1273 |
| 3 | Nanjing University | China | 55 | 1589 |
| 4 | Islamic Azad University | Iran | 55 | 474 |
| 5 | Southeast University | China | 49 | 2020 |
| 6 | Chinese Academy of Sciences | China | 47 | 1368 |
| 7 | Fudan University | China | 44 | 856 |
| 8 | Sun Yat-Sen University | China | 34 | 671 |
| 9 | Southern Medical University | China | 30 | 576 |
| 10 | Yonsei University | South Korea | 27 | 634 |
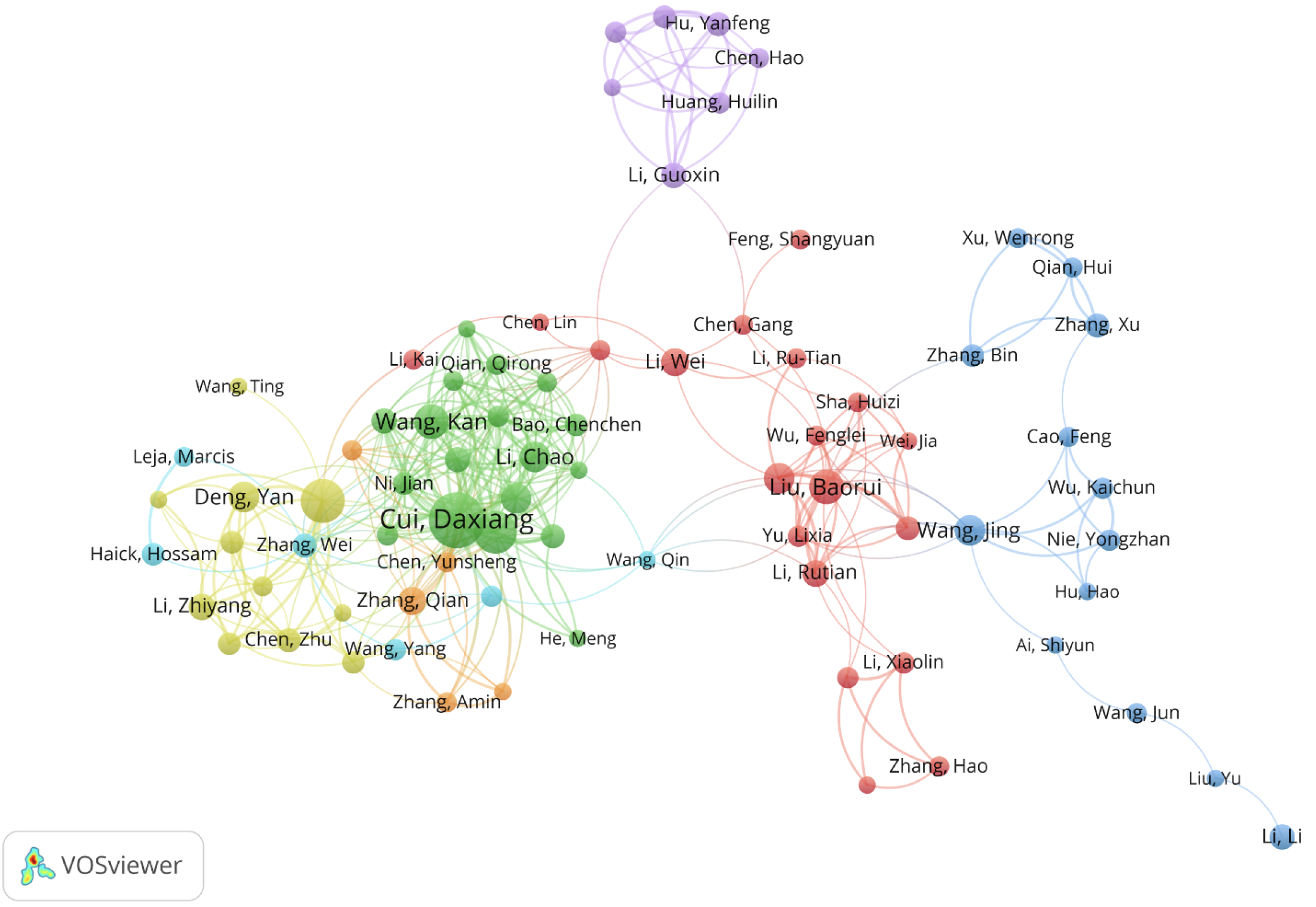
A total of 11209 authors have published in the field of nanomaterials in GC from 2000 to 2022. The 20 most productive authors are listed in Table 3. Da-Xiang Cui (47 publications, 2693 citations) published the most articles, followed by Nong-Yue He (29 publications, 1434 citations) and Chun-Lei Zhang (27 publications, 1915 citations). A co-authorship analysis was performed using VOSviewer for the current research area. When the minimum number of published articles was set to 5136 authors meet the threshold, and VOSviewer was used to establish the co-authorship network diagram (Figure 4). A total of 79 authors were split into seven clusters indicated by various colors. Among them, Da-Xiang Cui (n = 31), Chun-Lei Zhang (n = 26), Kan Wang (n = 22), and Jian Ni (n = 22) had the most partners.
| Rank | Author | Total publications | Total citations | Per citations |
| 1 | Da-Xiang Cui | 47 | 2693 | 57 |
| 2 | Nong-Yue He | 29 | 1434 | 49 |
| 3 | Chun-Lei Zhang | 27 | 1915 | 71 |
| 4 | Kan Wang | 19 | 1137 | 60 |
| 5 | Bao-Rui Liu | 19 | 447 | 24 |
| 6 | Chao Li | 15 | 731 | 49 |
| 7 | Qin Liu | 15 | 277 | 18 |
| 8 | Yan Deng | 14 | 1451 | 104 |
| 9 | Guo Gao | 14 | 1070 | 76 |
| 10 | Jing Wang | 14 | 235 | 17 |
| 11 | Qian Zhang | 12 | 400 | 33 |
| 12 | Wei Li | 12 | 166 | 14 |
| 13 | Ali Salehzadeh | 12 | 44 | 4 |
| 14 | Zhi-Yang Li | 11 | 375 | 34 |
| 15 | Rutian Li | 11 | 297 | 27 |
| 16 | Yong-Min Huh | 11 | 292 | 27 |
| 17 | Seungjoo Haam | 11 | 265 | 24 |
| 18 | Xiao Zhi | 10 | 583 | 58 |
| 19 | Fei Pan | 10 | 533 | 53 |
| 20 | Li Li | 10 | 160 | 16 |
From 2000 to 2022, articles on the application of nanomaterials in GC were published in 588 journals. Table 4 lists the 10 most productive journals, all of which have more than 20 publications. The most prolific journal was International Journal of Nanomedicine (64 publications), while the most cited journal was Biomaterials (2078 citations). Table 5 lists the articles with the top 10 citations, all of which have more than 210 citations. The most cited article was “Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of transforming growth factor β (TGF-β) signaling” by Kano et al[13] (345 citations), published in Proceedings of the National Academy of Sciences in 2007. In addition, according to RCA, the Impact Index Per Article values of the five most cited papers were significantly higher than those of the other publications.
| Rank | Journal | IF (2021) | Total publications | Total citations |
| 1 | International Journal of Nanomedicine | 7.033 | 64 | 2026 |
| 2 | Journal of Nanoscience and Nanotechnology | / | 44 | 746 |
| 3 | Journal of Biomedical Nanotechnology | 3.641 | 33 | 994 |
| 4 | Nanoscience and Nanotechnology Letters | / | 30 | 273 |
| 5 | International Journal of Pharmaceutics | 6.51 | 28 | 649 |
| 6 | Biomaterials | 15.304 | 28 | 2078 |
| 7 | International Journal of Biological Macromolecules | 8.025 | 25 | 535 |
| 8 | Scientific Reports | 4.997 | 24 | 720 |
| 9 | ACS Applied Materials & Interfaces | 10.383 | 23 | 636 |
| 10 | RSC Advances | 4.036 | 22 | 399 |
| Rank | Title | Journal | Impact Index Per Article | Citations | Average Citations | Publication year | Ref. |
| 1 | Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling | Proceedings of the National Academy of Sciences | 22.9 | 345 | 22 | 2007 | [13] |
| 2 | Folic acid-conjugated Silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy | Biomaterials | 26.7 | 333 | 28 | 2011 | [79] |
| 3 | Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules | ACS Nano | 35.7 | 285 | 48 | 2017 | [80] |
| 4 | Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression | Journal of Cancer Research and Clinical Oncology | 33.6 | 225 | 38 | 2017 | [81] |
| 5 | Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy | Biomaterials | 16.5 | 222 | 19 | 2011 | [82] |
| 6 | A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation | British Journal of Cancer | 13.1 | 219 | 14 | 2007 | [83] |
| 7 | Defined factors induce reprogramming of gastrointestinal cancer cells | Proceedings of the National Academy of Sciences | 15.6 | 217 | 17 | 2010 | [84] |
| 8 | Synthesis of novel biodegradable and self-assembling methoxy poly (ethylene glycol)–palmitate nanocarrier for curcumin delivery to cancer cells | Acta Biomaterialia | 11.3 | 196 | 13 | 2008 | [85] |
| 9 | Ultrasensitive Silicon Nanowire for Real-World Gas Sensing: Noninvasive Diagnosis of Cancer from Breath Volatolome | Nano Letters | 12.9 | 172 | 22 | 2015 | [86] |
| 10 | Gastric cancer detection based on blood plasma surface-enhanced Raman spectroscopy excited by polarized laser light | Biosensors and Bioelectronics | 12.4 | 171 | 14 | 2011 | [56] |
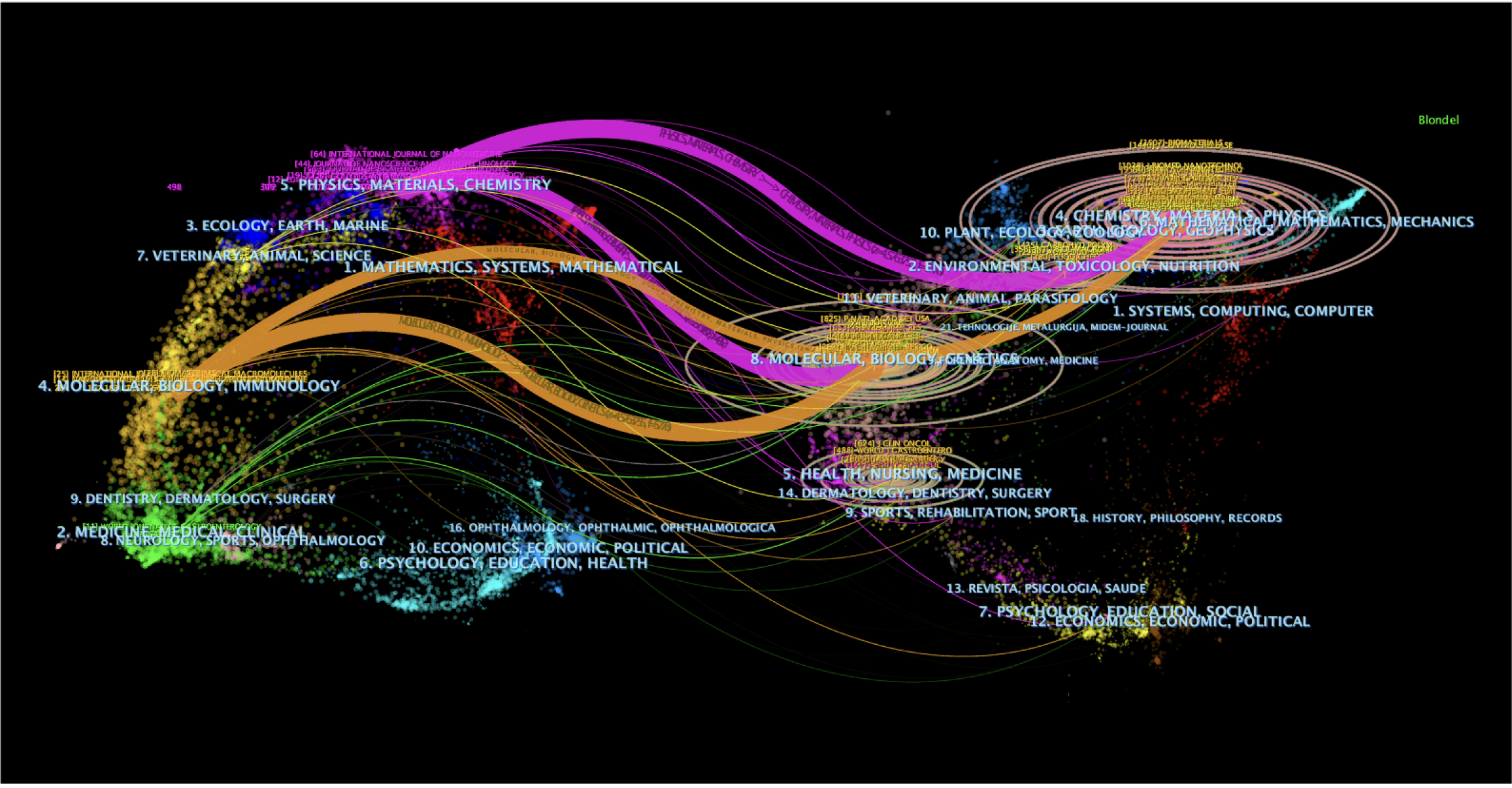
The citation status among journals was analyzed using CiteSpace and has been presented as a dual-map overlay of journals (Figure 5). The left half represents the literature published in journals, the right half represents the source journals in which those published studies were cited, and the curves from left to right are the citation path connection lines, which show the knowledge flow and connection in different research fields. As shown in Figure 5, the research into nanomaterials in GC is mainly distributed in the disciplines of Materials, Chemistry, Physics, Immunology, Biology, and Molecular, and the cited literature is also mainly from these subjects.
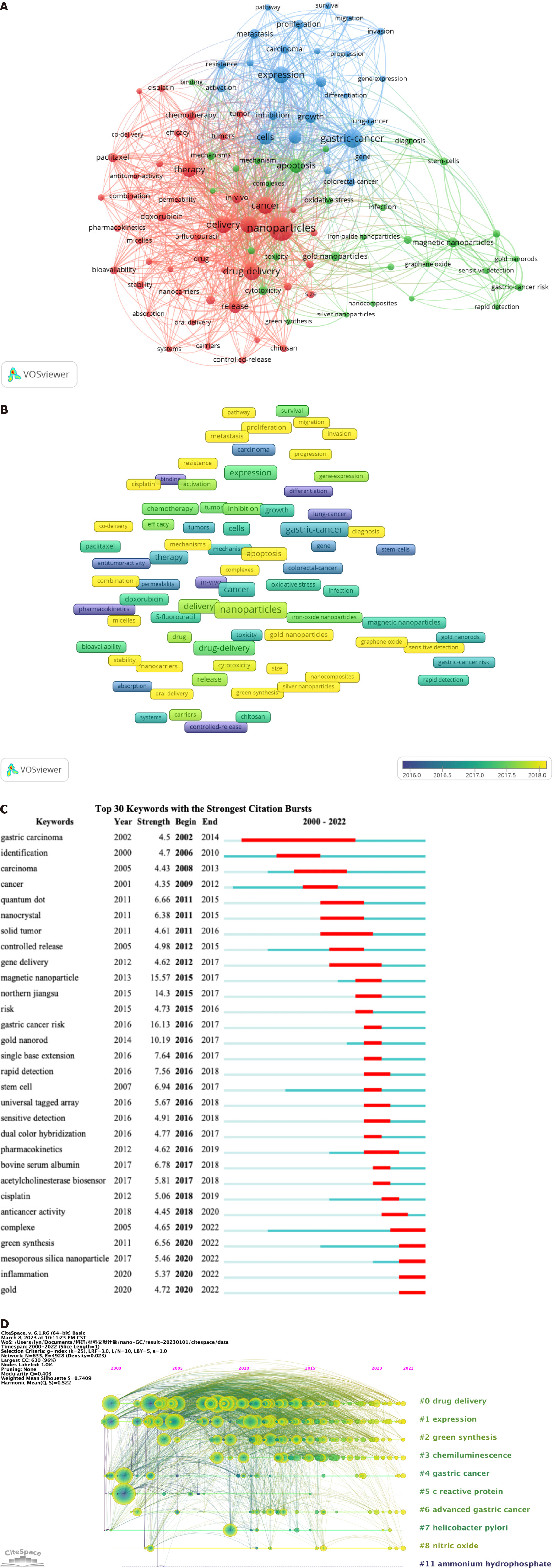
The primary subjects of publications can be covered by keywords; hence, keywords with high frequency are appropriate for bibliometric analysis. In the current study, VOSviewer was used for the extraction and clustering of the top 100 keywords in terms of occurrence frequency, as shown in Table 6 and Figure 6A. Based on the degree of similarity, VOSviewer automatically classified the keywords into three clusters, which are shown in red (cluster 1), green (cluster 2), and blue (cluster 3). Cluster 1 represented the application of nanomaterials in GC treatment and comprised 45 keywords; the central keywords were nanoparticles (n = 389), in vitro (n = 182), and drug delivery (n = 193). Cluster 2 represented the application and toxicity of nanomaterials in GC diagnosis and contained 30 keywords, such as apoptosis (n = 124), magnetic nanoparticles (n = 66), and cytotoxicity (n = 54). Cluster 3 represented the effects of nanomaterials on the biological behavior of GC cells and contained 25 keywords, such as GC (n = 219), expression (n = 190), and cells (n = 142).
| Cluster | Keywords | Counts | Rank | Cluster | Keywords | Counts | Rank |
| 1 | Nanoparticles | 389 | 1 | 2 | DNA | 42 | 35 |
| 1 | Drug-delivery | 193 | 3 | 2 | Stem-cells | 42 | 36 |
| 1 | Cancer | 187 | 5 | 2 | Northern jiangsu | 41 | 40 |
| 1 | Delivery | 182 | 6 | 2 | Toxicity | 40 | 43 |
| 1 | In-vitro | 182 | 7 | 2 | Gastric-cancer risk | 39 | 44 |
| 1 | Therapy | 134 | 9 | 2 | Diagnosis | 35 | 53 |
| 1 | Release | 104 | 11 | 2 | Antioxidant | 34 | 55 |
| 1 | In-vivo | 76 | 14 | 2 | Oxidative stress | 33 | 61 |
| 1 | Chemotherapy | 65 | 17 | 2 | Mechanism | 32 | 63 |
| 1 | Doxorubicin | 64 | 18 | 2 | Quantum dots | 32 | 64 |
| 1 | Paclitaxel | 55 | 23 | 2 | Infection | 32 | 65 |
| 1 | Design | 49 | 27 | 2 | Complexes | 30 | 70 |
| 1 | Stability | 48 | 28 | 2 | Graphene oxide | 29 | 72 |
| 1 | Bioavailability | 44 | 30 | 2 | Model | 28 | 74 |
| 1 | Drug | 44 | 31 | 2 | Proteins | 28 | 75 |
| 1 | Combination | 41 | 38 | 2 | Binding | 27 | 77 |
| 1 | Acid | 41 | 39 | 2 | Gold nanorods | 27 | 78 |
| 1 | System | 40 | 41 | 2 | Sensitive detection | 26 | 81 |
| 1 | Tumor | 40 | 42 | 2 | Iron-oxide nanoparticles | 25 | 87 |
| 1 | Efficacy | 38 | 45 | 2 | Silver nanoparticles | 25 | 88 |
| 1 | Tumors | 37 | 47 | 2 | Induction | 24 | 91 |
| 1 | 5-fluorouracil | 36 | 48 | 2 | Rapid detection | 23 | 95 |
| 1 | Chitosan | 36 | 49 | 2 | Nanocomposites | 23 | 96 |
| 1 | Size | 36 | 50 | 2 | Biomedical applications | 22 | 98 |
| 1 | Controlled-release | 35 | 52 | 2 | Green synthesis | 22 | 99 |
| 1 | Micelles | 34 | 54 | 3 | Gastric-cancer | 219 | 2 |
| 1 | Cisplatin | 33 | 57 | 3 | Expression | 190 | 4 |
| 1 | Nanocarriers | 33 | 58 | 3 | Cells | 142 | 8 |
| 1 | Pharmacokinetics | 33 | 59 | 3 | Breast-cancer | 104 | 12 |
| 1 | Cancer-cells | 33 | 60 | 3 | Growth | 90 | 13 |
| 1 | Carriers | 32 | 62 | 3 | Identification | 73 | 15 |
| 1 | Liposomes | 31 | 67 | 3 | Metastasis | 64 | 19 |
| 1 | Antitumor-activity | 30 | 68 | 3 | Carcinoma | 60 | 20 |
| 1 | Formulation | 30 | 69 | 3 | Proliferation | 57 | 22 |
| 1 | Oral delivery | 29 | 71 | 3 | Inhibition | 55 | 24 |
| 1 | Systems | 28 | 73 | 3 | Resistance | 52 | 26 |
| 1 | Polymeric micelles | 26 | 80 | 3 | Activation | 45 | 29 |
| 1 | Co-delivery | 25 | 84 | 3 | Lung-cancer | 44 | 33 |
| 1 | Microspheres | 25 | 85 | 3 | Gene | 43 | 34 |
| 1 | Inflammation | 25 | 86 | 3 | Colorectal-cancer | 42 | 37 |
| 1 | Permeability | 24 | 90 | 3 | Protein | 38 | 46 |
| 1 | Polymeric nanoparticles | 23 | 92 | 3 | Invasion | 36 | 51 |
| 1 | Multidrug-resistance | 23 | 93 | 3 | Adenocarcinoma | 34 | 56 |
| 1 | Ph | 23 | 94 | 3 | Survival | 32 | 66 |
| 1 | Absorption | 22 | 97 | 3 | Gene-expression | 28 | 76 |
| 2 | Apoptosis | 124 | 10 | 3 | Pathway | 27 | 79 |
| 2 | Magnetic nanoparticles | 66 | 16 | 3 | Micrornas | 26 | 82 |
| 2 | Gold nanoparticles | 57 | 21 | 3 | Progression | 26 | 83 |
| 2 | Cytotoxicity | 54 | 25 | 3 | Migration | 25 | 89 |
| 2 | Mechanisms | 44 | 32 | 3 | Differentiation | 22 | 100 |
To explore the trend in research hotspots over time, we analyzed the average appearing year (AAY) of the top 100 keywords using VOSviewer and displayed it as a heat map (Figure 6B). The results showed that the keywords that appeared in the earlier years included model (AAY: 2015.07), binding (AAY: 2015.67), and controlled-release (AAY: 2015.68), while the high-frequency keywords that appeared recently included antioxidant (AAY: 2019.50), green synthesis (AAY: 2019.38), silver nanoparticles (AAY: 2018.83), invasion (AAY: 2018.75), and mechanisms (AAY: 2018.74).
Keyword citation burst analysis can reflect the extent of acceptance and dissemination of the main topics of relevant research. We analyzed the keyword citation burst of the application of nanomaterials in GC from 2000 to 2022 using CiteSpace software to better reveal the knowledge structure. Figure 6C shows the keywords with the top 30 burst strengths, including GC risk (Strength = 16.04), magnetic nanoparticle (Strength = 15.93), northern Jiangsu (Strength = 14.2), and gold nanorod (Strength = 10.55), indicating that these topics are the best accepted. In addition, the keywords currently in the citation burst were complex (2019-2022), green synthesis (2020-2022), mesoporous silica nanoparticle (2020-2022), inflammation (2020-2022), and gold (2020-2022), indicating that these topics will be hotspots in the near future.
The keyword timeline view can describe the trend in research hotspots in a field over time. We analyzed the timeline view of research into the application of nanomaterials in GC in the 2000-2023 period using CiteSpace software (Figure 6D). All keywords were separated into 11 clusters, and the more keywords in the cluster the more important the topic in the field. The ranking of the clusters in the timeline view is determined by the number of citations. The top three clusters—#1, drug delivery; #2, expression; and #3, green synthesis—cover almost the entire timeline, indicating that they have always been the focus of research into the application of nanomaterials in GC.
In recent years, a vast number of studies have noted that the unique properties of nanomaterials can make them suitable for the diagnosis and treatment of GC. In the present study and to reflect the research trends, we analyzed the annual publication numbers and the distribution of publications by country, institution, journal, and author in relation to the application of nanomaterials in GC. The findings showed that the overall annual publication of related studies has been on an increasing trend in the 2000-2022 period, although there were slight fluctuations in some years. In addition, the number of publications generated in the last 10 years and the last 5 years accounted for 89.51% and 55.08% of all articles, respectively. This indicates that research into the application of nanomaterials in GC is an emerging and rapidly developing field. In addition, our analysis revealed that, from 2000 to 2022, 11209 authors from 2353 institutions in 86 countries/regions worldwide have published articles in this field. Of these, 1007 articles were published in China, reaching more than half of the total number (1007 of 1888). In addition, 8 of the 10 most productive institutions were in China, and all of the top 10 productive authors were from China. Taken together, these analyses suggest that the application of nanomaterials in GC has attracted extensive attention worldwide and that China is in a leading position in this field.
There have been extensive collaborations among relevant countries, institutions, and authors in research into the application of nanomaterials in GC. Therefore, we performed co-authorship analysis at the national, institutional, and author levels. At the national level, the United States and China were the two most collaborative countries. At the institutional level, the three most collaborative institutional clusters were centered on the Chinese Academy of Sciences, Kaohsiung Medical University, and Nanjing Medical University, respectively. Accordingly, clusters 1 and 3 mainly comprised institutions from China, including the University of Chinese Academy of Sciences, the Fourth Military Medical University, Southeast University, and Nanjing University, whereas cluster 2 was dominated by institutions from South Korea and China, including National Yang-Ming University, National University of Tainan, and Sungkyunkwan University. At the author level, the three largest cooperative clusters all comprised authors from China. Thus, we can conclude that cooperation among institutions and authors from different countries may be relatively limited in this field.
We also analyzed the citation frequencies of articles examining the application of nanomaterials in GC in terms of countries, institutions, journals, and authors to reflect the quality of publications. At the national and institutional levels, China and Shanghai Jiao Tong University, respectively, were in the leading position in terms of their total citations, consistent with the number of publications. In addition, among the 10 most productive journals, Biomaterials, International Journal of Nanomedicine, and ACS Nano had the most citations, and the impact factor of Biomaterials is also the highest (15.304), indicating its significant influence in the field. At the author level, the publications of the 20 most productive authors were all cited more than 120 times, and the per citation values all exceeded 12. Therefore, whether in quantitative or qualitative terms, the authors listed in Table 3 have made significant contributions to the research into the application of nanomaterials in GC, and their publications are of high academic value.
In the current study, the top 100 keywords of the retrieved articles were divided into three research clusters. The main topics of the clusters were as follows: (1) The application of nanomaterials in GC treatment; (2) The application and toxicity of nanomaterials in GC diagnosis; and (3) The effects of nanomaterials on the biological behavior of GC cells.
Cluster 1: The application of nanomaterials in GC treatment: This cluster contains 45 keywords. In this cluster, the keywords drug delivery, system, co-delivery, controlled-release, bioavailability, stability, and other keywords can be associated to represent the properties of nanomaterial-based drug delivery systems in GC. In addition, the keywords chemotherapy, doxorubicin, paclitaxel, 5-fluorouracil (5-FU), cisplatin, pharmacokinetics, and other keywords are interrelated to represent the application of nanomaterials in GC chemotherapy. Finally, the keywords liposomes, polymeric nanoparticles, micelles, chitosan, microspheres, and other keywords are interrelated to represent the main forms of nanocarriers applied in GC treatment in the above two technologies.
Nanomaterials are materials that have at least one dimension in three-dimensional space at the nanometer scale (1 nm-100 nm) or are composed of these materials as fundamental components[14]. The stomach is an ideal site for nanomaterial applications because of its ability to alter the characteristics of nanomaterials through changes in pH, pressure, and bacterial content[15]. Current applications of nanomaterials in the treatment of GC include gene therapy, immunotherapy, oxidative therapy, radiotherapy, chemotherapy, thermo-therapy, and photothermal therapy[16]. In the present study, through keyword co-occurrence analysis, we found that the hotspots of research into nanomaterials in GC treatment mainly focus on the features of nanomaterial-based drug delivery systems and on the application of nanomaterials in GC chemotherapy, as well as on the most widely used nanomaterials in these two technologies, including liposomes, polymeric nanoparticles, micelles, chitosan, and microspheres.
In recent years, nanomaterials have greatly evolved in the field of drug delivery and have been widely applied in cardiovascular disease, ophthalmic disease, and tumors[17-19]. Compared to traditional oncology drug delivery, the most prominent feature of the use of nanomaterials as carriers is the ability to target drug delivery into tumors and control the release of drugs[20]. This is a unique advantage in GC treatment. Before entering the target tumor cells, the nano-drug delivery system can protect the contained drug from the harsh environment, such as the high levels of proteases in the blood and the high acidity in the stomach, and control the time and dosage of drug release, reducing adverse effects and improving the biological stability and activity of drugs[21]. In addition, the same nanocarrier can be used to enable the co-delivery of diagnostic agents and therapeutic drugs or multiple drugs, thereby enabling the real-time readout of therapeutic effects and combination therapy that may be able to overcome multidrug resistance[22].
Chemotherapy is a valuable treatment for GC and is currently the only way to deliver anticancer drugs; however, conventional chemotherapy often leads to various adverse effects, such as anemia, alopecia, nephrotoxicity, and neurotoxicity. In addition, chemotherapeutic drugs are of limited stability and solubility and lack targeting in the human body, thereby making their role in cancer a double-edged sword. The use of nanocarriers to encapsulate drugs for targeted drug delivery to specific sites, thereby improving pharmacokinetics, is important to overcome the limitations of conventional chemotherapy[16].
Doxorubicin, paclitaxel, 5-FU, and cisplatin are drugs commonly used in the chemotherapy of GC, and studies have been conducted to develop nanomaterials to encapsulate these chemotherapeutic agents in targeted therapies for GC. Hemati et al[23,24] developed and optimized a novel cationic poly (ethylene glycol)-containing anticancer drug (doxorubicin or quercetin) and siRNA nanovesicles and demonstrated that co-delivery of doxorubicin or quercetin with the oncogene CDC20 siRNA strongly inhibited the growth of GC cells and has promising applications in GC treatment. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) can increase the solubility of paclitaxel and avoid infusion-related reactions similar to solvent-based paclitaxel with no premedication[25]. In addition, nab-paclitaxel can be administered at greater drug concentrations and more frequent doses with a shorter infusion time than solvent-based paclitaxel. Clinical trials have shown that the overall survival using solvent-based paclitaxel is not superior to that of nab-paclitaxel at the same dosing frequency; therefore, the advantages of nab-paclitaxel could make it a potential option for the second-line treatment of GC[26,27]. Azimee et al[28] developed a TiO2 nanoparticle with autophagic potential to promote the cytotoxicity and apoptotic impact of 5-FU on GC cells, thereby enhancing the effects of chemotherapy. They demonstrated the beneficial effects of TiO2 nanoparticles combined with chemotherapy in GC using in vitro models, laying the basis for the development of potential solutions to chemoresistance[28]. The existing research results suggest that the application of nanomaterials can effectively reduce the toxic effects of conventional GC chemotherapy and significantly improve the ability of chemotherapeutic drugs to eliminate tumors without damaging normal cells, and their application in clinical GC chemotherapy shows considerable promise.
Based on our analyses, the commonly used nanomaterials in the treatment of GC include liposomes, chitosan, and polymeric micelles. Liposomes are one of the most widely used nanomedicine carriers. The distinguishing benefit of liposomes over other nanomaterials is that they have both hydrophobic and hydrophilic cavities and can encapsulate both water-soluble and lipid-soluble drugs, which can improve drug delivery efficiency and therapeutic capacity, help overcome multidrug resistance, and limit adverse drug reactions[29-31]. Numerous studies have demonstrated that liposomes can be used as carriers in GC photodynamic therapy[32-34], RNA interference therapy[35-37], and anticancer drug combination therapy[38-40].
Chitosan is a bioactive polymer with promising applications in cancer therapy due to its functional properties, which include antibacterial activity, non-toxicity, ease of modification, and high biodegradability[41]. Chitosan has special mucosal adhesion and cationic properties that enhance its interaction with mucous membranes, thereby promoting transmucosal drug delivery[42]. Research on the application of chitosan in GC treatment has mainly focused on the potential of chitosan and its various derivatives to act as antitumor drugs by themselves and on their ability to enhance drug targeting and reduce resistance when coupled with different drugs, improving antitumor efficiency[42]. For example, Zhang et al[43] modified N-deoxycholic acid glycol chitosan with GX1 (a marker peptide of GC angiogenesis) and used it as a carrier coupled with the chemotherapeutic drug doxorubicin to prepare a GC vascular-targeting nanoparticle and demonstrated that this nanoparticle was more toxic to GC cells compared with free drugs. Another study developed a chitosan-gelatin-EGCG (CGE) nanocarrier to deliver siRNA of a novel 5-FU resistance-associated lncRNA, TMEM44-AS1 and demonstrated that this delivery system could effectively reverse 5-FU resistance in GC, enhancing the therapeutic effect of 5-FU[44].
Cluster 2: Application and toxicity of nanomaterials in GC diagnosis: This cluster contains 30 keywords, including magnetic nanoparticles, gold nanoparticles, diagnosis, graphene oxide, iron oxide nanoparticles, silver nanoparticles, and rapid detection. These keywords can be linked to represent the nanomaterials mainly used in GC diagnosis. Keywords such as cytotoxicity, apoptosis, mechanisms, and oxidative stress can also be linked to represent the cytotoxicity of the nanomaterials used in GC diagnosis.
In addition to the therapeutic potential, nanomaterials have shown considerable promise in the diagnosis of GC. There has been substantial research into the application of nanomaterials in GC diagnostic methods such as imaging, endoscopy, and detection of relevant biomarkers[45]. Furthermore, we found that the most popular nanomaterials applied in studies of GC diagnosis were mainly inorganic nanomaterials, including magnetic nanoparticles, graphene oxide, and gold nanoparticles. The advantages of inorganic nanomaterials lie in their smaller and more uniform particle size, ease of synthesis and modification, higher photothermal conversion efficiency, and favorable fluorescent properties[46]. However, compared with polymeric nanomaterials, inorganic nanomaterials are less biocompatible and difficult to clear away and may have long-term toxicity or induce cytotoxicity[47,48], which prevents their large-scale clinical application.
Magnetic nanoparticles are the most studied nanomaterials in the computed tomography (CT)/magnetic resonance (MR) imaging of tumors[49]. Studies have shown that a superparamagnetic iron oxide nanoparticle (SPION) is particularly suitable for the MR imaging of GC[50-52]. Wang et al[53] developed a SiO2-coated SPION as a core-shell nanoparticle labeled with near-infrared fluorescence (NIRF) dye and an anti-CD146 monoclonal antibody for MR/NIRF imaging of the MKN45 xenograft GC model, which could be distinguished in 30 min after the injection time point. Luo et al[50] prepared a folate-functionalized polyethyleneimine SPION complex and used it to deliver PD-L1 knockdown siRNA, ultimately demonstrating that this complex both can serve as a T2-weighted contrast agent for MR imaging of GC and can effectively target PD-L1 for knockdown therapy in GC. In summary, magnetic nanomaterials are useful for the rapid imaging and guided therapy of GC.
Gold and silver nanoparticles are the most studied noble metal nanomaterials in GC. Both metals have special plasmon resonance effects on their surfaces, which give them high photothermal conversion efficiency and strong absorption capacity for near-infrared light. Gold nanoparticles are often stable in vivo and tend to accumulate at the tumor site, which makes them attractive for the diagnostic imaging of GC, and studies have shown that gold nanoparticles can be used as excellent contrast agents for the CT/MR/photoacoustic imaging of GC[54]. In addition, gold nanoparticle-based biosensors have high accuracy and sensitivity, enabling them to detect GC biomarkers[45,55]. Research into silver nanoparticles in GC has mainly focused on their use in noninvasive cancer detection technologies[56-59]. For example, Feng et al[56] prepared a surface-enhanced Raman spectroscopy (SERS)-based method for plasma analysis by using silver nanoparticles as a SERS substrate mixed directly with plasma to enhance the Raman scattering of various biomolecular components and explored for the first time the influence of different laser polarizations on plasma SERS spectra. This demonstrated that plasma excited by left-rotating circularly polarized laser SERS spectroscopy is expected to be a reliable clinical diagnosis tool for noninvasive GC detection[56].
Compared with other nanomaterials, graphene oxide is a carbon-based nanomaterial with excellent electrical and optical properties, high mechanical strength, an extremely large surface area, and little cytotoxicity[60]. Research of graphene oxide in GC has only emerged in recent years, and it is often used in combination with other nanomaterials to enhance electrical conductivity and biocompatibility, particularly in combination with gold nanomaterials to make electrochemical sensors for the early diagnosis of GC. For example, Daneshpour et al[61] developed a dual-signal nanosensor based on a gold nanoparticle-quantum dot-magnetic nanoparticle-graphene oxide composite; this composite showed good performance in the quantitative analysis of miR-106a (GC oncogenic miRNA) and let-7a (GC suppressor miRNA), which has major implications for the screening of miRNA sequences and early diagnosis of GC. Zhang et al[62] developed a gold nanostar-modified graphene oxide nanocomposite (GO-AuNSs) and modified it with a layer of biocomplex rBSA-FA (coupling reduced bovine serum albumin with folic acid) to obtain a GO-AuNSs@rBSA-FA nanocomposite with sensitive detection of GC cells.
To promote the biomedical application of nanomaterials, it is necessary to fully understand their cytotoxicity. Numerous studies have explored the toxicity of inorganic nanomaterials in GC cells. The main and common mechanism of cytotoxicity produced by inorganic nanoparticles involves the induction of oxidative stress and the subsequent triggering of cell cycle arrest, cell death/apoptosis, and DNA damage. Other sources of toxicity also include the release of toxic metal ions, chemical instability, the release of excessive compounds, and surface pollutants[63]. For example, the cytotoxicity of gold nanoparticles is often induced by oxidative stress, endogenous reactive oxygen species (ROS) production, and degradation of the intracellular antioxidant pool, and the toxicity is related to the size of the nanoparticles and chemical ligands[64]. A high level of toxicity was observed in vivo for ultrasmall Fe3O4 nanoparticles (2.3 and 4.2 nm). The toxicity was related to the iron element and size. Ultrasmall nanoparticles (< 5 nm) can effectively induce the generation of ROS[65]. The toxicity of graphene oxide might be induced by lipid peroxidation, oxidative stress, and mitochondrial dysfunction[66]. The synthesis of nanomaterials often requires the use of highly toxic chemical raw materials and a large amount of energy. To avoid the generation of toxicity and pollution, research in recent years has gradually focused on the green synthesis of nanomaterials, that is, the use of environmentally friendly solvent systems, reducing agents, and non-toxic stabilizers to prepare nanomaterials[67]. Studies have pointed out that most of the gold, silver, copper, zinc oxide, and other nanomaterials synthesized using different plant extracts have been demonstrated to be effective free radical scavengers, which can prevent oxidative stress[68]. For example, Mi et al[69] prepared novel gold nanoparticles, cirsium japonicum mediated-AuNPs (CJ-AuNPs), through a biosynthetic process involving ethanol extracts from Cirsium japonicum (Herba cirsii) and verified that it can selectively kill GC AGS cells and cause oxidative stress and iron-dependent iron ptosis without systemic toxicity.
Cluster 3: Effects of nanomaterials on the biological behavior of GC cells: This cluster contains 25 keywords, including cells, proliferation, growth, metastasis, and invasion. These keywords are interrelated to represent the effects of nanomaterials on the biological behavior of GC cells.
Favorable histocompatibility and adjustability mean that nanomaterials have considerable potential in the field of cancer research. Furthermore, they allow personalized modification of different cell biological behaviors. According to our analyses, most studies in GC have focused on the use of nanomaterials themselves or their delivery of drugs or genes to achieve antitumor effects by specifically affecting proliferation, growth, metastasis, and invasive ability, by ameliorating the drug resistance of GC cells, or by activating/inhibiting certain signaling pathways. For example, a nanofiber integrated with epigenetic regulators inhibits GC cell proliferation and promotes apoptosis to exert antitumor effects[70]. A paclitaxel GX1-modified nanostructured lipid carrier could inhibit the growth of GC cells and reduce drug toxicity[71]. Due to the chemotherapy resistance and uncontrolled proliferation, GC stem cells are closely associated with the occurrence, growth, metastasis, and recurrence of GC. CD44/CD133-ATRA-PLPN (CD44 and CD133 antibodies conjugated to all-trans retinoic acid-loaded poly (propyleneglycolate-ethyleneglycolate)-lecithin PEG nanoparticles), targeting two markers of GC stem cells, CD44 and CD133, was reported by Chen et al[72] to have a strong inhibitory effect on the growth of GC stem cells. Yao et al[73] developed a new glioma-associated oncogene homolog 1 small-interfering RNA nanoparticle that targeted GC stem cells and demonstrated that it could significantly inhibit the malignant behaviors of GC stem cells and suppress cell migration and invasion by specifically blocking Hedgehog signaling in vivo and in vitro, showing significant effects on in vivo tumor recurrence and providing a promising new strategy for the targeted therapy of GC. In addition, nanomaterials themselves have multiple effects on GC cells. For example, studies have found that liposomes have unique inhibitory effects on the proliferation of GC cells[74,75] and that chitosan and some of its derivatives can play roles in inhibiting GC cell proliferation and promoting apoptosis, suggesting that these materials themselves have potential therapeutic effects in GC[76-78].
To explore the emerging frontiers in the application of nanomaterials in GC, we analyzed the AAY and citation burst of keywords for the retrieved studies (Figure 6B and C). The results showed that keywords such as antioxidants, green synthesis, silver nanoparticles, invasion, mechanisms, nanocomposites, microRNAs, multidrug resistance, stability, and complexes have emerged in recent years. In addition, the keywords gold, inflammation, mesoporous silica nanoparticle, green synthesis, and complex have been in the midst of a current citation burst, indicating that these topics are at the forefront of the research area and are likely to become future research hotspots. As mentioned earlier, nanomaterials have considerable biocompatibility and are easy to combine with other biomaterials and antitumor drugs to form various complexes or nanocomposites for comprehensive applications in the diagnosis and treatment of GC, which has major research potential. Gold, silver, and mesoporous silica nanoparticles represent novel nanomaterials that have gradually emerged in the GC field in recent years. Most of the current research is focused on the early diagnosis of GC, particularly the electrochemical receptors applied for noninvasive detection and early diagnosis, which have broad application prospects. Green synthesis is one of the effective ways to reduce the induced toxicity of nanomaterials. In addition, the topics of antioxidants, invasion, mechanisms, multidrug resistance, stability, and inflammation represent the main roles played by nanomaterials applied in GC and the key problems to be solved, reflecting the future research directions.
The present work comprised a bibliometric and visualization study and also involved a review with a comprehensive and systematic analysis. The articles included covered a wide range of topics. In general, research on the application of nanomaterials in GC is now in a rapid development phase, with China and the United States at the center of the field, and international collaboration should be strengthened between research institutions and researchers from different countries. Research into nanomaterial applications in GC is currently divided into three main parts: The application of nanomaterials in GC treatment; the application and toxicity of nanomaterials in GC diagnosis; and the effects of nanomaterials on the biological behavior of GC cells. Promising future research directions may be focused on several topics, including complexes, silver nanoparticles, and green synthesis. In conclusion, this bibliometric study provides a comprehensive review of research into the application of nanomaterials in GC and could serve as a reference for future researchers in this field.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozden S, Türkiye S-Editor: Luo ML L-Editor: A P-Editor: Zhang XD
| 1. | Rai A, Noor S, Ahmad SI, Alajmi MF, Hussain A, Abbas H, Hasan GM. Recent Advances and Implication of Bioengineered Nanomaterials in Cancer Theranostics. Medicina (Kaunas). 2021;57. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Zhu X, Su T, Wang S, Zhou H, Shi W. New Advances in Nano-Drug Delivery Systems: Helicobacter pylori and Gastric Cancer. Front Oncol. 2022;12:834934. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Doughty ACV, Hoover AR, Layton E, Murray CK, Howard EW, Chen WR. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials (Basel). 2019;12:779. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Quader S, Kataoka K. Nanomaterial-Enabled Cancer Therapy. Mol Ther. 2017;25:1501-1513. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Agarwal A, Durairajanayagam D, Tatagari S, Esteves SC, Harlev A, Henkel R, Roychoudhury S, Homa S, Puchalt NG, Ramasamy R, Majzoub A, Ly KD, Tvrda E, Assidi M, Kesari K, Sharma R, Banihani S, Ko E, Abu-Elmagd M, Gosalvez J, Bashiri A. Bibliometrics: tracking research impact by selecting the appropriate metrics. Asian J Androl. 2016;18:296-309. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724-728. [PubMed] [Cited in This Article: ] |
| 10. | Baishideng Publishing Group Inc. Reference Citation Analysis. Available from: https://www.referencecitationanalysis.com. [Cited in This Article: ] |
| 11. | Wang JL, Ma YJ, Ma L, Ma N, Guo DM, Ma LS. Baishideng's Reference Citation Analysis database announces the first Journal Article Influence Index of 104 core journals and a list of high-quality academic journals in orthopedics. World J Orthop. 2022;13:891-902. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Wang JL, Ma YJ, Ma L, Ma N, Guo DM, Ma LS. Baishideng's Reference Citation Analysis database announces the first Journal Article Influence Index of 101 core journals and a list of high-quality academic journals in gastroenterology and hepatology. World J Gastroenterol. 2022;28:5383-5394. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104:3460-3465. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Shi W, Guan X, Sun S, Han Y, Du X, Tang Y, Zhou W, Liu G. Nanoparticles decrease the byssal attachment strength of the thick shell mussel Mytilus coruscus. Chemosphere. 2020;257:127200. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Liang M, Li LD, Li L, Li S. Nanotechnology in diagnosis and therapy of gastrointestinal cancer. World J Clin Cases. 2022;10:5146-5155. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Nagaraju GP, Srivani G, Dariya B, Chalikonda G, Farran B, Behera SK, Alam A, Kamal MA. Nanoparticles guided drug delivery and imaging in gastric cancer. Semin Cancer Biol. 2021;69:69-76. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Arayne MS, Sultana N, Qureshi F. Review: nanoparticles in delivery of cardiovascular drugs. Pak J Pharm Sci. 2007;20:340-348. [PubMed] [Cited in This Article: ] |
| 18. | Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine (Lond). 2017;12:683-702. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Xin Y, Huang M, Guo WW, Huang Q, Zhang LZ, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16:134. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5:216rv4. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16-20. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320-12364. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Hemati M, Haghiralsadat F, Jafary F, Moosavizadeh S, Moradi A. Targeting cell cycle protein in gastric cancer with CDC20siRNA and anticancer drugs (doxorubicin and quercetin) co-loaded cationic PEGylated nanoniosomes. Int J Nanomedicine. 2019;14:6575-6585. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Hemati M, Haghiralsadat F, Yazdian F, Jafari F, Moradi A, Malekpour-Dehkordi Z. Development and characterization of a novel cationic PEGylated niosome-encapsulated forms of doxorubicin, quercetin and siRNA for the treatment of cancer by using combination therapy. Artif Cells Nanomed Biotechnol. 2019;47:1295-1311. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Roviello G, Conter FU, Mini E, Generali D, Traversini M, Lavacchi D, Nobili S, Sobhani N. Nanoparticle albumin-bound paclitaxel: a big nano for the treatment of gastric cancer. Cancer Chemother Pharmacol. 2019;84:669-677. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K, Ueda S, Yoshida K, Shimodaira H, Nishina T, Tsuda M, Kurokawa Y, Tamura T, Sasaki Y, Morita S, Koizumi W. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277-287. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Bando H, Shimodaira H, Fujitani K, Takashima A, Yamaguchi K, Nakayama N, Takahashi T, Oki E, Azuma M, Nishina T, Hironaka S, Komatsu Y, Shitara K. A phase II study of nab-paclitaxel in combination with ramucirumab in patients with previously treated advanced gastric cancer. Eur J Cancer. 2018;91:86-91. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Azimee S, Rahmati M, Fahimi H, Moosavi MA. TiO(2) nanoparticles enhance the chemotherapeutic effects of 5-fluorouracil in human AGS gastric cancer cells via autophagy blockade. Life Sci. 2020;248:117466. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Hong C, Wang D, Liang J, Guo Y, Zhu Y, Xia J, Qin J, Zhan H, Wang J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics. 2019;9:4437-4449. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Zhang C, Zhang S, Zhi D, Cui J. Cancer Treatment with Liposomes Based Drugs and Genes Co-delivery Systems. Curr Med Chem. 2018;25:3319-3332. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Li Y, Cong H, Wang S, Yu B, Shen Y. Liposomes modified with bio-substances for cancer treatment. Biomater Sci. 2020;8:6442-6468. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Xin J, Wang S, Wang B, Wang J, Zhang L, Xin B, Shen L, Zhang Z, Yao C. AlPcS(4)-PDT for gastric cancer therapy using gold nanorod, cationic liposome, and Pluronic(®) F127 nanomicellar drug carriers. Int J Nanomedicine. 2018;13:2017-2036. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Namiki Y, Fuchigami T, Tada N, Kawamura R, Matsunuma S, Kitamoto Y, Nakagawa M. Nanomedicine for cancer: lipid-based nanostructures for drug delivery and monitoring. Acc Chem Res. 2011;44:1080-1093. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Namiki Y, Namiki T, Date M, Yanagihara K, Yashiro M, Takahashi H. Enhanced photodynamic antitumor effect on gastric cancer by a novel photosensitive stealth liposome. Pharmacol Res. 2004;50:65-76. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Babu A, Munshi A, Ramesh R. Combinatorial therapeutic approaches with RNAi and anticancer drugs using nanodrug delivery systems. Drug Dev Ind Pharm. 2017;43:1391-1401. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Yang F, Zheng Z, Xue X, Zheng L, Qin J, Li H, Zhou Y, Fang G. Targeted eradication of gastric cancer stem cells by CD44 targeting USP22 small interfering RNA-loaded nanoliposomes. Future Oncol. 2019;15:281-295. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Ando H, Fukushima M, Eshima K, Hasui T, Shimizu T, Ishima Y, Huang CL, Wada H, Ishida T. A novel intraperitoneal therapy for gastric cancer with DFP-10825, a unique RNAi therapeutic targeting thymidylate synthase, in a peritoneally disseminated xenograft model. Cancer Med. 2019;8:7313-7321. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Sun Y, Xie Y, Tang H, Ren Z, Luan X, Zhang Y, Zhu M, Lv Z, Bao H, Li Y, Liu R, Shen Y, Zheng Y, Pei J. In vitro and in vivo Evaluation of a Novel Estrogen-Targeted PEGylated Oxaliplatin Liposome for Gastric Cancer. Int J Nanomedicine. 2021;16:8279-8303. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Yang CL, Chao YJ, Wang HC, Hou YC, Chen CG, Chang CC, Shan YS. Local ablation of gastric cancer by reconstituted apolipoprotein B lipoparticles carrying epigenetic drugs. Nanomedicine. 2021;37:102450. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Lu M, Wang T, Wang J. Effects of paclitaxel liposome and capecitabine in the treatment of advanced gastric cancer by clinical observation. Int J Clin Pharmacol Ther. 2016;54:693-697. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K. Chitosan as a bioactive polymer: Processing, properties and applications. Int J Biol Macromol. 2017;105:1358-1368. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Shafabakhsh R, Yousefi B, Asemi Z, Nikfar B, Mansournia MA, Hallajzadeh J. Chitosan: A compound for drug delivery system in gastric cancer-a review. Carbohydr Polym. 2020;242:116403. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Zhang E, Xing R, Liu S, Li K, Qin Y, Yu H, Li P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int J Biol Macromol. 2019;126:662-672. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Zhou M, Dong J, Huang J, Ye W, Zheng Z, Huang K, Pan Y, Cen J, Liang Y, Shu G, Ye S, Lu X, Zhang J. Chitosan-Gelatin-EGCG Nanoparticle-Meditated LncRNA TMEM44-AS1 Silencing to Activate the P53 Signaling Pathway for the Synergistic Reversal of 5-FU Resistance in Gastric Cancer. Adv Sci (Weinh). 2022;9:e2105077. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Li R, Liu B, Gao J. The application of nanoparticles in diagnosis and theranostics of gastric cancer. Cancer Lett. 2017;386:123-130. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Wang Z, Guo W, Kuang X, Hou S, Liu H. Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective. Asian J Pharm Sci. 2017;12:498-508. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int J Pharm. 2018;539:104-111. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Wang F, Li C, Cheng J, Yuan Z. Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents. Int J Environ Res Public Health. 2016;13. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Bakhtiary Z, Saei AA, Hajipour MJ, Raoufi M, Vermesh O, Mahmoudi M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomedicine. 2016;12:287-307. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Luo X, Peng X, Hou J, Wu S, Shen J, Wang L. Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer. Int J Nanomedicine. 2017;12:5331-5343. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Chen Y, Lian G, Liao C, Wang W, Zeng L, Qian C, Huang K, Shuai X. Characterization of polyethylene glycol-grafted polyethylenimine and superparamagnetic iron oxide nanoparticles (PEG-g-PEI-SPION) as an MRI-visible vector for siRNA delivery in gastric cancer in vitro and in vivo. J Gastroenterol. 2013;48:809-821. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Tokuhara T, Tanigawa N, Matsuki M, Nomura E, Mabuchi H, Lee SW, Tatsumi Y, Nishimura H, Yoshinaka R, Kurisu Y, Narabayashi I. Evaluation of lymph node metastases in gastric cancer using magnetic resonance imaging with ultrasmall superparamagnetic iron oxide (USPIO): diagnostic performance in post-contrast images using new diagnostic criteria. Gastric Cancer. 2008;11:194-200. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Wang P, Qu Y, Li C, Yin L, Shen C, Chen W, Yang S, Bian X, Fang D. Bio-functionalized dense-silica nanoparticles for MR/NIRF imaging of CD146 in gastric cancer. Int J Nanomedicine. 2015;10:749-763. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Yang Z, Wang D, Zhang C, Liu H, Hao M, Kan S, Liu D, Liu W. The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer. Front Oncol. 2021;11:819329. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Căinap C, Nagy V, Gherman A, Cetean S, Laszlo I, Constantin AM, Căinap S. Classic tumor markers in gastric cancer. Current standards and limitations. Clujul Med. 2015;88:111-115. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Feng S, Chen R, Lin J, Pan J, Wu Y, Li Y, Chen J, Zeng H. Gastric cancer detection based on blood plasma surface-enhanced Raman spectroscopy excited by polarized laser light. Biosens Bioelectron. 2011;26:3167-3174. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Zhang Z, Liu Y, Liu P, Yang L, Jiang X, Luo D, Yang D. Non-invasive detection of gastric cancer relevant d-amino acids with luminescent DNA/silver nanoclusters. Nanoscale. 2017;9:19367-19373. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Huang L, Zhu Y, Xu C, Cai Y, Yi Y, Li K, Ren X, Jiang D, Ge Y, Liu X, Sun W, Zhang Q, Wang Y. Noninvasive Diagnosis of Gastric Cancer Based on Breath Analysis with a Tubular Surface-Enhanced Raman Scattering Sensor. ACS Sens. 2022;7:1439-1450. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Lin J, Chen R, Feng S, Pan J, Li Y, Chen G, Cheng M, Huang Z, Yu Y, Zeng H. A novel blood plasma analysis technique combining membrane electrophoresis with silver nanoparticle-based SERS spectroscopy for potential applications in noninvasive cancer detection. Nanomedicine. 2011;7:655-663. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Chen J, He GM, Xian GY, Su XQ, Yu LL, Yao F. Mechanistic biosynthesis of SN-38 coated reduced graphene oxide sheets for photothermal treatment and care of patients with gastric cancer. J Photochem Photobiol B. 2020;204:111736. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Daneshpour M, Karimi B, Omidfar K. Simultaneous detection of gastric cancer-involved miR-106a and let-7a through a dual-signal-marked electrochemical nanobiosensor. Biosens Bioelectron. 2018;109:197-205. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Zhang A, Liu Q, Huang Z, Zhang Q, Wang R, Cui D. Electrochemical Cytosensor Based on a Gold Nanostar-Decorated Graphene Oxide Platform for Gastric Cancer Cell Detection. Sensors (Basel). 2022;22. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Li J, Chang X, Chen X, Gu Z, Zhao F, Chai Z, Zhao Y. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnol Adv. 2014;32:727-743. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U, Jahnen-Dechent W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5:2067-2076. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Wu L, Wen W, Wang X, Huang D, Cao J, Qi X, Shen S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part Fibre Toxicol. 2022;19:24. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Zhang J, Cao HY, Wang JQ, Wu GD, Wang L. Graphene Oxide and Reduced Graphene Oxide Exhibit Cardiotoxicity Through the Regulation of Lipid Peroxidation, Oxidative Stress, and Mitochondrial Dysfunction. Front Cell Dev Biol. 2021;9:616888. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Benelli G. Green Synthesis of Nanomaterials. Nanomaterials (Basel). 2019;9. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Balkrishna A, Kumar A, Arya V, Rohela A, Verma R, Nepovimova E, Krejcar O, Kumar D, Thakur N, Kuca K. Phytoantioxidant Functionalized Nanoparticles: A Green Approach to Combat Nanoparticle-Induced Oxidative Stress. Oxid Med Cell Longev. 2021;2021:3155962. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Mi XJ, Park HR, Dhandapani S, Lee S, Kim YJ. Biologically synthesis of gold nanoparticles using Cirsium japonicum var. maackii extract and the study of anti-cancer properties on AGS gastric cancer cells. Int J Biol Sci. 2022;18:5809-5826. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Wang L, Zhang C, Hong Y, Li X, Li T, Gao A, Pan S, Liu B, Jin H, Cui D. Integrating Epigenetic Modulators in Nanofibers for Synergistic Gastric Cancer Therapy via Epigenetic Reprogramming. Nano Lett. 2021;21:298-307. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Jian Y, Zhao M, Cao J, Fan T, Bu W, Yang Y, Li W, Zhang W, Qiao Y, Wang J, Wen A. A Gastric Cancer Peptide GX1-Modified Nano-Lipid Carriers Encapsulating Paclitaxel: Design and Evaluation of Anti-Tumor Activity. Drug Des Devel Ther. 2020;14:2355-2370. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Chen H, Lin J, Shan Y, Zhengmao L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomed Pharmacother. 2019;115:108857. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Yao H, Sun L, Li J, Zhou X, Li R, Shao R, Zhang Y, Li L. A Novel Therapeutic siRNA Nanoparticle Designed for Dual-Targeting CD44 and Gli1 of Gastric Cancer Stem Cells. Int J Nanomedicine. 2020;15:7013-7034. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Yang F, Zheng Z, Zheng L, Qin J, Li H, Xue X, Gao J, Fang G. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. Onco Targets Ther. 2018;11:6811-6825. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Li Q, Gao Y, Xu ZG, Jiang H, Yu YY, Zhu ZG. Effect of antisense oligodeoxynucleotide targeted against NF-κB/P65 on cell proliferation and tumorigenesis of gastric cancer. Clin Exp Med. 2013;13:11-19. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Qi LF, Xu ZR, Li Y, Jiang X, Han XY. In vitro effects of chitosan nanoparticles on proliferation of human gastric carcinoma cell line MGC803 cells. World J Gastroenterol. 2005;11:5136-5141. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Dong XD, Yu J, Meng FQ, Feng YY, Ji HY, Liu A. Antitumor effects of seleno-short-chain chitosan (SSCC) against human gastric cancer BGC-823 cells. Cytotechnology. 2019;71:1095-1108. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Chi J, Jiang Z, Qiao J, Zhang W, Peng Y, Liu W, Han B. Antitumor evaluation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics. Int J Biol Macromol. 2019;136:1-12. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, He M, Li Z, Gao G, Gao B, Fu S, Cui D. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials. 2011;32:9796-9809. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Nakhleh MK, Amal H, Jeries R, Broza YY, Aboud M, Gharra A, Ivgi H, Khatib S, Badarneh S, Har-Shai L, Glass-Marmor L, Lejbkowicz I, Miller A, Badarny S, Winer R, Finberg J, Cohen-Kaminsky S, Perros F, Montani D, Girerd B, Garcia G, Simonneau G, Nakhoul F, Baram S, Salim R, Hakim M, Gruber M, Ronen O, Marshak T, Doweck I, Nativ O, Bahouth Z, Shi DY, Zhang W, Hua QL, Pan YY, Tao L, Liu H, Karban A, Koifman E, Rainis T, Skapars R, Sivins A, Ancans G, Liepniece-Karele I, Kikuste I, Lasina I, Tolmanis I, Johnson D, Millstone SZ, Fulton J, Wells JW, Wilf LH, Humbert M, Leja M, Peled N, Haick H. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano. 2017;11:112-125. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, Zhang P, Qian H, Jiang PC, Xu WR, Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991-1004. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Huang P, Li Z, Lin J, Yang D, Gao G, Xu C, Bao L, Zhang C, Wang K, Song H, Hu H, Cui D. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials. 2011;32:3447-3458. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, Ueno H, Muro K, Yamada Y, Okusaka T, Shirao K, Shimada Y, Nakahama H, Matsumura Y. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br J Cancer. 2007;97:170-176. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, Sekimoto M, Doki Y, Mori M. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci U S A. 2010;107:40-45. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752-1761. [PubMed] [DOI] [Cited in This Article: ] |