Published online Jan 15, 2024. doi: 10.4239/wjd.v15.i1.72
Peer-review started: October 11, 2023
First decision: November 2, 2023
Revised: November 14, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: January 15, 2024
Intracranial atherosclerosis, a leading cause of stroke, involves arterial plaque formation. This study explores the link between plaque remodelling patterns and diabetes using high-resolution vessel wall imaging (HR-VWI).
To investigate the factors of intracranial atherosclerotic remodelling patterns and the relationship between intracranial atherosclerotic remodelling and diabetes mellitus using HR-VWI.
Ninety-four patients diagnosed with middle cerebral artery or basilar artery atherosclerosis were enrolled. Their basic clinical data were collected, and HR-VWI was performed. The vascular area at the plaque (VAMLN) and normal reference vessel (VAreference) were delineated and measured using image postprocessing software, and the Remodelling index (RI) was calculated. According to the value of the RI, the patients were divided into a positive remodelling (PR) group, intermediate remodelling (IR) group, negative remodelling (NR) group, PR group and non-PR (N-PR) group.
The PR group exhibited a higher prevalence of diabetes and serum cholesterol levels than the IR and NR groups [45.2%, 4.54 (4.16, 5.93) vs 25%, 4.80 ± 1.22 and 16.4%, 4.14 (3.53, 4.75), respectively, P < 0.05]. The diabetes incidence was also significantly greater in the PR group than in the N-PR group (45.2% vs 17.5%, P < 0.05). Furthermore, the PR group displayed elevated serum triglyceride and cholesterol levels compared to the N-PR group [1.64 (1.23, 2.33) and 4.54 (4.16, 5.93) vs 4.54 (4.16, 5.93) and 4.24 (3.53, 4.89), P < 0.05]. Logistic regression analysis revealed diabetes mellitus as an independent influencing factor in plaque-PR [odds ratio (95% confidence interval): 3.718 (1.207-11.454), P < 0.05].
HR-VWI can clearly show the morphology and signal characteristics of intracranial vascular walls and plaques. Intracranial atherosclerotic plaques in diabetic patients are more likely to show PR, suggesting poor plaque stability and a greater risk of stroke.
Core Tip: High-resolution vessel wall imaging provides clear visualization of intracranial vascular walls and plaques. Diabetic patients with intracranial atherosclerotic plaques are more likely to display positive remodelling, indicating unstable plaques and a heightened risk of stroke. These findings contribute to the basis for preventing ischaemic stroke.
- Citation: Mo YQ, Luo HY, Zhang HW, Liu YF, Deng K, Liu XL, Huang B, Lin F. Investigating the relationship between intracranial atherosclerotic plaque remodelling and diabetes using high-resolution vessel wall imaging. World J Diabetes 2024; 15(1): 72-80
- URL: https://www.wjgnet.com/1948-9358/full/v15/i1/72.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i1.72
Intracranial atherosclerotic disease is one of the main causes of ischaemic stroke in the world, accounting for approximately 10% of transient ischaemic attacks and 30%-50% of ischaemic strokes[1]. It is the most common factor among Asian people[2]. The adaptive changes in the structure and function of blood vessels that can adapt to changes in the internal and external environment are called vascular remodelling, which is a common and important pathological mechanism in atherosclerotic diseases, and the remodelling mode of atherosclerotic plaques is closely related to the occurrence of stroke. Positive remodelling (PR) is an outwards compensatory remodelling where the arterial wall grows outwards in an attempt to maintain a constant lumen diameter. For a long time, it was believed that the degree of stenosis can accurately reflect the risk of ischaemic stroke[3-5]. Previous studies have revealed that lesions without significant luminal stenosis can also lead to acute events[6,7], as summarized in a recent meta-analysis study in which approximately 50% of acute/subacute ischaemic events were due to this type of lesion[6]. Research[8,9] has pointed out that the PR of plaques is more dangerous and more likely to cause acute ischaemic stroke.
Previous studies[10-13] have found that there are specific vascular remodelling phenomena in the coronary and carotid arteries of diabetic patients. However, due to the deep location and small lumen of intracranial arteries and limitations of imaging techniques, the relationship between intracranial arterial remodelling and diabetes is still unclear. In recent years, with the development of magnetic resonance technology and the emergence of high-resolution (HR) vascular wall imaging, a clear and multidimensional display of the intracranial vascular wall has been achieved.
Therefore, in this study, HR wall imaging (HR-VWI) was used to display the remodelling characteristics of bilateral middle cerebral arteries and basilar arteries and to explore the factors of intracranial vascular remodelling and its relationship with diabetes.
This is a retrospective study. Patients were recruited from Shenzhen Second People's Hospital from December 2019 to March 2022. All patients met the following inclusion and exclusion criteria.
Inclusion criteria: The patient had a preliminary clinical diagnosis of ischaemic stroke or transient ischaemic attack in the corresponding blood supply areas of the bilateral middle cerebral arteries or basilar arteries and had a diagnosis of cerebral atherosclerosis on computed tomography angiography or magnetic resonance angiography (MRA). The patient had stable vital signs, was conscious, had no obvious restlessness and was expected to cooperate well with the HR magnetic resonance imaging (HR-MRI) examination.
Exclusion criteria: Nonatherosclerotic vascular diseases, such as arteritis, moyamoya disease and dissection, were present; the patient had contraindications for magnetic resonance examination (such as claustrophobia, cardiac pacemaker, nerve stimulator, drug pump, electronic cochlea, defibrillator, heart stent, artificial heart valve, metal clip after aneurysm surgery and other metal implants); the image quality was degraded due to motion or flow artefacts and could not be used for diagnosis; the basic clinical data of the patients were collected and included sex, age, blood pressure, history of diabetes, total cholesterol, triglycerides, etc. The study was approved by the local institutional review board.
All patient imaging tests were performed using a Siemens Prisma 3.0 T (Siemens, Germany) magnetic resonance scanner with a 32-channel head coil.
The scanning protocols comprised conventional brain and cerebrovascular imaging. Conventional brain imaging encompassed T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and fluid-attenuated inversion-recovery (FLAIR). Cerebrovascular imaging involved 3-dimensional time-of-flight MRA (3D TOF MRA) and HR vascular wall imaging technology, specifically the three-dimensional sampling perfection with application-optimized contrasts using different flip angle evolution (3D-SPACE) sequence for HR-VWI. The contrast medium was meglumine gadolinium pyrospermate (Gd-DTPA) and was given via a bolus in an elbow vein at an amount of 0.2 mL/kg body weight. The imaging parameters of these sequences were as follows: (1) T1WI: Repetition time (TR)/ echo time (TE): 2000/7.4 ms, field of view (FOV) 220 × 220; slice thickness 5 mm, and slice number 20; (2) T2WI: TR/TE: 4000/117 ms, FOV 220 × 220; slice thickness 5 mm, and slice number 20; (3) FLAIR: TR/TE: 9000/81 ms, FOV 220 × 220; slice thickness 5 mm, and slice number 20; (4) 3D-TOF MRA: TR/TE: 21/3.4 ms, FOV 200 × 200; slice thickness 0.7 mm, and slice number 40; and (5) 3D-SPACE: Sagittal imaging orientation, TR/TE: 900/14 ms, FOV 240 × 240; slice thickness 0.6 mm, and slice number 224.
The 3D SPACE data (DICOM format) were imported into an image postprocessing workstation (Siemens Syngo Via workstation), and all parameter measurements were performed on the platform.
The images were analysed in the workstation by two senior neuroimaging physicians jointly, without knowledge of the patient information, and disagreements in conclusions were discussed and agreed upon.
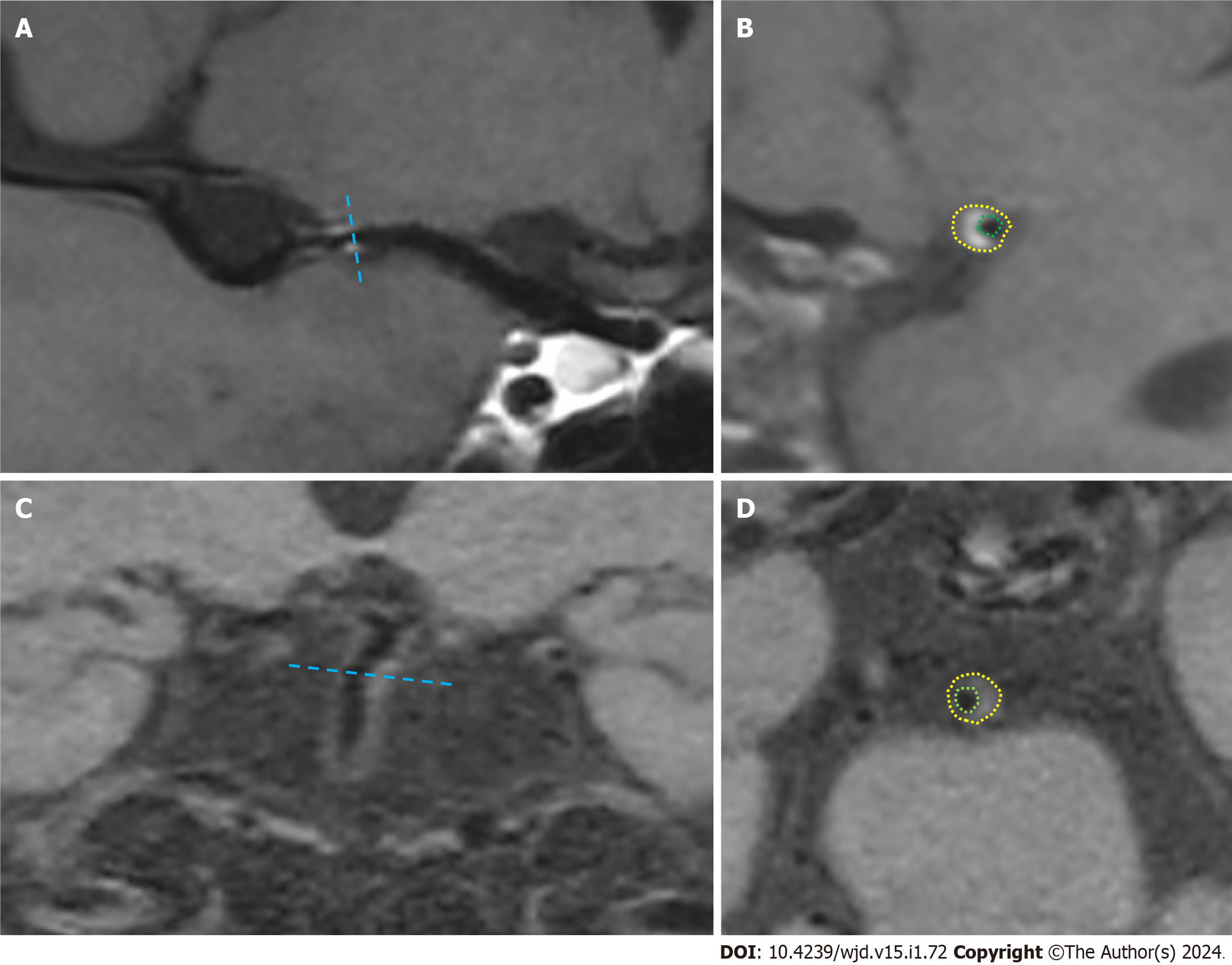
The image quality was first evaluated to exclude patients with poor image quality that could not meet the diagnostic criteria. By curved planar reformation or multi-planar reconstruction reconstruction, the most stenotic site of the lumen at the plaque was selected as the target site in the transverse axis perpendicular to the vessel alignment, and the reference site was selected at the normal segment proximal to the target site (if the proximal reference site was not available, the adjacent distal site was used). The images of the narrowest site of the lumen and the reference site were magnified to 300%, and the vascular boundaries were manually traced by using the drawing tool according to the specific morphology of the narrowed vessel to obtain the vascular area at the narrowest point of the vessel (VAMLN) and the vascular area of the reference vessel (VAreference), as shown in Figure 1. Then, the remodelling index (RI) was calculated according to the formula: RI = VAMLN/VAreference. Based on the values of the RI, the plaque remodelling pattern was classified as follows: RI > 1.05 for PR, 0.95 < RI ≤ 1.05 for intermediate remodelling (IR), and RI ≤ 0.95 for negative remodelling (NR).
All data were analysed by using the SPSS 26.0 package (Chicago, IL, United States). Variables underwent normality testing via the Kolmogorov-Smirnov test. Quantitative data are expressed as the mean ± SD, while nonnormally distributed data are presented as the median with interquartile range. Independent sample t tests were employed for normally distributed and homogenous variance quantitative data comparisons; otherwise, the Mann-Whitney U test was used.
Categorical values were summarized by counts and percentages. The chi-square test (or Fisher’s exact test when appropriate) was applied for categorical data comparisons. Multivariate logistic regression was conducted to identify independent factors associated with plaque-PR. Statistical significance was defined as P < 0.05.
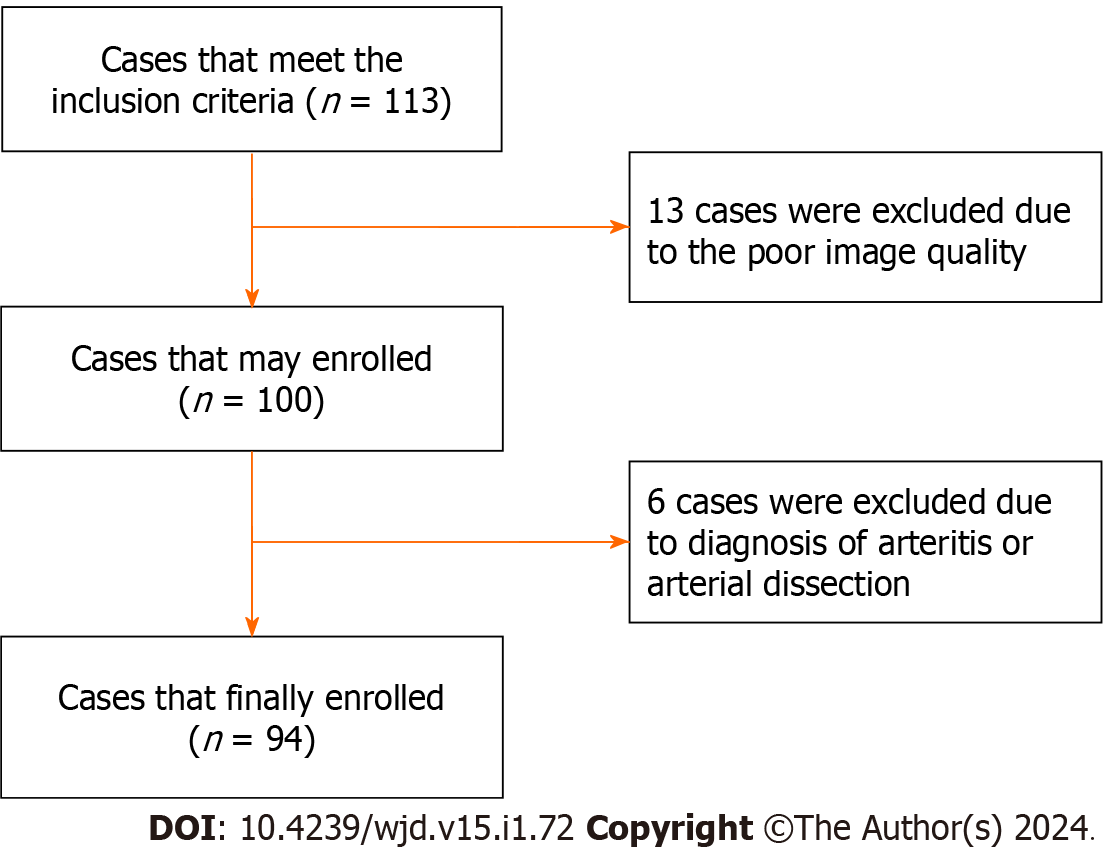
According to the inclusion criteria, we collected 113 patients. However, 13 patients were excluded due to quality reasons, and 6 patients were also excluded that were diagnosed with arteritis or arterial dissection. Finally, a total of 94 patients were enrolled in this study (Figure 2), and they had an age range of 29 to 82 years and a mean age of 55.56 ± 12.168 years. Among them, there were 65 males, with an age range of 29 to 82 years, and the average age was 52.72 ± 11.905 years. There were 29 female patients, with an age range of 31 to 82 years, and the average age was 61.93 ± 10.347 years. There were 31 patients in the PR group, 8 patients in the IR group, and 55 patients in the NR group, in which the IR group and NR group were merged into the non-PR (N-PR) group with a total of 63 patients.
The univariate analysis showed that there were statistically significant differences in the prevalence of diabetes and serum cholesterol levels among the three groups [14 (45.2%) and 4.54 (4.16, 5.93) vs 2 (25%) and 4.80 ± 1.22 vs 9 (16.4%) and 4.14 (3.53,4.75), respectively, P < 0.05]. There was no significant difference in age, sex, hypertension history or triglycerides among the three groups. Table 1 for the comparison of clinical data characteristics between the three groups of patients.
| PR group (n = 31) | IR group (n = 8) | NR group (n = 55) | F/H/χ2 value | P value | |
| Age | 57.61 ± 12.77 | 56.00 ± 14.99 | 54.35 ± 11.46 | 0.716 | 0.491 |
| Sex (%) | 20 (64.5) | 5 (62.5) | 40 (72.7) | 0.808 | 0.668 |
| Hypertension (%) | 22 (71) | 4 (50) | 37 (67.3) | 1.269 | 0.530 |
| Diabetes (%) | 14 (45.2) | 2 (25) | 9 (16.4) | 8.433 | 0.015 |
| Triglyceride | 1.88 ± 0.97 | 1.61 (1.10, 1.67) | 1.64 (1.09, 2.33) | 5.029 | 0.081 |
| Cholesterol | 4.54 (4.16, 5.93) | 4.80 ± 1.22 | 4.14 (3.53, 4.75) | 3.159 | 0.019 |
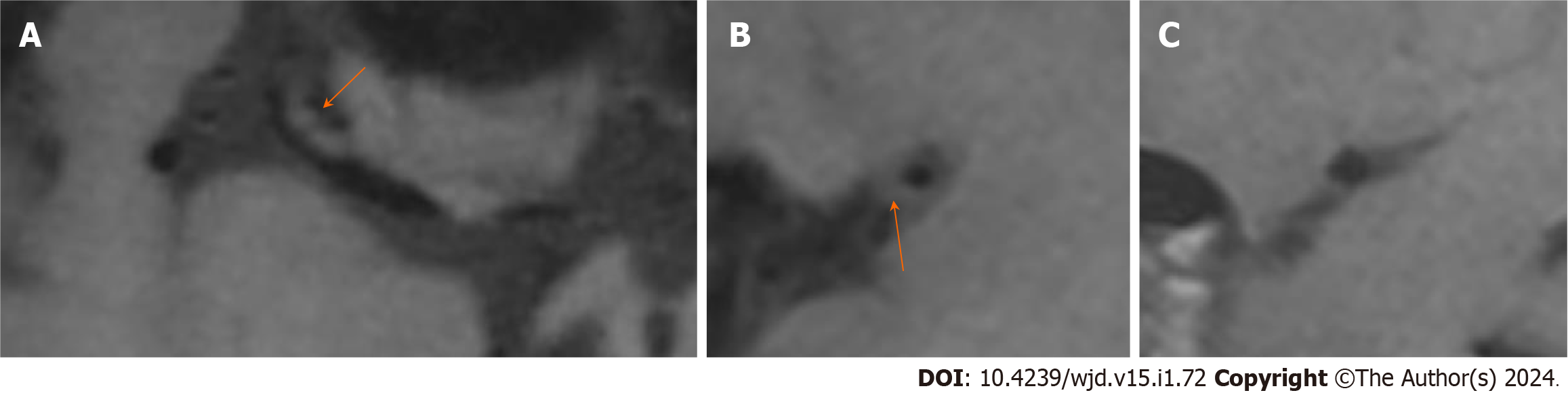
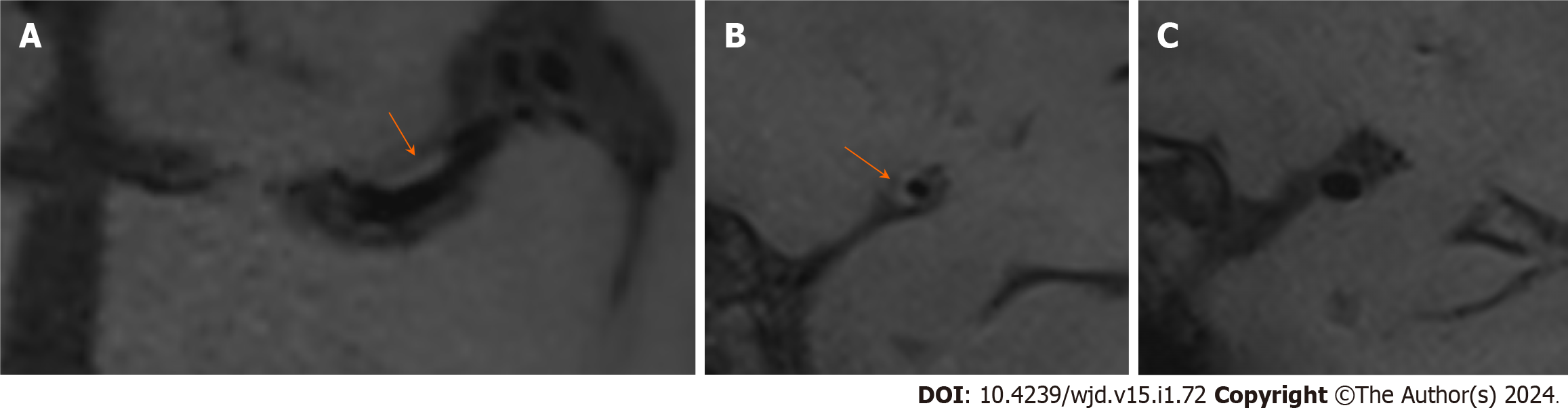
The univariate analysis showed that there were significant differences in the prevalence of diabetes, triglycerides and cholesterol between the two groups. The number of patients with diabetes in the PR group was greater than that in the N-PR group [14 (45.2%) vs 11 (17.5%), P < 0.05]. Compared with the N-PR group, the patients in the PR group had higher serum triglyceride and cholesterol values [1.64 (1.23, 2.33) and 4.54 (4.16, 5.93) vs 1.55 (1.06, 1.67) and 4.24 (3.53, 4.89), respectively, P < 0.05]. There was no significant difference in age, sex or hypertension history between the PR group and the N-PR group. Table 2 for the comparison of the clinical data characteristics and Figures 3 and 4 for the comparison of the image features between the two groups of patients.
| PR group (n = 31) | N-PR group (n = 63) | t/z/χ2 value | P value | |
| Age | 57.61 ± 12.77 | 54.56 ± 11.83 | 1.147 | 0.254 |
| Sex (%) | 20 (64.5) | 45 (71.4) | 0.465 | 0.495 |
| Hypertension (%) | 22 (71) | 41 (65.1) | 0.326 | 0.568 |
| Diabetes (%) | 14 (45.2) | 11 (17.5) | 8.166 | 0.004 |
| Triglyceride | 1.64 (1.23, 2.33) | 1.55 (1.06, 1.67) | 2.191 | 0.028 |
| Cholesterol | 4.54 (4.16, 5.93) | 4.24 (3.53, 4.89) | 2.452 | 0.014 |
The indicators that had statistical significance in the univariate analysis and that may affect the vascular remodelling pattern, including age, sex, diabetes, hypertension and triglycerides, were included in the binary logistic regression analysis to explore the important factors affecting the remodelling pattern.
The results showed that diabetes was an independent feature of plaque-PR [odds ratio (95% confidence interval): 3.718 (1.207-11.454), P < 0.05]. There was no significant correlation between hypertension, triglycerides, sex and age and PR of plaque (P > 0.05). The independent factor analysis of patient characteristics and plaque-PR is shown in Table 3.
| OR (95%CI) | P value | |
| Diabetes | 3.718 (1.207-11.454) | 0.022 |
| Hypertension | 1.077 (0.377-3.079) | 0.890 |
| Sex | 0.558 (0.189-1.644) | 0.290 |
| Age | 0.996 (0.952-1.042) | 0.860 |
| Triglyceride | 1.977 (0.979-3.995) | 0.057 |
In our study, we used HR-VWI to explore the remodelling patterns of the responsible plaques of intracranial arteries and their influencing factors in patients with ischaemic stroke or transient ischaemic attack. This proved that HR-VWI can noninvasively and accurately show the morphological characteristics and signal characteristics of intracranial artery plaques and can be used as an effective means to evaluate the vessel walls of intracranial atherosclerosis patients. The results showed that the prevalence of diabetes was higher in the group with PR of intracranial responsible plaques, and diabetes was an independent factor affecting the PR of plaques, which revealed that hyperglycaemia might promote the formation of PR of plaques.
An increasing number of studies have proven that arterial remodelling is an important pathological mechanism commonly present in atherosclerotic diseases. Arterial remodelling can occur in coronary arteries, renal arteries, femoral arteries, carotid arteries, etc. However, different arteries have different abilities and methods of remodelling. Arterial remodelling includes both PR (arterial dilation) and negative remodelling (artery constriction). PR is outwards compensatory remodelling, in which the arterial wall grows outwards in an attempt to maintain a constant lumen diameter, while negative remodelling is defined as local contraction of the vessel size[14].
Previous studies have often evaluated the severity of intracranial atherosclerotic plaques or the probability of ischaemic stroke by the degree or number of vascular stenoses. In recent years, studies have shown that the stability of the plaque itself is an important factor affecting the occurrence of stroke[15]. Intracranial atherosclerosis should be evaluated not only according to the degree of lumen stenosis but also according to the characteristics of the vascular wall. Plaques that do not cause lumen stenosis can also lead to stroke. The pattern of plaque remodelling is closely related to plaque instability. PR of arteries is more dangerous than negative remodelling and is more likely to cause acute ischaemic stroke[16,17].
In diabetes, vascular remodelling can be caused by irritation of the vascular wall due to hyperglycaemia, insulin resistance, endothelial cell dysfunction, nonenzymatic glycosylation and other factors. Watase et al[13] examined 557 plaques of common carotid arteries and internal carotid arteries and found that carotid artery plaques in diabetic patients showed more PR than those in nondiabetic patients. Terashima et al[12] also found that PR in the coronary artery is associated with diabetes. The results of our study also indicated that the PR of intracranial arterial plaques was related to diabetes, and the prevalence of diabetes in the PR group was higher than that in the nonPR group. Moreover, our study also shows that diabetes is an independent factor in the PR of intracranial plaques. It is suggested that hyperglycaemia may promote the formation of plaque-PR. Pathological studies have confirmed that positively remodelled plaques have a large lipid core and accumulate macrophages and inflammatory cells[18,19]. We speculate that hyperglycaemia may promote severe vascular wall inflammatory infiltration or the formation of a lipid core, thus increasing the risk of plaque rupture. This helps prompt clinicians to intervene early regarding blood glucose management in diabetic patients to prevent the occurrence of stroke.
Contrary to the results of our study, Zhang et al[16] found that the remodelling pattern of the middle cerebral artery was not associated with diabetes. The reasons why our study differed from their study may be related to the different inclusion criteria of patients because their study included asymptomatic patients with suspected middle cerebral atherosclerosis.
Although some studies have shown that[20] hypertension is related to damage to structural and functional cerebrovascular health and can promote an increase in the thickness of the middle layer of intracranial arteries, plaque and stenosis, the relationship between intracranial plaque remodelling and hypertension is still controversial. Guo et al[21] found that there was no statistically significant difference in the blood pressure indicators of the basilar artery between the PR group and the N-PR group, which was consistent with the results of this study, indicating that hyper
There are still some deficiencies in this study. First, the sample size in this study was small, and there was a lack of pathological controls. Therefore, in future research, the sample size needs to be expanded, and the possible highest number of pathological samples need to be obtained. Second, the parameters of the blood vessel wall and plaque were evaluated manually, and measurement error was inevitable. In addition, we did not conduct a follow-up study to evaluate whether the treatment effect of the PR group was better than that of the non-PR group, which will be explored in further studies in the future.
In conclusion, HR-VWI can clearly show the morphology and signal characteristics of intracranial vascular canal walls and plaques. Patients with diabetes mellitus are more likely to experience PR of intracranial atherosclerotic plaques, suggesting poor plaque stability and a greater risk of stroke, and the results of this study may provide a basis for the prevention of ischaemic stroke and may help reduce the incidence and severity of ischaemic stroke by predicting dangerous plaques.
Intracranial atherosclerosis, a leading cause of stroke, involves arterial plaque formation.
This study explores the link between plaque remodelling patterns and diabetes using high-resolution vessel wall imaging (HR-VWI).
To investigate the factors of intracranial atherosclerotic remodelling patterns and the relationship between intracranial atherosclerotic remodelling and diabetes mellitus using HR-VWI.
Exploratory techniques were employed in this investigation, focusing on unraveling the intricate relationship between intracranial atherosclerosis and diabetes. A cohort of 94 individuals diagnosed with atherosclerosis in the middle cerebral artery or basilar artery was assembled for scrutiny. Rigorous data collection ensued, supplemented by HR-VWI. Employing sophisticated image postprocessing, the vascular area at the plaque and normal reference vessel were meticulously measured. The remodelling index (RI) served as a pivotal metric, categorizing patients into distinct remodelling groups. Statistical analyses illuminated pronounced associations between positive remodelling (PR) and heightened diabetes prevalence, substantiating the critical role of HR-VWI in delineating vascular nuances.
The study's findings illuminate compelling insights into the intricate dynamics of intracranial atherosclerosis. Notably, the PR group, identified through the RI, exhibited a significantly higher diabetes prevalence (45.2%) compared to intermediate remodelling and negative remodelling groups. This statistical distinction underscores the intimate connection between diabetes and atherosclerotic plaque characteristics. Additionally, the PR group demonstrated elevated serum cholesterol and triglyceride levels, reinforcing the correlation. Logistic regression analysis identified diabetes mellitus as an independent influencer in plaque-PR, further emphasizing its significance. The conclusive link between diabetic status and increased PR highlights the potential vulnerability and heightened stroke risk associated with intracranial atherosclerotic plaques in diabetic patients.
In summation, our research underscores the pivotal role of HR-VWI in elucidating the intricate dynamics of intracranial atherosclerosis. The PR observed in diabetic patients accentuates a concerning association, indicative of heightened plaque instability and an augmented risk of stroke. The distinct patterns unveiled through the RI delineate varying degrees of atherosclerotic changes, with the PR group exhibiting a pronounced correlation with diabetes mellitus. These findings not only enhance our understanding of plaque characteristics but also emphasize the critical importance of HR-VWI in identifying at-risk individuals. This study contributes valuable insights that may inform targeted interventions for diabetic patients with intracranial atherosclerosis, potentially mitigating the risk of stroke.
Our research opens avenues for further exploration and clinical implications. The identified correlation between PR and diabetes in intracranial atherosclerosis prompts future investigations into the underlying mechanisms. Understanding the intricate interplay between diabetes and plaque dynamics can inform tailored preventive strategies. Additionally, this study underscores the significance of HR-VWI as a diagnostic tool, suggesting its potential integration into routine clinical assessments for at-risk populations. Exploring therapeutic interventions that target plaque stability in diabetic patients may emerge as a crucial research direction, aiming to mitigate the heightened stroke risk. Overall, our findings pave the way for multidisciplinary collaborations and advancements in both diagnostic approaches and preventive measures for individuals with intracranial atherosclerosis and diabetes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ankrah AO, Netherlands; Cai L, United States; Mahmoud MZ, Saudi Arabia S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
| 1. | Battistella V, Elkind M. Intracranial atherosclerotic disease. Eur J Neurol. 2014;21:956-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 2. | Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, Pu C, Jia J, Zhang T, Liu X, Zhang S, Xie P, Fan D, Ji X, Wong KL, Wang L; China Stroke Study Collaboration. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 645] [Cited by in F6Publishing: 778] [Article Influence: 194.5] [Reference Citation Analysis (0)] |
| 3. | Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ; Warfarin Aspirin Symptomatic Intracranial Disease Trial Investigators. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 520] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 4. | Turan TN, Makki AA, Tsappidi S, Cotsonis G, Lynn MJ, Cloft HJ, Chimowitz MI; WASID Investigators. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010;41:1636-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Heck D, Jost A. Carotid stenosis, stroke, and carotid artery revascularization. Prog Cardiovasc Dis. 2021;65:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Liu X, Wu X, Degnan AJ, Malhotra A, Zhu C. Culprit intracranial plaque without substantial stenosis in acute ischemic stroke on vessel wall MRI: A systematic review. Atherosclerosis. 2019;287:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Wang M, Wu F, Yang Y, Miao H, Fan Z, Ji X, Li D, Guo X, Yang Q. Quantitative assessment of symptomatic intracranial atherosclerosis and lenticulostriate arteries in recent stroke patients using whole-brain high-resolution cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2018;20:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Yu DD, Pu YH, Pan YS, Zou XY, Soo Y, Leung T, Liu LP, Wang DZ, Wong KS, Wang YL, Wang YJ; Chinese Intracranial Atherosclerosis (CICAS) Study Group. High Blood Pressure Increases the Risk of Poor Outcome at Discharge and 12-month Follow-up in Patients with Symptomatic Intracranial Large Artery Stenosis and Occlusions: Subgroup analysis of the CICAS Study. CNS Neurosci Ther. 2015;21:530-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Yang M, Ma N, Liu L, Wang A, Jing J, Hou Z, Liu Y, Lou X, Miao Z, Wang Y. Intracranial collaterals and arterial wall features in severe symptomatic vertebrobasilar stenosis. Neurol Res. 2020;42:649-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Huang J, Jiao S, Song Y, Chen Y, Zhang J, Zhang C, Gong T, Chen M. Association between type 2 diabetes mellitus, especially recently uncontrolled glycemia, and intracranial plaque characteristics: A high-resolution magnetic resonance imaging study. J Diabetes Investig. 2020;11:1278-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Reddy HK, Koshy SK, Foerst J, Sturek M. Remodeling of coronary arteries in diabetic patients-an intravascular ultrasound study. Echocardiography. 2004;21:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Terashima M, Nguyen PK, Rubin GD, Meyer CH, Shimakawa A, Nishimura DG, Ehara S, Iribarren C, Courtney BK, Go AS, Hlatky MA, Fortmann SP, McConnell MV. Right coronary wall CMR in the older asymptomatic advance cohort: positive remodeling and associations with type 2 diabetes and coronary calcium. J Cardiovasc Magn Reson. 2010;12:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Watase H, Sun J, Hippe DS, Balu N, Li F, Zhao X, Mani V, Fayad ZA, Fuster V, Hatsukami TS, Yuan C. Carotid Artery Remodeling Is Segment Specific: An In Vivo Study by Vessel Wall Magnetic Resonance Imaging. Arterioscler Thromb Vasc Biol. 2018;38:927-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Sun J, Feng XR, Yang X, Feng PY, Liu YB, Yang HX, Zhang TZ. Correlation between characteristics of intracranial atherosclerotic plaques and ischemic stroke in high-resolution vascular wall MRI. Acta Radiol. 2023;64:732-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Spacek M, Zemanek D, Hutyra M, Sluka M, Taborsky M. Vulnerable atherosclerotic plaque - a review of current concepts and advanced imaging. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162:10-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Zhang DF, Chen YC, Chen H, Zhang WD, Sun J, Mao CN, Su W, Wang P, Yin X. A High-Resolution MRI Study of Relationship between Remodeling Patterns and Ischemic Stroke in Patients with Atherosclerotic Middle Cerebral Artery Stenosis. Front Aging Neurosci. 2017;9:140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Zhao DL, Deng G, Xie B, Ju S, Yang M, Chen XH, Teng GJ. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci. 2015;22:700-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | de Weert TT, Ouhlous M, Zondervan PE, Hendriks JM, Dippel DW, van Sambeek MR, van der Lugt A. In vitro characterization of atherosclerotic carotid plaque with multidetector computed tomography and histopathological correlation. Eur Radiol. 2005;15:1906-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Varrassi M, Sferra R, Gravina GL, Pompili S, Fidanza RC, Ventura M, Splendiani A, Barile A, Vetuschi A, Di Cesare E. Carotid artery plaque characterization with a wide-detector computed tomography using a dedicated post-processing 3D analysis: comparison with histology. Radiol Med. 2019;124:795-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 20. | Liu W, Huang X, Liu X, Ortega D, Chen L, Chen Z, Sun J, Wang L, Hatsukami TS, Yuan C, Li H, Yang J. Uncontrolled hypertension associates with subclinical cerebrovascular health globally: a multimodal imaging study. Eur Radiol. 2021;31:2233-2241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Guo R, Zhang X, Zhu X, Liu Z, Xie S. Morphologic characteristics of severe basilar artery atherosclerotic stenosis on 3D high-resolution MRI. BMC Neurol. 2018;18:206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |