Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.664
Peer-review started: December 20, 2022
First decision: January 9, 2023
Revised: January 11, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 27, 2023
Hepatic venous pressure gradient (HVPG) is the gold standard for diagnosis of portal hypertension (PH), invasiveness and potential risks in the process of measurement limited its widespread use.
To investigate the correlation of computed tomography (CT) perfusion parameters with HVPG in PH, and quantitatively assess the blood supply changes in liver and spleen parenchyma before and after transjugular intrahepatic portosystemic shunt (TIPS).
Twenty-four PH related gastrointestinal bleeding patients were recruited in this study, and all patients were performed perfusion CT before and after TIPS surgery within 2 wk. Quantitative parameters of CT perfusion, including liver blood volume (LBV), liver blood flow (LBF), hepatic arterial fraction (HAF), spleen blood volume (SBV) and spleen blood flow (SBF), were measured and compared before and after TIPS, and the quantitative parameters between clinically significant PH (CSPH) and non-CSPH (NCSPH) group were also compared. Then the correlation of CT perfusion parameters with HVPG were analyzed, with statistical significance as P < 0.05.
For all 24 PH patients after TIPS, CT perfusion parameters demonstrated decreased LBV, increased HAF, SBV and SBF, with no statistical difference in LBF. Compared with NCSPH, CSPH showed higher HAF, with no difference in other CT perfusion parameters. HAF before TIPS showed positive correlation with HVPG (r = 0.530, P = 0.008), while no correlation was found in other CT perfusion parameters with HVPG and Child-Pugh scores.
HAF, an index of CT perfusion, was positive correlation with HVPG, and higher in CSPH than NCSPH before TIPS. While increased HAF, SBF and SBV, and decreased LBV, were found after TIPS, which accommodates a potential non-invasive imaging tool for evaluation of PH.
Core Tip: Transjugular intrahepatic portosystemic shunt (TIPS) is an effective therapy for portal hypertension (PH) related gastro-esophageal variceal bleeding (GEVB). However, the decrease of liver blood supply after TIPS led to a decline in the liver detoxification, which increased potential risk for the occurrence of various complications such as hepatic encephalopathy, liver dysfunction and even liver failure. This study explored the feasibility of computed tomography perfusion imaging in quantitatively evaluating the changes in liver and spleen blood supply in PH related GEVB before and after TIPS, and making correlation analysis between perfusion parameters, hepatic venous pressure gradient and Child-Pugh scores were evaluated.
- Citation: Dong J, Zhang Y, Wu YF, Yue ZD, Fan ZH, Zhang CY, Liu FQ, Wang L. Computed tomography perfusion in differentiating portal hypertension: A correlation study with hepatic venous pressure gradient. World J Gastrointest Surg 2023; 15(4): 664-673
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/664.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.664
Portal hypertension (PH) is a main manifestation of increased portal pressure caused by various factors[1-3]. It is commonly caused by liver fibrosis, which leads to a series of complications, such as bleeding from gastroesophageal varices, ascites, infection, etc. Some patients progress to liver cancer, with poor prognosis[4]. The diagnostic gold standard for PH is hepatic venous pressure gradient (HVPG) higher than 5 mmHg, and it has been reported that when HVPG was higher than 10 mmHg, patients were classified into clinically significant PH (CSPH)[1,4,5], with the increased rate of complications and poor 1-year survival rate[5,6]. Transjugular intrahepatic portosystemic shunt (TIPS) is an effective technique for the treatment of PH related complications compared to drug therapy and surgical treatment, especially for patients with gastroesophageal variceal bleeding[5,7]. However, the complications after TIPS, such as hepatic encephalopathy, liver dysfunction and even liver failure[8-11], were also concerned for most patients. Therefore, it is necessary to make non-invasively quantitative evaluation of HVPG and liver blood supply changes after TIPS, which is useful for precise treatment for PH patients, especially for TIPS surgery[12].
Multi-modal imaging, including computed tomography (CT) perfusion, functional magnetic resonance imaging and contrast enhanced ultrasonography, have been applied in the assessment of liver cirrhosis, which demonstrated the capability in non-invasive assessment of HVPG and liver blood supply changes[13-15]. It is reported that CT perfusion can acquire quantitative indices such as blood flow and blood volume through continuous dynamic scanning, which can be used to quantify the blood perfusion of target organs, such as stroke, liver, lung tumors, etc.[16,17] and it has also been applied in assessment of PH related liver cirrhosis. Therefore, our study is to explore the feasibility of CT perfusion imaging in quantitatively evaluating HVPG, and the blood supply changes in liver and spleen for PH patients, and make correlation analysis between perfusion CT parameters and HVPG.
This prospective study was approved by the Institutional Ethics Committee in our hospital, and all written informed consents were obtained from each participate. All patients with recurrent gastroesophageal variceal bleeding caused by PH were recruited for TIPS surgery therapy from January to June of 2018. The inclusion criteria were: (1) Patients with PH related recurrent gastroesophageal bleeding, and prepared to be performed with TIPS surgery; (2) Child-Pugh score and perfusion CT were evaluated within 2 wk before and after TIPS. The exclusive criteria were: (a) Any other etiology causing gastrointestinal bleeding; (b) Primary and/or secondary liver tumors; and (c) Iatrogenic factors causing liver blood supply changes, such as partial hepatectomy, splenectomy, and TIPS before etc; (3) Portal vein and/or hepatic vein lesions affecting liver blood supply, such as portal vein thrombosis leading stenosis higher than 75%, portal vein cavernous transformation, Budd-Chiari syndrome, etc; (4) Dysfunction in vital organs, such as cardiac, renal, respiratory failure; and (5) Any factors made the perfusion CT unavailable, such as motion and metal artifacts, allergic to contrast media.
Liver and spleen perfusion CT were performed on Revolution CT (GE Healthcare, WI), with the scanning parameters as follows[17-20]: 100 kVp tube voltage, 50 to 200 mA tube current with automated tube current modulations, 14 of noise index, 5 mm slice thickness, 1.0 s rotation speed, 0.992:1 helical pitch, and ASIR was set at proportion of 80%. Contrast media (Omnipaque, 350) volume was fixed as 50 mL, with 50 mL saline chaser, and were injected in 5 mL/s rate through antecubital with a power injector (Stellant, Medtron, Saarbrucken, Germany). The CT perfusion scanning time was set as 80 s, which included three parts of acquisition as follows: 10 scans with 1 s interscan gap firstly, 12 scans with 2 s interscan gap secondly, and 4 scans with 4 s interscan gap finally for each patient. All patients were compressed with a restriction band to reduce respiratory motion, and were instructed to avoid deep and irregular breathing during the procedure, which reduced respiratory motion artifacts.
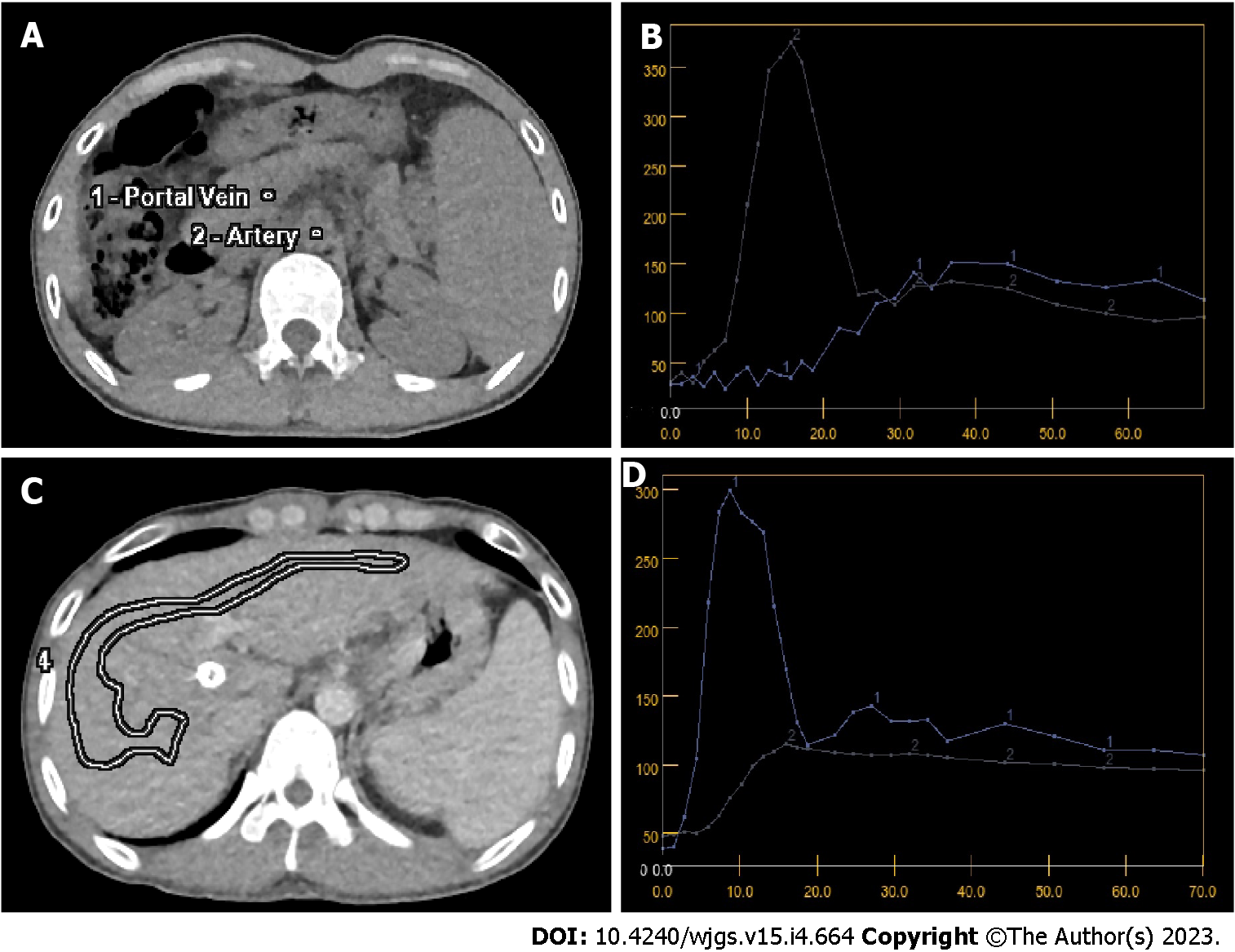
All perfusion CT raw data were reconstructed with a 2.5 mm slice thickness, and then the images were sent to a commercial software (CT Perfusion 4D AW 4.7, GE healthcare) for post-processions. First, the images mis-registration between different scanning phase were corrected by iterative registration reconstruction technique. Then, the constructed images were sent to commercial software for post-procession, and regions of interest were selected in different area of liver and spleen separately, and then quantitative parameters of perfusion were calculated, including liver blood volume (LBV), liver blood flow (LBF), hepatic arterial fraction (HAF), splenic blood volume (SBV), and splenic blood flow (SBF). The post-processions have been performed for three times, and the results were averaged as the final parameters.
All patients were performed with TIPS surgery within 2 wk after CT perfusion, and HVPG was measured in the process of TIPS surgery in accordance with guideline[21]. The process was as follows: Firstly, patients were in fast state for more than 8 h before TIPS surgery, and then local anesthesia was performed to cannulate the right internal jugular vein using Seldinger technique, and all the patients were placed a 5-French balloon catheter (Edwards Lifesciences LLC, United States) into the right hepatic vein for measurement of free and wedged hepatic venous pressures, the process were performed for three times at the same point, and the average of the difference between wedged and free hepatic venous pressure were recorded as the final results of HVPG. Then, TIPS surgery would be performed, and all patients underwent perfusion CT within 2 wk after TIPS. The therapeutic effect would be assessed after 3-mo follow-up, with complications recorded, including hemorrhage, hepatic encephalopathy (HE), liver dysfunction, bile leakage.
All statistical analysis was performed with Statistical Package for Social Sciences (SPSS) version 24.0 program. Quantitative results were described as mean ± SD. Normal distribution tests were analyzed with Kolmogorov-Smirnov test. Quantitative indices before and after TIPS, including LBV, HAF, LBF, SBV, were compared with pair-sample t-test, and the pearson correlation analyses were performed with CT perfusion parameters with HVPG and Child-Pugh scores, and a value of P < 0.05 was considered as statistical significance.
Thirty-seven patients were recruited in this study at first, and all written informed consents were signed, and 13 patients were excluded finally for the following reasons, including 3 patients with portal vein stenosis higher than 75% caused by portal vein thrombosis, 3 patients with splenectomy, 2 patients with portal vein cavernous transformation, 2 patients diagnosed as hepatic cell carcinoma and 2 patients with severe artifacts caused by respiratory motion in CT perfusion acquisitions, and 1 patients with serious complications of hepatic coma after TIPS. Finally, 24 patients (3/21, female/male) were enrolled in this study, including 11 hepatitis B, 7 alcoholic liver diseases, 3 drug induced hepatitis, 1 autoimmune hepatitis, 1 primary sclerosing cholangitis and 1 idiopathic PH. Patient general data were listed in Table 1.
| Characteristic | Value |
| Sex (female/male), n | 3/21 |
| Age (yr), mean ± SD | 51.3 ± 9.7 |
| Height (cm), mean ± SD | 168.7 ± 6.1 |
| Weight (kg), mean ± SD | 63.4 ± 12.5 |
| Previous episodes of variceal bleeding, mean ± SD | 3 ± 2 |
| Treatment history, n (%) | |
| β blockade only | 2 (8.3) |
| Sclera therapy only | 4 (16.7) |
| β blockade and sclerotherapy | 18 (75.0) |
| Child-Pugh stage, n (%) | |
| A | 5 (20.8) |
| B | 18 (75.0) |
| C | 1 (4.2) |
| Ascites, n (%) | |
| None | 16 (66.7) |
| Mild | 2 (8.3) |
| Severe | 6 (25.0) |
| HVPG in mmHg, n (%) | |
| < 10 | 11 (45.8) |
| ≥ 10 | 13 (54.2) |
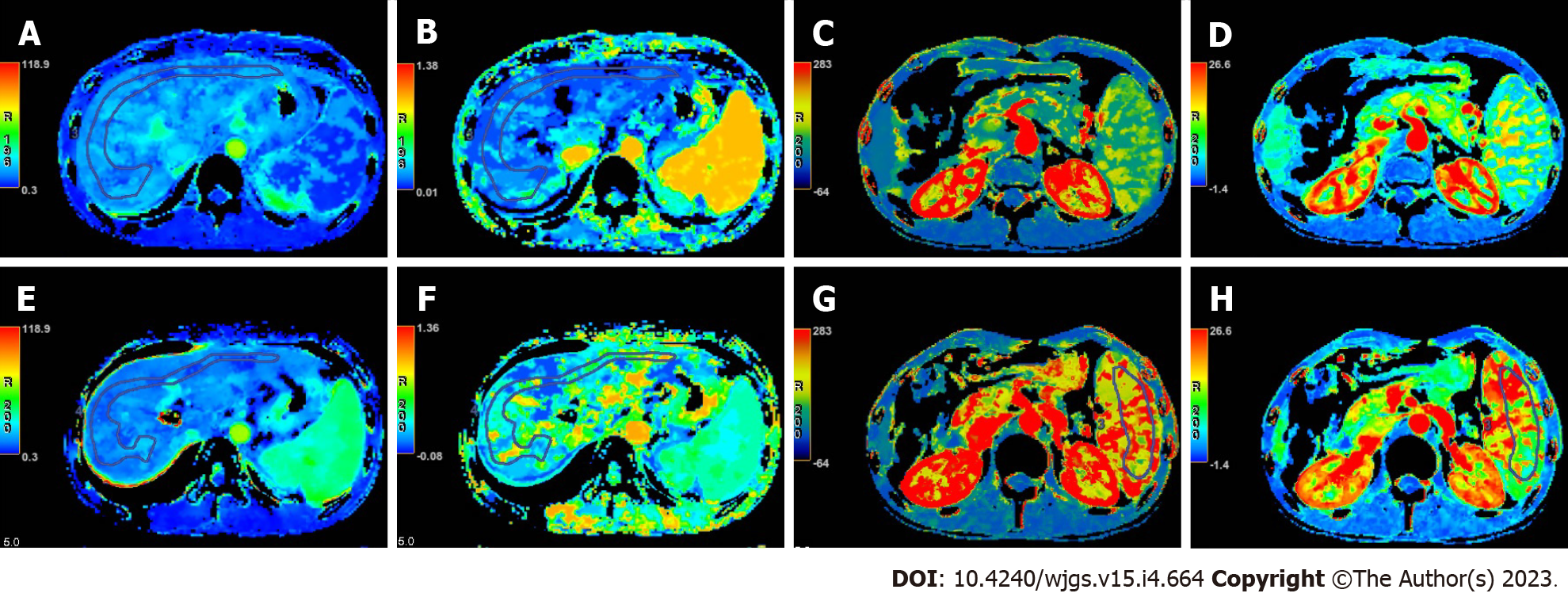
The quantitative parameters of CT perfusion before and after TIPS were compared in Table 2. After TIPS, decreased LBV and increased HAF were found, while no difference was shown in LBF. Whereas for SBF and SBV, both spleen blood flow and blood volume increased significantly after TIPS. The baseline of CT maps and time density curves were shown in Figure 1, and the comparison of colored perfusion maps were shown in Figure 2.
| Parameters | Before TIPS | After TIPS | P value |
| Liver parenchyma | |||
| LBF | 97.2 ± 32.2 | 85.8 ± 37.9 | 0.232 |
| LBV | 19.2 ± 5.3 | 12.1 ± 2.4 | < 0.001 |
| HAF (× 10-2) | 12.3 ± 6.1 | 55.3 ± 9.9 | < 0.001 |
| Spleen parenchyma | |||
| SBF | 107.6 ± 32.1 | 160.6 ± 33.1 | < 0.001 |
| SBV | 12.1 ± 3.0 | 18.7 ± 3.4 | < 0.001 |
The comparison of the results of CSPH group (n = 13) with non-CSPH (NCSPH) group (n = 11) was shown in Table 3. Before TIPS, there were no significant differences in the blood flow and blood volume of liver and spleen between the CSPH group and the NCSPH group, while the HAF in NCSPH group was much lower than that in CSPH group. After TIPS, no difference was found between the CSPH and NCSPH groups for all CT perfusion parameters.
| Parameters | NCSPH (n = 11) | CSPH (n = 13) | P value |
| Before TIPS | |||
| Liver parenchyma | |||
| LBF | 103.6 ± 23.3 | 91.7 ± 38.3 | 0.377 |
| LBV | 19.5 ± 2.9 | 18.9 ± 6.9 | 0.796 |
| HAF (× 10-2) | 5.4 ± 2.8 | 18.2 ± 10.9 | 0.001 |
| Spleen parenchyma | |||
| SBF | 95.9 ± 25.1 | 117.4 ± 34.9 | 0.102 |
| SBV | 12.4 ± 3.5 | 12.0 ± 2.7 | 0.747 |
| After TIPS | |||
| Liver parenchyma | |||
| LBF | 77.5 ± 33.9 | 92.8 ± 41.0 | 0.336 |
| LBV | 11.3 ± 2.4 | 12.7 ± 2.2 | 0.165 |
| HAF (× 10-2) | 54.4 ± 11.4 | 56.0 ± 8.8 | 0.711 |
| Spleen parenchyma | |||
| SBF | 150.0 ± 35.7 | 169.9 ± 29.1 | 0.153 |
| SBV | 19.2 ± 3.8 | 18.3 ± 3.0 | 0.522 |
Among CT perfusion parameters, preoperative HAF was correlated with HVPG (r = 0.530, P = 0.008), suggesting that hepatic arterial perfusion parameters were positively correlated with HVPG. Whereas other perfusion parameters before TIPS, including LBF, LBV, SBF and SBV were not correlated with HVPG. All CT perfusion parameters, including liver perfusion parameters LBF, LBV and HAF, and spleen perfusion parameters SBF and SBV, were not correlated with the Child-Pugh score before TIPS. Besides, no correction was found between HVPG and Child-Pugh score.
After TIPS placement, all patients received 2-wk and 3-mo reexamination to evaluate the curative effect. All patients had patency of TIPS stents, and no obvious thrombosis was found, no bleeding occurred again, and the ascites were relieved apparently. In terms of complications, there were 4 patients with HE and 1 patient with hepatic insufficiency within 3 mo, all of which occurred within 2 wk after operation. Among them, there was 1 patient with mild HE, 2 patients with moderate HE and 1 patient with severe HE, and they recovered well after liver protection therapy. One patient with hepatic insufficiency occurred within 3 d after the operation, and recovered within 2 wk after rapid correction.
CT perfusion demonstrated capacity in quantitatively evaluating the liver blood supply changes after TIPS surgery. Our results suggested that LBV decreased significantly after TIPS procedure (19.2 ± 5.3 vs 12.1 ± 2.4, P < 0.01), while HAF increased (12.3 ± 6.1 vs 56.0 ± 8.8, P < 0.01) significantly. These results showed that the total liver blood supply decreased, but the proportion of hepatic artery blood supply increased after TIPS. It is well-known that TIPS is one of the effective methods for the treatment of PH[4,5,7,22], such as PH related gastro-esophageal variceal bleeding, refractory ascites, etc[1,4,23]. The reason of this results can be explained as follows: The liver is a dual blood supply organ, and, the proportion of hepatic artery is only about 20%-30%, while the portal vein is more than 70% under normal circumstances. For PH patients after TIPS surgery, part of the portal vein blood supply would be drained directly into inferior vena cava through TIPS stent, so the total blood from portal vein system decreased significantly, and the PH would be alleviated[14,20,22,24]. Then, the compensatory hepatic artery blood supply would be increased, which resulted in the obvious increase in hepatic artery supply proportion[25,26]. However, since the compensated blood supply from hepatic artery is not enough to compensate for the decrease in portal venous blood supply, so the effective blood supply of the liver parenchyma still decreased[13,14,20,24,27]. The decrease of total effective blood supply in liver parenchyma increased the potential risk of the occurrence of hepatic dysfunction, hepatic encephalopathy, and even liver failure after TIPS[8-10].
Spleen parenchyma blood supply changes could also be assessed with CT perfusion. Our results suggested that SBF (107.6 ± 32.1 vs 160.6 ± 33.1, P < 0.01) and SBV (12.1 ± 3.0 vs 18.7 ± 3.4, P < 0.01) increased significantly after TIPS. For patients with PH, due to the presence of varicose veins, the formation of collateral circulation and a large amount of ascites caused by the high pressure of the portal venous system, the amount of blood returning to the liver is blocked, thereby reducing the effective circulating blood volume of the body[1,4,13,26]. After TIPS, portal venous blood flow can be directly returned to the systemic circulation through the TIPS shunt, which increases the effective circulating blood volume. Therefore, the arterial blood supply is significantly increased after TIPS[24]. As we all know, the blood supply of the spleen only comes from the arterial system, which explains the increase of SBV and SBF, and may also be an important factor for the increase of HAF in liver.
CT perfusion showed capacity in discriminating CSPH and NCSPH group. It is reported that the rate of various complications in CSPH group is significantly higher than NCSPH group[28]. So, it is necessary to discriminate CSPH and NCSPH non-invasively. Our results suggested that before TIPS, HAF in CSPH group was significantly higher than NCSPH group (18.2 ± 10.9 vs 5.4 ± 2.8, P < 0.01), which means the arterial proportion of liver blood supply in CSPH is much higher than NCSPH group. but no statistical difference was found in LBF and LBV, which means HAF is the only index of CT perfusion in discriminating CSPH with NCSPH before TIPS surgery. However, after TIPS surgery, all CT perfusion parameters, including LBF, LBV and HAF showed no statistical difference between CSPH and NCSPH group. This is because part of the portal vein blood supply into liver had been conducted into inferior vena cava directly through TIPS shunt, so the hemodynamics of liver blood supply had been changed after TIPS. Therefore, HAF of CT perfusion can be used to discriminate CSPH and NCSPH before TIPS.
HAF in CT perfusion could be used as a non-invasive index to assess HVPG. Our results showed that HAF had a positive correlation (r = 0.553, P = 0.008) with HVPG, indicating that with the increase of HVPG, the proportion of hepatic artery blood supply increased gradually in the liver parenchyma. It is reported that HVPG is a surrogate for portal vein pressure[28], so with the increase of HVPG, the portal venous pressure would also increase, and then the resistance of liver blood supply would increase significantly. However, since the hepatic artery blood pressure is much higher than portal vein pressure, so the liver arterial blood supply would continuously drain into liver parenchyma, and the compensated arterial supply even increased with the increase of portal vein pressure and HVPG[20,26]. This is why HAF demonstrated positive correlation with HVPG. However, HVPG showed no correlation with LBV, LBF, SBV and SBF, and this is because many factors can influence the effective blood supply in liver parenchyma, which is partially consistent with the previous studies[14,19]. In addition, all CT perfusion parameters of the liver showed no correlation with the Child-Pugh score, suggesting that the Child-Pugh score can only be used to evaluate the liver function, and can’t reflect the blood supply in the liver parenchyma.
In addition, all TIPS stents were re-examined 2 wk and 3 mo after TIPS surgery in this study, and no shunt restenosis occurred. In terms of complications, there were 4 patients with HE, and 1 patient with hepatic insufficiency, and hepatic insufficiency occurred before operation and 1 d after operation. It was speculated that many factors, such as a long course of disease, recurrent preoperative gastrointestinal bleeding, severe refractory ascites with large amount of hydrothorax, could be correlated with the occurrence of these complications. After reviewing the patient’s data in our study, 4 HE patients showed more than 20% decrease in LBV and LBF, and 40% increase in HAF after TIPS, which is much higher than other patients without complication. Therefore, CT perfusion accommodates a potential predictor for complications after TIPS in PH patients. However, due to the limitation of the sample size, it needs to expand the sample size of the study on whether LBV and LBF can be used as preoperative predictors for postoperative complications after TIPS.
There are some limitations in our studies. Firstly, the sample size is small; secondly, the portal vein thrombosis is a factor leading to the decreased hepatic blood flow, and whether their blood perfusion of the liver will change needs to be further studied; thirdly, many other factors affecting the effective blood volume of liver parenchyma have not been discussed, such as the presence of venous collaterals in the liver, umbilical vein patency, gastric-renal shunt, spleen-kidney shunt; fourthly, Laennec type of pathological grading in liver fibrosis should be included to conduct multivariate analysis for further exploration.
In conclusion, HAF, an index of CT perfusion, showed potential capability in noninvasive assessment of HVPG, and demonstrated capability in discriminating CSPH with NCSPH before TIPS surgery, which is important for patients with higher risk. For PH patients after TIPS surgery, the blood volume in liver parenchyma would be decreased significantly, which can be quantitatively assessed with CT perfusion, and it accommodates a potential predictor for complications after TIPS in PH patients, and it is useful for making full evaluations and even take precautions before surgery.
The gold standard for diagnosis of portal hypertension (PH) is the value of hepatic venous pressure gradient (HVPG), which is also widely used in risk stratification for these patients. However, HVPG were limited for the potential risks and invasiveness during the acquisitions, so it is necessary to develop a non-invasive method to assess HVPG. In our study, computed tomography (CT) perfusion was applied to evaluate the blood supply changes before and after transjugular intrahepatic portosystemic shunt (TIPS) surgery, and to investigate the feasibility in non-invasive evaluation of HVPG.
We explore this research to evaluate the feasibility of CT perfusion as the non-invasive surrogate for HVPG, and assess the liver blood supply changes after TIPS, which had the potential application in predicting the occurrence of complications.
The aiming of this study is to investigate the correlation of CT perfusion parameters with HVPG in PH, and quantitatively assess the blood supply changes in liver and spleen parenchyma before and after TIPS.
We prospectively recruited 24 PH patients who were performed TIPS surgery for treatment of gastroesophageal bleeding in our hospital. All the patients underwent CT perfusion before and after TIPS surgery. Quantitative parameters, including liver blood volume (LBV), liver blood flow (LBF), hepatic arterial fraction (HAF), spleen blood volume (SBV) and spleen blood flow (SBF), were compared before and after TIPS, and the correlation with HVPG was also analyzed.
After TIPS, decreased LBV, increased HAF, SBV and SBF were found. HAF before TIPS showed positive correlation with HVPG (r = 0.530, P = 0.008).
HAF demonstrated potential use in discriminating clinically significant PH (CSPH) than non-CSPH before TIPS. While increased HAF, SBF and SBV, and decreased LBV were found after TIPS, which accommodates a potential non-invasive imaging tool for evaluation of PH.
Multi-modality research of baseline assessment for PH, including anatomical information, lab results, ultrasonography and functional magnetic resonance imaging should be explored in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ott G, Germany; van Kester MS, Netherlands S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1139] [Cited by in F6Publishing: 1188] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 2. | Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 777] [Cited by in F6Publishing: 887] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 3. | Jalan R, D'Amico G, Trebicka J, Moreau R, Angeli P, Arroyo V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J Hepatol. 2021;75 Suppl 1:S14-S26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 435] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 5. | Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 6. | Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 267] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 7. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 826] [Cited by in F6Publishing: 758] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 8. | Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Silva-Junior G, Martinez J, Genescà J, Bureau C, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud L, Ferreira CN, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Weiss E, Catalina MV, Erasmus HP, Uschner FE, Schulz M, Brol MJ, Praktiknjo M, Chang J, Krag A, Nevens F, Calleja JL, Robic MA, Conejo I, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Pavesi M, Garcia-Pagán JC, Jansen C, Bañares R; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 9. | Bettinger D, Sturm L, Pfaff L, Hahn F, Kloeckner R, Volkwein L, Praktiknjo M, Lv Y, Han G, Huber JP, Boettler T, Reincke M, Klinger C, Caca K, Heinzow H, Seifert LL, Weiss KH, Rupp C, Piecha F, Kluwe J, Zipprich A, Luxenburger H, Neumann-Haefelin C, Schmidt A, Jansen C, Meyer C, Uschner FE, Brol MJ, Trebicka J, Rössle M, Thimme R, Schultheiss M. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74:1362-1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 10. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 11. | Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A, Peñate M, Cruz A, Peña E. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Magaz M, Baiges A, Hernández-Gea V. Precision medicine in variceal bleeding: Are we there yet? J Hepatol. 2020;72:774-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Zhou X, Luo Y, Peng YL, Cai W, Lu Q, Lin L, Sha XX, Li YZ, Zhu M. Hepatic perfusion disorder associated with focal liver lesions: contrast-enhanced US patterns--correlation study with contrast-enhanced CT. Radiology. 2011;260:274-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Preibsch H, Spira D, Thaiss WM, Syha R, Nikolaou K, Ketelsen D, Lauer UM, Horger M. Impact of transjugular intrahepatic portosystemic shunt implantation on liver perfusion measured by volume perfusion CT. Acta Radiol. 2017;58:1167-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kim H, Booth CJ, Pinus AB, Chen P, Lee A, Qiu M, Whitlock M, Murphy PS, Constable RT. Induced hepatic fibrosis in rats: hepatic steatosis, macromolecule content, perfusion parameters, and their correlations--preliminary MR imaging in rats. Radiology. 2008;247:696-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Kanda T, Yoshikawa T, Ohno Y, Kanata N, Koyama H, Takenaka D, Sugimura K. CT hepatic perfusion measurement: comparison of three analytic methods. Eur J Radiol. 2012;81:2075-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Talakić E, Schaffellner S, Kniepeiss D, Mueller H, Stauber R, Quehenberger F, Schoellnast H. CT perfusion imaging of the liver and the spleen in patients with cirrhosis: Is there a correlation between perfusion and portal venous hypertension? Eur Radiol. 2017;27:4173-4180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176:667-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 22. | Mezawa S, Homma H, Ohta H, Masuko E, Doi T, Miyanishi K, Takada K, Kukitsu T, Sato T, Niitsu Y. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol. 2001;96:1155-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Weidekamm C, Cejna M, Kramer L, Peck-Radosavljevic M, Bader TR. Effects of TIPS on liver perfusion measured by dynamic CT. AJR Am J Roentgenol. 2005;184:505-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Motosugi U, Ichikawa T, Sou H, Morisaka H, Sano K, Araki T. Multi-organ perfusion CT in the abdomen using a 320-detector row CT scanner: preliminary results of perfusion changes in the liver, spleen, and pancreas of cirrhotic patients. Eur J Radiol. 2012;81:2533-2537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Radeleff B, Sommer CM, Heye T, Lopez-Benitez R, Sauer P, Schmidt J, Kauczor HU, Richter GM. Acute increase in hepatic arterial flow during TIPS identified by intravascular flow measurements. Cardiovasc Intervent Radiol. 2009;32:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Gülberg V, Haag K, Rössle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatology. 2002;35:630-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Tsushima Y, Endo K. Portal perfusion measurement on dynamic CT in patients with liver cirrhosis. AJR Am J Roentgenol. 2005;185:813; author reply 813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Ferrusquía-Acosta J, Bassegoda O, Turco L, Reverter E, Pellone M, Bianchini M, Pérez-Campuzano V, Ripoll E, García-Criado Á, Graupera I, García-Pagán JC, Schepis F, Senzolo M, Hernández-Gea V. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J Hepatol. 2021;74:811-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |