Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1774
Peer-review started: March 23, 2023
First decision: April 26, 2023
Revised: May 9, 2023
Accepted: May 30, 2023
Article in press: May 30, 2023
Published online: August 27, 2023
Due to the chronic progressive disease characteristics of primary biliary cholangitis (PBC), patients with advanced PBC should not be ignored. Most prognostic score studies have focused on early stage PBC.
To compare the prognostic value of various risk scores in advanced PBC to help PBC patients obtain more monitoring and assessment.
This study considered patients diagnosed with PBC during hospitalization between 2015 and 2021. The clinical stage was primarily middle and late, and patients usually took ursodeoxycholic acid (UDCA) after diagnosis. The discriminatory performance of the scores was assessed with concordance statistics at baseline and after 1 year of UDCA treatment. Telephone follow-up was conducted to analyze the course and disease-associated outcomes. The follow-up deadline was December 31, 2021. We compared the risk score indexes between those patients who reached a composite end point of death or liver transplantation (LT) and those who remained alive at the deadline. The combined performance of prognostic scores in estimating the risk of death or LT after 1 year of UDCA treatment was assessed using Cox regression analyses. Predictive accuracy was evaluated by comparing predicted and actual survival through Kaplan-Meier analyses.
We included 397 patients who were first diagnosed with PBC during hospitalization and received UDCA treatment; most disease stages were advanced. After an average of 6.4 ± 1.4 years of follow-up, 82 patients had died, and 4 patients had undergone LT. After receiving UDCA treatment for 1 year, the score with the best discrimination performance was the Mayo, with a concordance statistic of 0.740 (95% confidence interval: 0.690-0.791). The albumin-bilirubin, GLOBE, and Mayo scores tended to overestimate transplant-free survival. Comparing 7 years of calibration results showed that the Mayo score was the best model.
The Mayo, GLOBE, UK-PBC, and ALBI scores demonstrated comparable discriminating performance for advanced stage PBC. The Mayo score showed optimal discriminatory performance and excellent predictive accuracy.
Core Tip: Primary biliary cholangitis (PBC) is a chronic progressive liver disease that destroys the intrahepatic small bile ducts. PBC in the middle and late stages cannot be ignored. The present study enrolled patients first diagnosed with PBC during hospitalization whose disease stages were primarily in the middle and late stages. We compared the prognostic value of various risk scores in PBC patients with advanced disease stages so that a significant proportion would undergo monitoring, disease evaluation, and timely treatment.
- Citation: Feng J, Xu JM, Fu HY, Xie N, Bao WM, Tang YM. Prognostic scores in primary biliary cholangitis patients with advanced disease. World J Gastrointest Surg 2023; 15(8): 1774-1783
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1774.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1774
Primary biliary cholangitis (PBC) is a chronic progressive liver disease that causes the gradual destruction of the intrahepatic small bile ducts[1]. Preclinical PBC may present with specific diagnostic antibodies (anti-mitochondrial antibody, AMA) but remain asymptomatic with normal liver function for over a decade. Approximately 50%-60% are asymptomatic at diagnosis[2]. Ursodeoxycholic acid (UDCA) is the first-line treatment. It increases long-term survival. However, approximately 40% of patients with PBC have incomplete responses, and these patients progress rapidly to the middle and late stages of disease after early diagnosis and treatment[3]. Because of the chronic progressive disease characteristics, PBC patients in the middle and late stages should not be ignored.
Over the past 20 years, several risk-scoring models for PBC have been proposed as tools to estimate the risk of adverse outcomes and to guide management[4]. The most influential scores are GLOBE and UK-PBC, developed for early PBC patients. Recent studies reported that these scores accurately predict outcomes in patients treated with UDCA treatment at various disease stages[5-7]. However, their application to middle and late stage PBC patients remains to be studied. The Mayo score was developed to determine the timing of liver transplantation (LT) in PBC and is now a model for predicting PBC survival[8-10]. The aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 index (FIB-4) are non-invasive fibrosis scores based on biochemical indicators[11]. All parameters, including aminotransferase, platelets, and age, are associated with PBC outcomes[12,13]. The albumin-bilirubin (ALBI) score was initially developed to assess liver function in hepatocellular carcinoma patients[14]. The total bilirubin (TBil) and albumin in the score are associated with PBC progression, and some studies have used them to predict PBC outcomes[15,16]. There are few studies on the efficacy and differences of the various prognostic scoring systems in PBC patients, especially in patients in advanced stages[17-19].
Some patients with decompensated cirrhosis return to a clinical state consistent with compensated cirrhosis when they undergo appropriate etiological and symptomatic supportive treatment, named the “recompensation phenomenon”[20]. Portal hypertension and systemic inflammation can lead to the progression of decompensated cirrhosis. Recently, studies have been performed on the mechanism and clinical feasibility of reversing decompensation and recompensation in cirrhosis[21-23]. These findings led to updating the stage evaluation concept and an outcomes estimate system for decompensated cirrhosis.
The present study enrolled patients diagnosed with PBC during hospitalization whose disease stages were in the middle and late stages. We compared the effectiveness and differences of various prognostic scoring systems to optimize monitoring, disease evaluation, and timely treatment for advanced stage PBC.
Patient data were derived from nine hospitals in Yunnan Province, China. Patients whose disease was on the first page of the medical record were diagnosed with PBC (ICD-10 code K74.3) and were treated with UDCA after diagnosis. The diagnostic criteria were as follows: elevated serum alkaline phosphatase; AMA-positive or AMA-negative when there were PBC-specific autoantibodies such as spl00 and gp210; histological evidence suggesting non-suppurative destructive cholangitis; and interlobular bile duct injury. PBC can be diagnosed when two criteria are met, and the diagnostic criteria met the 2018 American Association for the Study of Liver Diseases guidelines[24].
Patients were excluded if they underwent follow-up for less than 6 mo or if the dates of treatment initiation or major clinical events were unknown.
Clinical data were obtained from 397 PBC patients diagnosed during hospitalization from May 1, 2015 to December 31, 2021. Clinical data collected from these patients included age, sex, ethnicity, date of PBC diagnosis, past medical and personal histories, clinical manifestations, liver disease complications, liver biopsy results, imaging results, gastroscopy results, and laboratory values (immunological tests, serum biochemistries, complete blood counts, and coagulation times). UDCA (13-15 mg/kg/day) was prescribed after diagnosis, and laboratory results were collected at the 1-year follow-up. Current guidelines and the reports from centers worldwide state that biochemical improvement after 1 year of UDCA treatment accurately predicts long-term outcomes and survival[24-26]; therefore, we collected laboratory results at a 1-year follow-up for prognostic assessment.
All patients were followed up by telephone with a deadline of December 31, 2021. Endpoint events were liver-related death or LT. No endpoint event was non-transplantation survival. Classification of the disease stage was according to the patient’s clinical characteristics and examination data. A cirrhosis diagnosis was based on liver imaging examination (B-ultrasound, computed tomography), liver biopsy, or liver transient elastic imaging in the medical records. The diagnosis standard was derived from the 2020 guidelines[27]. We divided the patients into groups without cirrhosis, compensated cirrhosis, and decompensated cirrhosis.
This study was performed per the Declaration of Helsinki. The Ethics Committee of the second affiliated hospital of Kunming Medical University approved the study (approval No. YJ-2022-14). Each participating center approved the protocol. We analyzed all data anonymously.
The baseline time was the start of UDCA treatment, and the primary endpoint was a composite of death or LT. Patients not meeting this endpoint during follow-up were censored at their final follow-up visit. The formulas of prognostic scores can be found in the Supplementary material. These scores were computed at baseline and after 1 year of UDCA treatment. These risk scores were descriptive statistics to compare patients that did or did not meet the composite endpoint.
Predictive validity was based on model discrimination and calibration. Cox proportional hazard regression analyses were performed to assess the discriminative performance of the risk scoring models at baseline and after UDCA treatment for 1 year. The overall discriminative performance of these models was calculated using the concordance (C)-statistic. Combining these predictive models when assessing the risk of death or LT based on data collected following UDCA treatment for 1 year was further evaluated using Cox regression analyses. C-statistic values were also assessed for various combinations of risk prediction models.
A graphical approach was used to assess model calibration by comparing Kaplan-Meier transplant-free survival estimates produced by these risk prediction models after 1 year of UDCA treatment.
All analyses were performed using R v 4.2.1. To account for missing values, the predictive mean matching of the mice package was applied to interpolate the missing data of laboratory results using multiple interpolation methods. Continuous data were expressed as the median and interquartile range. P < 0.05 was the threshold of significance.
We enrolled 397 PBC patients initially diagnosed while hospitalized and underwent UDCA treatment. The mean age was 56.84 (standard deviation 11.2) years and included 343 (86.4%) females. The specific staging, clinical, and biochemical characteristics at the beginning of UDCA treatment are displayed in Table 1.
| Baseline cohort characteristic | Value |
| Age at diagnosis, yr | 56.84 (11.2) |
| Female, n (%) | 343 (86.4) |
| Year of diagnosis, range | 2015 to 2021 |
| AMA M2+, n (%) | 296 (74.6) |
| AMA M2-, gp210+, n (%) | 98 (24.7) |
| AMA M2-, sp100+, n (%) | 99 (24.9) |
| Liver biopsy cases, n (pathological stage, using the Scheuer classification) | 5 (I), 23 (II), 3 (I-II), 5 (II-III), 14 (III), 4 (III-IV), 77 (IV) |
| PLT as × 109/L | 88 (142, 207) |
| PT in s | 12.2 (13.3, 14.9) |
| INR | 0.96 (1.06, 1.23) |
| ALB in g/L | 29.9 (35.1, 39.9) |
| ALT in U/L | 27 (52, 98) |
| AST in U/L | 40 (68, 120) |
| TBil in mol/L | 15.8 (24.3, 52.3) |
| CREA in mmol/L | 50 (58, 68) |
| ALP in IU/L | 122 (204, 351) |
| GGT in IU/L | 56 (166, 356) |
| GLOBE | 0.34 (1.56, 2.65) |
| UK-PBC | 0.02 (0.07, 0.26) |
| APRI | 0.69 (1.40, 2.54) |
| FIB4 | 2.28 (4.36, 6.93) |
| ALBI | -2.51 (-2.03, -1.39) |
| Mayo | 1.44 (2.33, 3.52) |
| Without cirrhosis, baseline time/end of the follow-up, n (%) | 80 (20.2)/50 (12.6) |
| Compensated cirrhosis | 43 (10.9%)/56 (14.1%) |
| Decompensated cirrhosis | 274 (69.0%)/288 (72.5%) |
| Death | 82 |
| Liver-related death | 79 |
| LT | 4 |
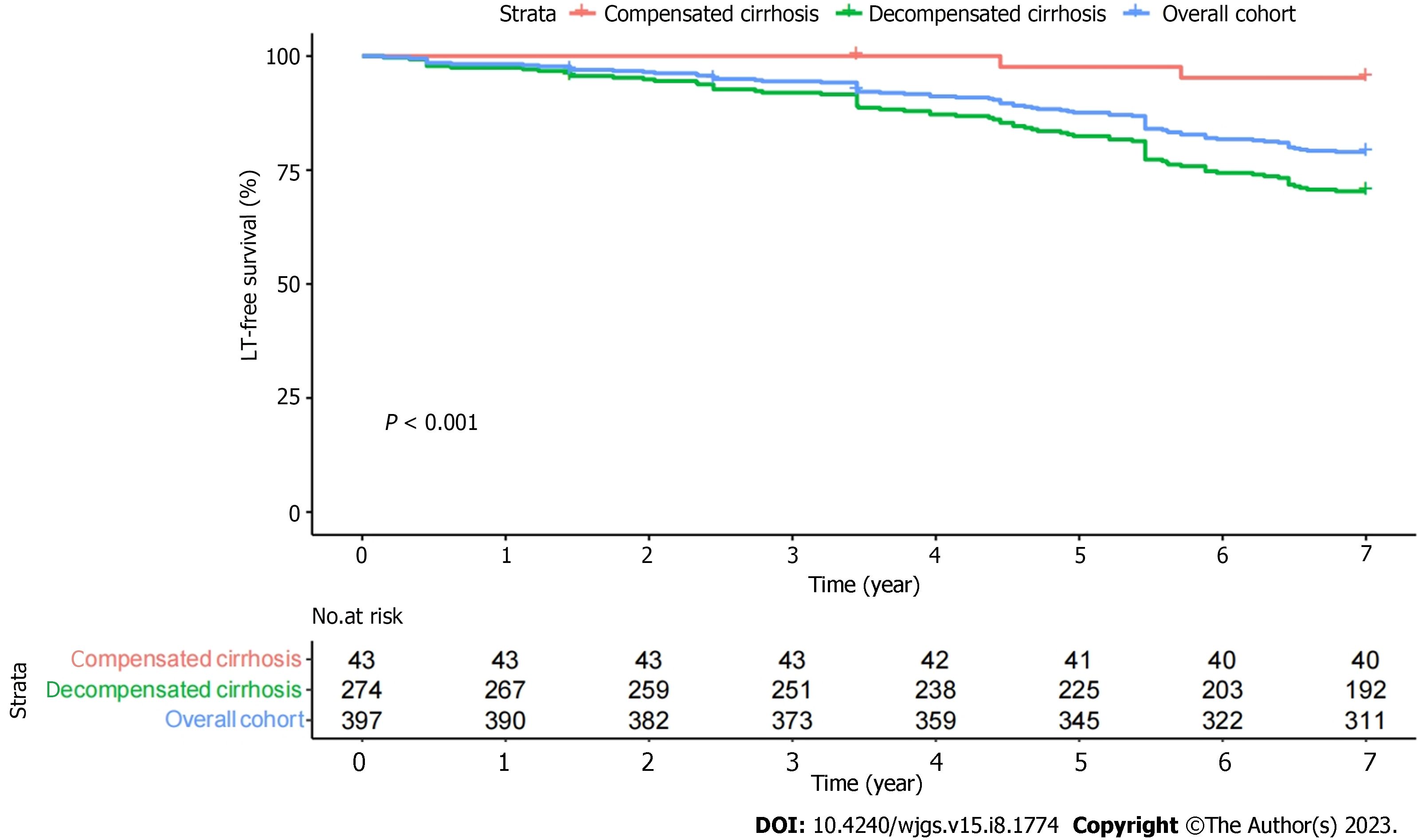
The patients were followed for 6.4 ± 1.4 years, with 3 patients lost to follow-up at the final follow-up. During follow-up, 86 experienced a clinical endpoint: 4 patients underwent LT; and 82 patients died. Liver disease was related to the cause of death in 79/82 (96.3%) patients. The 3-, 5-, and 7-year transplant-free survival rates were 94.0%, 86.9%, and 78.3%, respectively (Figure 1). Advanced stages correlated with lower survival (P < 0.001).
At the start of UDCA therapy, 80 (20.2%) patients had no cirrhosis, 43 (10.9%) patients had compensated cirrhosis, and 274 (69.0%) patients had decompensated cirrhosis.
The overall discriminative performance of the Mayo, APRI, FIB-4, and ALBI models was assessed at baseline based on C-statistic values when used to predict death or LT. GLOBE and UK-PBC scores were based on values measured at baseline and after UDCA treatment for 1 year. The baseline C-statistic values for the Mayo and ALBI scores were 0.702 [95% confidence interval (CI): 0.653-0.751] and 0.705 (95%CI: 0.656-0.755), respectively, while the FIB-4 and APRI scores showed poorer performance (Table 2).
| C-statistic at various follow-up time points (95%CI) | ||
| Risk prediction model | Baseline | 1 yr of UDCA |
| GLOBE | 0.731 (0.681-0.782) | |
| UK-PBC | 0.727 (0.678-0.776) | |
| APRI | 0.592 (0.536-0.647) | 0.347 (0.296-0.398) |
| FIB4 | 0.648 (0.593-0.704) | 0.680 (0.628-0.732) |
| ALBI | 0.705 (0.656-0.755) | 0.725 (0.672-0.778) |
| Mayo | 0.702 (0.653-0.751) | 0.740 (0.690-0.791) |
Following UDCA treatment for 1 year, the C-statistic values for Mayo, GLOBE, UK-PBC, and ALBI scores were 0.740 (95%CI: 0.678-0.776), 0.731 (95%CI: 0.681-0.782), 0.727 (95%CI: 0.678-0.776), and 0.725 (95%CI: 0.672-0.778), respectively. In contrast, the FIB-4 score showed poorer discriminatory power, and the APRI scores showed virtually no discriminatory performance (Table 2; Supplementary Figure 1).
There were no significant differences between the GLOBE, UK-PBC, Mayo, and ALBI scores concerning predictive performance at the start of UDCA treatment or 1 year after (Supplementary Table 1).
Cox regression analyses were used to evaluate the availability of combining predictive models when assessing the odds of death or LT based on data collected following UDCA treatment for 1 year. In univariate Cox regression analyses, the UK-PBC, ALBI, GLOBE, and Mayo scores were all significantly associated with death or LT (P < 0.001) (Table 3). The hazard ratio of UK-PBC was the largest (hazard ratio: 6.046, 95%CI: 3.479-10.510). In multivariate analysis, only the GLOBE scores remained significantly associated with death or LT (Table 3).
| Univariate analyses | Multivariable analyses | |||||
| Prognostic score | Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value |
| GLOBE | 1.703 | 1.474-1.967 | < 0.001 | 1.582 | 1.029-2.433 | 0.037 |
| UK-PBC | 6.046 | 3.479-10.510 | < 0.001 | 1.012 | 0.330-3.102 | 0.983 |
| APRI | 0.998 | 0.971-1.024 | 0.860 | 0.918 | 0.812-1.038 | 0.171 |
| FIB4 | 1.005 | 0.997-1.013 | 0.242 | 1.008 | 0.991-1.024 | 0.369 |
| ALBI | 2.546 | 1.955-3.316 | < 0.001 | 1.194 | 0.595-2.40 | 0.618 |
| Mayo | 1.495 | 1.348-1.676 | < 0.001 | 1.022 | 0.697-1.497 | 0.913 |
Adding the UK-PBC, APRI, FIB-4, Mayo, and ALBI scores to the GLOBE score did not significantly improve the discriminative performance, with a C-statistic value that remained at 0.73 (Supplementary Table 2). The C-statistics of all scores before adding are displayed in Table 1.
Combining the UK-PBC score with the APRI, FIB-4, and ALBI scores did not cause a significant increase in discrimination performance. The C-statistic remained at 0.72 (Supplementary Table 2); only with the addition of the Mayo score did the C-statistic increase (+0.02).
The most significant increase in C-statistic values was observed when the Mayo score was combined with the others. The APRI score increased to 0.740 (95%CI: 0.689-0.791), and the FIB-4 score increased to 0.741 (95%CI: 0.69-0.791) (Supplementary Table 2)
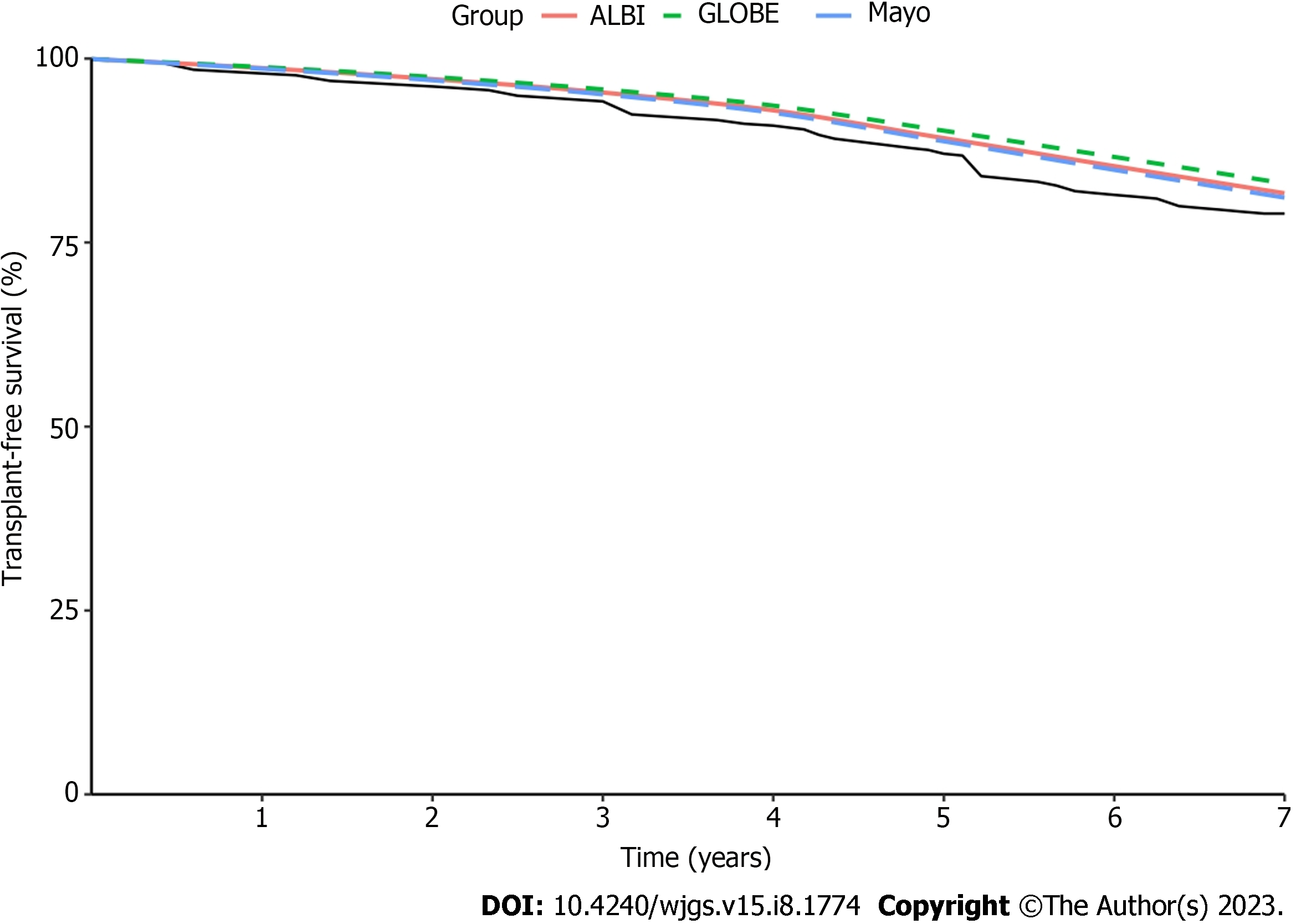
The ALBI, GLOBE, and Mayo scores with superior discriminatory performance were selected to evaluate the predicted and observed survival (Figure 2). The UK-PBC score was omitted from the analyses because it primarily predicts liver-related death rather than transplant-free survival[28]. The three risk prediction models tended to overestimate transplant-free survival. They showed good calibration for short-term survival; the deviation from observed survival at 1 year to 3 years for ALBI, GLOBE, and Mayo was < 0.2%. After 3 years, the deviation tended to be greater yearly. The most significant deviation was for the GLOBE score (2.0%-4.3%), and the most minor was for the Mayo score (1.0%-2.4%). When these scores were evaluated at yearly intervals for up to 7 years, the deviation of the GLOBE score was the greatest, and the Mayo score was the most minor. By comparison, the Mayo score demonstrated the best calibration.
We assessed the PBC-specific scores GLOBE, UK-PBC, and Mayo and compared the ALBI, APRI, and FIB-4 scores. These analyses revealed that the ALBI and Mayo scores showed adequate discriminatory performance and good predictive accuracy at baseline. The Mayo score demonstrated superior discriminatory performance and calibration singly and combined with other risk models, suggesting that this score is the best risk prediction model for predicting liver-related death or LT in PBC patients in the advanced stage. These findings also suggested that the performance of the PBC-specific risk scores was superior to other prognostic scores for advanced PBC.
Models with a C-statistic value greater than 0.7 are considered good prognostic models. The Mayo score was the only model consistently reaching this threshold at baseline and 1 year of UDCA treatment. The C-statistic of the Mayo score was greater after patients received UDCA for 1 year, suggesting an increase in discriminatory performance with prolonged UDCA treatment. The next most effective predictive models were the GLOBE, UK-PBC, and ALBI scores, with no significant differences in predicting liver-related death or LT following UDCA treatment for 1 year.
The Mayo score exhibited consistently better discriminative performance than other scores in this PBC patient cohort. The Mayo score is a traditional risk prediction model developed for PBC patients, primarily developed to evaluate untreated PBC patients. However, this study enrolled patients that had undergone UDCA treatment and were in an advanced stage. The Mayo score has previously been linked to transplant-free survival among patients that underwent UDCA treatment, enabling their stratification into low- and high-risk groups based on the original thresholds[29,30]. However, the reliance of this scoring model on ascites, which can be subjective, may limit its clinical applicability.
In this study, the parameter was derived from the results of imaging examinations during hospitalization, and this examination is a routine item of these hospitalized patients. Therefore, the judgment of the parameter of ascites was relatively objective. In theory, the superior discriminatory performance of the Mayo score may be promoted by ascites and prothrombin time, which are the most relevant parameters in late stage PBC; other parameters are TBil and ALB, which also are indicators of significant changes in patients with more advanced stages. Based on these characteristics, the Mayo score may be more applicable for prognosis assessment in advanced stage PBC patients. Our study verified this point, but the actual evidence remains to be further verified in a large population or more studies.
The discriminatory performance of the GLOBE, UK-PBC, and ALBI scores is secondary to the Mayo score. Both the UK-PBC and GLOBE scores were developed as PBC-specific scoring systems and have previously been applied to evaluate the prognosis of early PBC patients. Our cohort was mainly late stage patients, and the results were inferior to the Mayo score. The ALBI score is calculated using two indicators (TBil, ALB), which are validated biomarkers associated with PBC disease progression[31-33]. APRI and FIB-4 scores had inferior discriminatory performance in this study, while the two were liver fibrosis scores based on biochemical indicators. However, this study’s poor performance may be because most patients had cirrhosis without significant differences in the progression of liver fibrosis, which is not applicable to predicting advanced PBC patients.
The different combinations of prognostic models were evaluated for their ability to predict death or LT. The study results showed that GLOBE and UK-PBC were relatively stable, with little change in the C-statistic when other scores were added. Moreover, the univariate and multivariate Cox regression analyses of all predictive models also support this point. The highest C-statistic value increases were observed when the Mayo scores were combined with the other scores. The results demonstrated that the GLOBE and UK-PBC score models have good stability and are applicable for prognosis assessment exclusively. While the APRI and FIB-4 scores were applied to combine with other scores, the best discriminatory performance was combined with the Mayo score.
We chose the ALBI, GLOBE, and Mayo sores for model calibration, which had superior discriminatory performance after UDCA therapy for 1 year, while the UK-PBC model was omitted because it predicts liver-related death and not transplant-free survival[28]. These scores all tended to overestimate the transplant-free survival rate, with better calibration at 1-3 years. The deviation tended to increase yearly after 3 years. In the 1-7-year interval, the deviation of the GLOBE score was the greatest, and the Mayo score was the most minor. In contrast, the best model calibration was the Mayo score. These findings suggested that the Mayo score has the best prediction performance and accuracy for advanced PBC patients.
This study has several limitations. First, we did not have a large study cohort, and the comparison of prognostic scores was calculated at baseline and 1 year later. This limitation indicates the need for verification using large sample sizes and prospective studies. Second, this was a retrospective analysis; some of the included data were missing. We applied predictive mean matching to interpolate the missing values. Third, while the UK-PBC risk score was developed to predict liver-related death and not transplant-free survival (unlike the other score models), the same analyses used the endpoints and indicated similar discriminatory performance. Despite the limitations, the study is significant because of the lack of the comparison of prognostic scores in advanced PBC patients.
The Mayo, GLOBE, UK-PBC, and ALBI scores had excellent prediction performance for death and LT. Mayo scores had the best prediction efficacy in discriminating performance and predicting outcomes. The significance of this study was that it enables advanced PBC patients to be monitored and assessed closely in clinical practice to delay PBC progression.
Due to the chronic progressive disease characteristics of primary biliary cholangitis (PBC), patients with advanced PBC should not be ignored. Most prognostic score studies have focused on early stage PBC.
This study was designed to compare the prognostic value of different risk scores in the PBC patients with advanced disease stages.
To determine the best prognostic score to ensure that the clinical majority of PBC patients get more monitoring and assessment.
The discriminatory performance of the scores was assessed with concordance statistics at baseline and after 1 year of ursodeoxycholic acid (UDCA) treatment. The combined performance of prognostic scores in estimating the risk of death or liver transplantation after 1 year of UDCA treatment was assessed using Cox regression analyses. Predictive accuracy was evaluated by comparing predicted and actual survival through Kaplan-Meier analyses.
After receiving UDCA treatment for 1 year, the score with the best discrimination performance was the Mayo score, with a concordance statistic of 0.740 (95% confidence interval: 0.690-0.791). The ALBI, GLOBE, and Mayo scores tended to overestimate transplant-free survival. Comparing 7 years of calibration results showed that the Mayo score was the best model.
The Mayo, GLOBE, UK-PBC, and ALBI scores demonstrated comparable discriminating performance for advanced stage PBC. The Mayo score showed optimal discriminatory performance and excellent predictive accuracy.
There is a need for verification of our results with larger sample sizes and prospective studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology.
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kreisel W, Germany; Manesis EK, Greece S-Editor: Yan JP L-Editor: Filipodia P-Editor: Cai YX
| 1. | Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396:1915-1926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Galoosian A, Hanlon C, Zhang J, Holt EW, Yimam KK. Clinical Updates in Primary Biliary Cholangitis: Trends, Epidemiology, Diagnostics, and New Therapeutic Approaches. J Clin Transl Hepatol. 2020;8:49-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Shah RA, Kowdley KV. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol. 2020;5:306-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, Patanwala I, Pereira SP, Thain C, Thorburn D, Tiniakos D, Walmsley M, Webster G, Jones DEJ. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568-1594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Alomari M, Covut F, Al Momani L, Chadalavada P, Hitawala A, Young MF, Romero-Marrero C. Evaluation of the United Kingdom-primary biliary cholangitis and global primary biliary cholangitis group prognostic models for primary biliary cholangitis patients treated with ursodeoxycholic acid in the U.S. population. JGH Open. 2020;4:132-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Yoo JJ, Cho EJ, Lee B, Kim SG, Kim YS, Lee YB, Lee JH, Yu SJ, Kim YJ, Yoon JH. Prognostic Value of Biochemical Response Models for Primary Biliary Cholangitis and the Additional Role of the Neutrophil-to-Lymphocyte Ratio. Gut Liver. 2018;12:714-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Chang JI, Kim JH, Sinn DH, Cho JY, Kim KM, Oh JH, Park Y, Sohn W, Goh MJ, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Clinical Outcomes and Validation of Ursodeoxycholic Acid Response Scores in Patients with Korean Primary Biliary Cholangitis: A Multicenter Cohort Study. Gut Liver. 2023;. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 8. | Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 516] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Weinmann A, Sattler T, Unold HP, Grambihler A, Teufel A, Koch S, Schuchmann M, Biesterfeld S, Wörns MA, Galle PR, Schulze-Bergkamen H. Predictive scores in primary biliary cirrhosis: a retrospective single center analysis of 204 patients. J Clin Gastroenterol. 2015;49:438-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Lammers WJ, Kowdley KV, van Buuren HR. Predicting outcome in primary biliary cirrhosis. Ann Hepatol. 2014;13:316-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Thandassery RB, Al Kaabi S, Soofi ME, Mohiuddin SA, John AK, Al Mohannadi M, Al Ejji K, Yakoob R, Derbala MF, Wani H, Sharma M, Al Dweik N, Butt MT, Kamel YM, Sultan K, Pasic F, Singh R. Mean Platelet Volume, Red Cell Distribution Width to Platelet Count Ratio, Globulin Platelet Index, and 16 Other Indirect Noninvasive Fibrosis Scores: How Much Do Routine Blood Tests Tell About Liver Fibrosis in Chronic Hepatitis C? J Clin Gastroenterol. 2016;50:518-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HL, Floreani A, Ponsioen CY, Mayo MJ, Invernizzi P, Battezzati PM, Parés A, Burroughs AK, Mason AL, Kowdley KV, Kumagi T, Harms MH, Trivedi PJ, Poupon R, Cheung A, Lleo A, Caballeria L, Hansen BE, van Buuren HR; Global PBC Study Group. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology. 2015;149:1804-1812.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 275] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 13. | Olmez S, Sayar S, Avcioglu U, Tenlik İ, Ozaslan E, Koseoglu HT, Altiparmak E. The relationship between liver histology and noninvasive markers in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:773-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1174] [Cited by in F6Publishing: 1640] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 15. | Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, Chan HL, To KF. New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Fujita K, Nomura T, Morishita A, Shi T, Oura K, Tani J, Kobara H, Tsutsui K, Himoto T, Masaki T. Prediction of Transplant-Free Survival through Albumin-Bilirubin Score in Primary Biliary Cholangitis. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Efe C, Taşçilar K, Henriksson I, Lytvyak E, Alalkim F, Trivedi H, Eren F, Eliasson J, Beretta-Piccoli BT, Fischer J, Calişkan AR, Chayanupatkul M, Coppo C, Ytting H, Purnak T, Muratori L, Werner M, Muratori P, Rorsman F, Önnerhag K, Günşar F, Nilsson E, Heurgué-Berlot A, Güzelbulut F, Demir N, Gönen C, Semela D, Aladağ M, Kiyici M, Schiano TD, Montano-Loza AJ, Berg T, Ozaslan E, Yoshida EM, Bonder A, Marschall HU, Wahlin S. Validation of Risk Scoring Systems in Ursodeoxycholic Acid-Treated Patients With Primary Biliary Cholangitis. Am J Gastroenterol. 2019;114:1101-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Goet JC, Murillo Perez CF, Harms MH, Floreani A, Cazzagon N, Bruns T, Prechter F, Dalekos GN, Verhelst X, Gatselis NK, Lindor KD, Lammers WJ, Gulamhusein A, Reig A, Carbone M, Nevens F, Hirschfield GM, van der Meer AJ, van Buuren HR, Hansen BE, Parés A; GLOBAL PBC Study Group. A Comparison of Prognostic Scores (Mayo, UK-PBC, and GLOBE) in Primary Biliary Cholangitis. Am J Gastroenterol. 2021;116:1514-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Scaravaglio M, Carbone M. Prognostic Scoring Systems in Primary Biliary Cholangitis: An Update. Clin Liver Dis. 2022;26:629-642. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 20. | Balcar L, Semmler G, Pomej K, Simbrunner B, Bauer D, Hartl L, Jachs M, Paternostro R, Bucsics T, Pinter M, Trauner M, Mandorfer M, Reiberger T, Scheiner B. Patterns of acute decompensation in hospitalized patients with cirrhosis and course of acute-on-chronic liver failure. United European Gastroenterol J. 2021;9:427-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, Klein S, Welsch C, Schäfer L, Jansen C, Claria J, Alcaraz-Quiles J, Arroyo V, Moreau R, Fernandez J, Bendtsen F, Mehta G, Gluud LL, Møller S, Praktiknjo M, Trebicka J. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021;70:379-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Hoshi H, Chu PS, Yoshida A, Taniki N, Morikawa R, Yamataka K, Noguchi F, Kasuga R, Tabuchi T, Ebinuma H, Saito H, Kanai T, Nakamoto N. Vulnerability to recurrent episodes of acute decompensation/acute-on-chronic liver failure characterizes those triggered by indeterminate precipitants in patients with liver cirrhosis. PLoS One. 2021;16:e0250062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 112] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 24. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 25. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 719] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 26. | Leung KK, Deeb M, Hirschfield GM. Review article: pathophysiology and management of primary biliary cholangitis. Aliment Pharmacol Ther. 2020;52:1150-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, Taniai M, Terai S, Nishikawa H, Hiasa Y, Hidaka H, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56:593-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 28. | Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, Griffiths L, Lim R, Trembling P, Williamson K, Wareham NJ, Aldersley M, Bathgate A, Burroughs AK, Heneghan MA, Neuberger JM, Thorburn D, Hirschfield GM, Cordell HJ, Alexander GJ, Jones DE, Sandford RN, Mells GF; UK-PBC Consortium. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 29. | Kilmurry MR, Heathcote EJ, Cauch-Dudek K, O'Rourke K, Bailey RJ, Blendis LM, Ghent CN, Minuk GY, Pappas SC, Scully LJ, Steinbrecher UP, Sutherland LR, Williams CN, Worobetz LJ. Is the Mayo model for predicting survival useful after the introduction of ursodeoxycholic acid treatment for primary biliary cirrhosis? Hepatology. 1996;23:1148-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, Dickson ER. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 1999;19:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, Ponsioen CY, Floreani A, Corpechot C, Mayo MJ, Battezzati PM, Parés A, Nevens F, Burroughs AK, Kowdley KV, Trivedi PJ, Kumagi T, Cheung A, Lleo A, Imam MH, Boonstra K, Cazzagon N, Franceschet I, Poupon R, Caballeria L, Pieri G, Kanwar PS, Lindor KD, Hansen BE; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-49.e5; quiz e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 32. | Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20:137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 241] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Bonnand AM, Heathcote EJ, Lindor KD, Poupon RE. Clinical significance of serum bilirubin levels under ursodeoxycholic acid therapy in patients with primary biliary cirrhosis. Hepatology. 1999;29:39-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |