Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.995
Peer-review started: January 30, 2023
First decision: March 24, 2023
Revised: April 8, 2023
Accepted: April 27, 2023
Article in press: April 27, 2023
Published online: July 15, 2023
Advanced glycation end products (AGEs) are a heterogeneous collection of compounds formed during industrial processing and home cooking through a sequence of nonenzymatic glycation reactions. The modern western diet is full of heat-treated foods that contribute to AGE intake. Foods high in AGEs in the contemporary diet include processed cereal products. Due to industrialization and marketing strategies, restaurant meals are modified rather than being traditionally or conventionally cooked. Fried, grilled, baked, and boiled foods have the greatest AGE levels. Higher AGE-content foods include dry nuts, roasted walnuts, sunflower seeds, fried chicken, bacon, and beef. Animal proteins and processed plant foods contain furosine, acrylamide, heterocyclic amines, and 5-hydroxymethylfurfural. Furosine (2-furoil-methyl-lysine) is an amino acid found in cooked meat products and other processed foods. High concentrations of carboxymethyl-lysine, carboxyethyl-lysine, and methylglyoxal-O are found in heat-treated nonvegetarian foods, peanut butter, and cereal items. Increased plasma levels of AGEs, which are harmful chemicals that lead to age-related diseases and physiological aging, diabetes, and autoimmune/inflammatory rheumatic diseases such as systemic lupus erythematosus and rheumatoid arthritis. AGEs in the pathophysiology of metabolic diseases have been linked to individuals with diabetes mellitus who have peripheral nerves with high amounts of AGEs and diabetes has been linked to increased myelin glycation. Insulin resistance and hyperglycemia can impact numerous human tissues and organs, leading to long-term difficulties in a number of systems and organs, including the cardiovascular system. Plasma AGE levels are linked to all-cause mortality in individuals with diabetes who have fatal or nonfatal coronary artery disease, such as ventricular dysfunction. High levels of tissue AGEs are independently associated with cardiac systolic dysfunction in diabetic patients with heart failure compared with diabetic patients without heart failure. It is widely recognized that AGEs and oxidative stress play a key role in the cardiovascular complications of diabetes because they both influence and are impacted by oxidative stress. All chronic illnesses involve protein, lipid, or nucleic acid modifications including crosslinked and nondegradable aggregates known as AGEs. Endogenous AGE formation or dietary AGE uptake can result in additional protein modifications and stimulation of several inflammatory signaling pathways. Many of these systems, however, require additional explanation because they are not entirely obvious. This review summarizes the current evidence regarding dietary sources of AGEs and metabolism-related complications associated with AGEs.
Core Tip: All chronic illnesses involve protein, lipid, or nucleic acid modifications, including crosslinked and nondegradable aggregates known as advanced-glycation end products (AGEs). Endogenous AGE formation or dietary AGE uptake can result in additional protein modifications and stimulation of several inflammatory signaling pathways. Many of these systems, however, require additional explanation because they are not entirely obvious. This review summarizes the current evidence regarding dietary sources of AGEs and metabolism related complications associated with AGEs.
- Citation: Khan MI, Ashfaq F, Alsayegh AA, Hamouda A, Khatoon F, Altamimi TN, Alhodieb FS, Beg MMA. Advanced glycation end product signaling and metabolic complications: Dietary approach. World J Diabetes 2023; 14(7): 995-1012
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/995.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.995
Advanced-glycation end products (AGEs) are heterogeneous compounds formed when glucose or other saccharides posttranslationally alter macromolecules such as proteins, lipids, and nucleic acids without the use of enzymes (fructose and pentose). Age-related illnesses and physiological aging are associated with higher plasma amounts of AGEs, which are toxic chemicals[1,2], causing diabetes mellitus (DM)[3], and autoimmune/inflammatory rheumatic diseases including systemic lupus erythematosus[4], rheumatoid arthritis[5], systemic sclerosis[6], and psoriasis[7]. More than 20 different AGEs have been discovered in dietary items, human blood, and tissues. These AGEs can be arbitrarily classified as fluorescent or nonfluorescent[8]. The three nonfluorescent substances that are most significant are pyrraline, carboxymethyl-lysine (CML), and carboxyethyl-lysine (CEL)[9]. The two fluorescent AGEs of most significance are pentosidine and methylglyoxal-lysine dimer (MOLD)[10]. The presence of lysine residue in the molecules serves as the primary distinguishing property of AGEs. The AGEs are discharged from the kidneys after being catabolized in renal proximal tubular cells on a metabolic level[11]. AGE formation after binding with AGE receptor (RAGE) can result in metabolic burdens such as hyperglycemia, hyperlipidemia, oxidative stress, inflammatory responses, and endothelial dysfunction[12]. AGE formation may be accelerated by a number of environmental factors such as sedentary lifestyles, high-carbohydrate and high-calorie diets, food cooked at high temperatures, and cigarette smoke[13]. Dietary AGE concentrations in a variety of commercial cow-based, goat-based, and soy-based infant formulas were measured using ultra-performance liquid chromatography-mass spectrometry, the degree of protein glycation in infant formulas is determined by the protein source, protein composition, and the number and type of carbohydrates. The soy-based formula studied contained significantly more arginine and arginine-derived dietary AGEs (dAGEs) than the cow- and goat-based formulas. The concentrations of dAGEs in infant formula with hydrolyzed proteins were higher than those in infant formula containing intact proteins, and lactose-containing formula was more susceptible to glycation than sucrose- and maltodextrin-containing formula[14]. Bakery products, with respect to their formation during baking, generate AGE content and have health effects. Phenolic components added to the formulation in bakery products greatly decrease the formation of AGEs; among these, ferulic acid showed the most significant lowering effect on AGEs. Dihydromyricetin outperformed the flavanones evaluated in the model cookie system in terms of AGE reduction. Furthermore, the addition of components that reduce water activity, such as dietary fiber, and the high temperature used in baking both enhance the formation of AGEs and the addition of fat, sugar, and protein-rich ingredients to bakery product formulations usually increases the AGE content. As a result, the food industry should concentrate on optimizing food production to reduce AGE formation while maintaining bakery product safety and organoleptic properties[15]. In light of this, AGEs may establish a clear connection between modern nutrition and health[16].
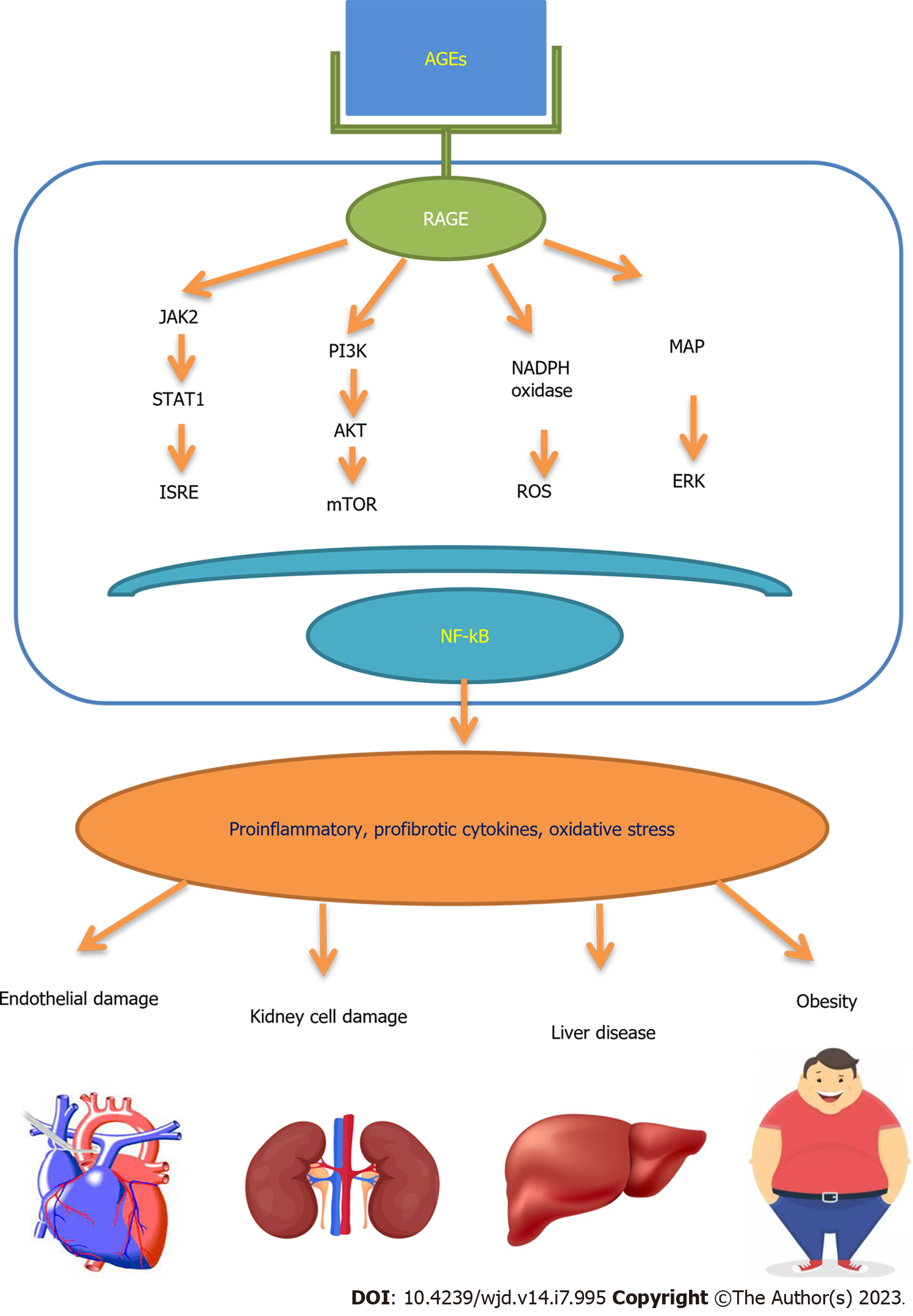
Among the various AGEs receptors presently identified, RAGE is a critical receptor for AGEs to exert the main mechanism of cells and new pattern recognition receptor RAGE is a one of the members of the immunoglobulin superfamily. Numerous cells, including macrophages, mesangial cells, and endothelial cells, have RAGE receptors expressed on their surfaces[17], which can join forces with AGEs to create the AGE-RAGE axis, which activates intracellular signaling pathways and starts a chain of intracellular events.
The AGE-RAGE interaction has been demonstrated in experimental investigations to alter cell signaling, stimulate gene expression, generate oxidative stress, and cause the release of proinflammatory chemicals[18]. RAGE expression levels are extremely low in healthy individuals, but when the body’s cells are stimulated or under stress, RAGE expression levels in damaged cells are markedly elevated. In light of this, RAGE is crucial for understanding how numerous diseases progress, including diabetes, Alzheimer’s disease, vascular damage, and tumors. RAGE can also identify a variety of ligands, including some endogenous ligands like S100/calgranulins and high mobility group box-1[19], which interact with RAGE after being released by injured cells, activating some signaling pathways to enhance tissue damage and inflammation[20]. Nuclear factor-light-chain enhancer of activated B cells, also known as NF-κB, is translocated into the nucleus as a result of RAGE activation, which upregulates RAGE expression in a hyperglycemic environment[21].
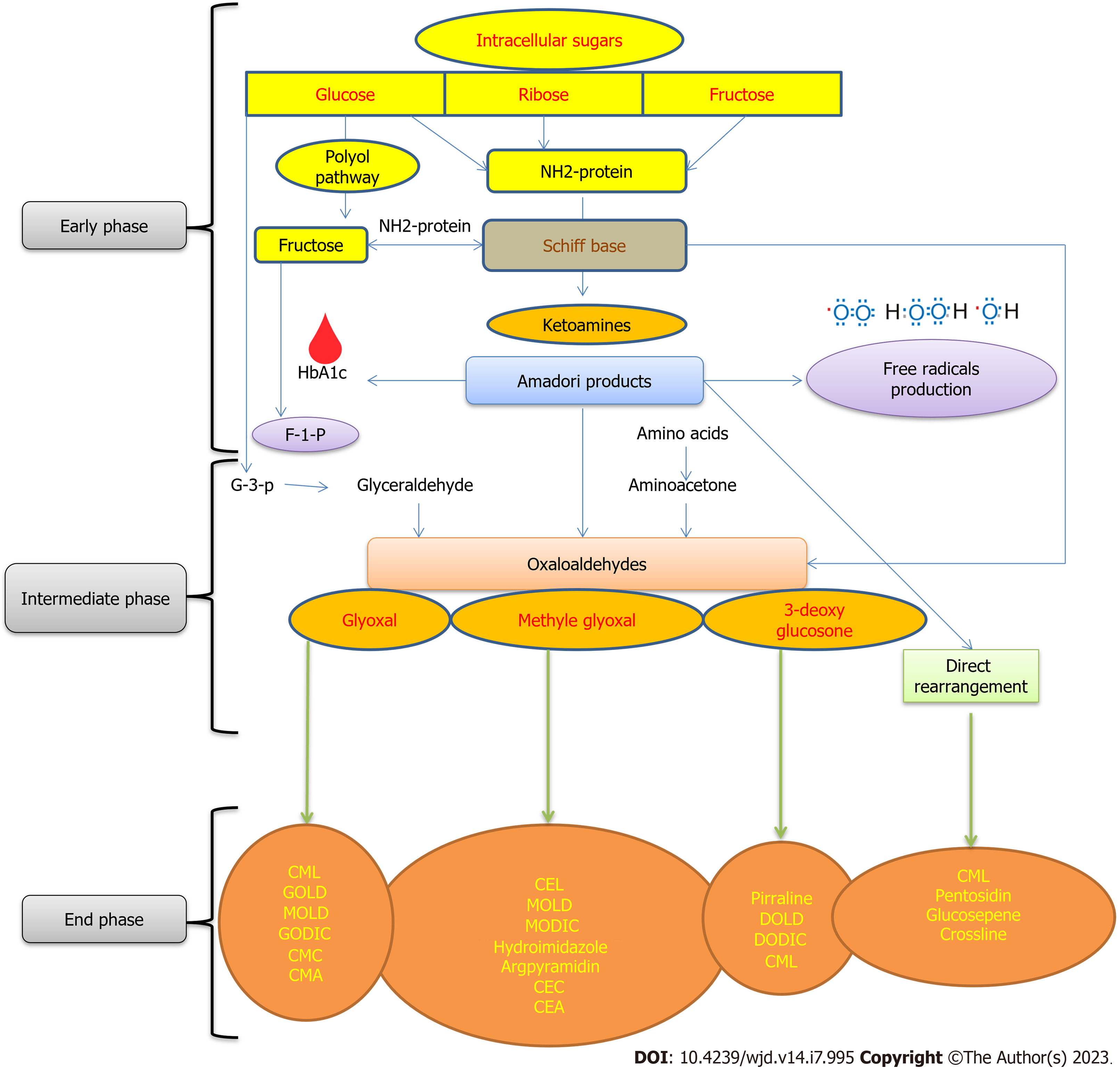
To create AGEs, the Maillard reaction (MR) proceeds through a series of processes. The primary regulators of AGEs formation including glycation of cellular and tissue proteins, are the rate of protein turnover, degree of hyperglycemia, and degree of oxidative stress[22]. The next sections explain the three stages of AGEs development in vivo (Figure 1).
The carbonyl group of reducing sugars such as glucose, fructose, or ribose reacts with the amino groups of proteins, primarily lysine and arginine residues, to create a Schiff base, which can also be formed via the polyol route. This unstable Schiff base is further modified to produce more stable ketoamines known as amadori products (APs), which can create free radicals and irreversible crosslinks with proteins and peptides. However, APs are still reversible, dependent on the minimal substrate concentration and time[23].
Glyoxal (GO), methylglyoxal (MG), and 3-deoxyglucosone (3-DG) are examples of AP that undergo additional chemical rearrangements in the presence of transitional metal ions to form active carbonyl intermediate groups known as dicarbonyls, which are precursors for the production of AGEs at an advanced stage. The previous phase’s glucose, fructose, and Schiff base can also be transformed and stored into dicarbonyls, which are known as “carbonyl stress,” and which have a propensity to react with amino and sulfhydryl groups of proteins to cause browning and crosslinking[24,25].
Dicarbonyls are eventually directly rearranged with AP and proteins as a result of multiple chemical modifications such as oxidation, nonoxidation, hydration, dehydration, glycation, glycosylation, fructosylation, and acid hydrolysis to create stable, irreversible AGEs such as DOLD, GOLD, MG-derived imidazolium crosslinking, and 3-deoxygluco. Table 1 depicts the key characteristics, sources, modes of production, and pathophysiology of the various forms of AGEs.
| Type of AGEs | Country | Food products | AGE level | Ref. |
| Acrylamide | Poland | French fries | 63-2175 μg/kg | [29] |
| Potato chips | 113-3647 | |||
| Crispbread | 65-1271 μg/kg | |||
| Crackers | 566-2017 μg/kg | |||
| Daily Bread | 35-110 μg/kg | |||
| United States | Biscuits | 5-1796 μg/kg | [30] | |
| India | Potato chips | 1456.5 μg/kg | [31] | |
| Biscuits bakery | 126-665 μg/kg | [32] | ||
| French fries | 825.96-1143.15 | |||
| Saudi Arabia | Biscuits | 90-182 μg/kg | [33] | |
| Chocolate pies | 439 μg/kg | |||
| Furan | Brazil | Biscuits | 38.1-105.3 μg/kg | [34] |
| Belgium | Jarred baby food | 61.7 μg/kg | [35] | |
| Spain | Vegetable-based baby food | 10.9 to 143.0 μg/kg | [36] | |
| Fruit-based baby food | 7.7 to 32.1 | |||
| Germany | Ready to drink coffee | 2-108 μg/kg | [37] | |
| Denmark | Instant coffee powder | 39-1330 μg/kg | [38] | |
| Dried fruits | 387 μg/kg | |||
| HMF | Malaysia | Stored honey | 118.47-1139.95 mg/kg | [39] |
| Bangaldesh | Stored honey | 3-703 mg/kg | [40] | |
| Turkey | Traditionally coffee | 213-239 mg/kg | [41] | |
| Brazil | Corn syrup | 406-2121 mg/kg | [42] | |
| Cane syrup | 109-893 mg/kg | |||
| Polish Market | Roasted coffee | 348 mg/kg | [43] | |
| Instant coffee | 3351 mg/kg | |||
| Fruit juices | 1-110 mg/L | |||
| Cola-carbonated drinks | 2-40 mg/L | |||
| Syria | Instant coffee | 526-1800 mg/kg | [44] | |
| GO | Italy | Sugar cookies | 362 mg/kg | [45] |
| Spain | Commercial cookies | 4.8-26.0 mg/kg | [46] | |
| Netherlands | Apple molasses | 0.01-37.00 mg/kg | [47] | |
| MGO | Italy | Sugar cookies | 293.0 mg/kg | [45] |
| Turkey | Dried apricots | 20-41 mg/kg | [48] | |
| Netherlands | Dutch spiced cake, rusk, apple molasses | 0.04-736.00 mg/kg | [47] | |
| Spain | Commercial cookies | 3.7-81.4 mg/kg | [46] | |
| Furosine | Denmark | Standard infant formula | 1700-2800 mg/kg P | [49] |
| Netherland | Standard infant formula | 4719-6394 mg/kg P | [50] | |
| China | Charcoal-flavored milk | 593.2 mg/100 g protein | [51] | |
| Branded fermented milk | 25.40-1661.05 mg/100 g P | [52] | ||
| Posturized milk | 12.58-61.80 mg/100 g P | [53] | ||
| Raw milk | 8.85 mg/100 g P | |||
| CML | United States | Fried beef | 20.03 mg/100 g P | [54] |
| Baked beef | 14.31 mg/100 g P | |||
| Fried chicken breast | 17.17 mg/100 g P | |||
| Baked chicken breast | 13.58 mg/100 g P | |||
| China | Ground beef | 3.00-19.96 mg/100 g P | [55] | |
| Fish | 0.66-2.00 mg/100 g P | [56] | ||
| Sea food (dry) | 44.8-439.0 mg/100 g P | [57] | ||
| Canned saury fishes | 250-1608 mg/100 g P | [58] | ||
| CEL | China | Fish | 3.08 mg/100 g P | [56] |
| Canned saury fishes | 721-3653 mg/100 g P | [58] |
The present narrative review of the literature was performed based on the data search from PubMed, Google Scholar, Scopus, The National Library of Medicine database, and Web of Science, at the beginning of 2023 focusing on keywords on AGEs, AGE generation, pathways, foods containing AGEs, and food sources for AGEs. The entire articles were screened for duplicate information and removed sequentially.
Research retrieved information from various reputed biomedical reports/articles published until 2023. The information from prestigious journals using keywords such as AGEs, AGE production through sequential pathways, food items containing AGEs, and country data on AGEs was systematically compiled into tables and presented as narrative review. Based on the scientific search engine, the articles were screened for relevant information available in AGEs research and review articles, which were compiled into tables and figures and presented in the current review article.
People are modifying restaurant meals rather than traditional/conventionally cooked due to industrialization and marketing methods (Table 1). Nonvegetarian food contains more dietary AGEs than vegetarian food. Age level is directly influenced by cooking temperature and time[26]. The foods with the highest AGEs are those that are fried, barbecued, baked, or boiled[27]. Dry-heat processed foods like crackers, chips, and cookies have the highest AGEs level per gram of food in this group. This is most likely due to the addition of components such as butter, oil, cheese, eggs, and nuts, which significantly enhance AGE formation during dry-heat processing[28] (Table 1)[29-58].
Worldwide, potatoes are a sustainable dietary alternative and source of energy from carbohydrates for all age groups. This root food is readily available all year, and boiling potatoes makes MR products more likely to occur[59]. Nonenzymatically, the reducing sugars glucose and fructose react with asparagine to make N-glucoside, which then produces melanoidin and the end product of the Schiff base reaction, which is decarboxylated to form acrylamide (ACR)[60]. Bread, coffee, fried potatoes, baked goods, and bread are the main sources of ACR[61], and browning increases its concentration[62]. The highest ACR production in diverse foods occurs at 120 °C[63]. Products made from cereal, coffee, and cocoa beans include 3-aminopropionamide[64] subsequently transformed into ACR in an aqueous MR[65]. Due to the fact that the MR occurs at the bread’s surface, the ACR concentration is higher in the crust and lower in the crumb. Similar to this, fried chips with a double layer of chips create a large amount of ACR[66]. Fried potatoes can expose you to an estimated 272-570 g/kg ACR, as can bread goods (75-1044 g/kg) and breakfast cereals (149 g/kg)[67].
Furan has a planar enol-carbonyl structure, a cyclic dicarbonyl structure, and a caramel-like scent due to the MR[68]. It is created through a number of processes, including thermal deterioration, oxidation of polyunsaturated fatty acids, and the MR, which is the thermal rearrangement of carbohydrates in the presence of amino acids[69]. Acetaldehyde and glycolaldehyde are produced through the breakdown of serine and cysteine amino acids, and the addition of an aldol group allows for the production of furans[70]. Numerous chemical processes, such as the Strecker reaction and the oxidation of polyunsaturated fatty acids, take place during the heat processing of food[71]. High concentrations of furan are directly correlated with higher cooking temperatures (150-200 °C), yet some furan is vaporized when cooking in an open pan[72]. Reports from the Fromberg et al[73] study in open vessel cooking, furan is reduced by 50%, and chocolate has a low concentration. Furans provide meals with a variety of flavors and aromas including sweet, fruity, nutty, meaty, and burnt. In the course of manufacturing infant foods, cereal, coffee, preserved foods, meat, and fish, furan, and its derivatives are created[74]. Studies have shown that coffee is one of the most widely consumed nonalcoholic beverages, with little negative effect[75]. The processing of coffee and its products is thought to contribute the largest furan concentration, followed by baked cookies, bread, and chips. Furan levels are also high in packaged and bottled meals[76]. Due to variation in macronutrient ratios and processing methods, furan concentration in foods for infants varies. Infant meals with a meat foundation as opposed to ones with mixed fruits contain higher levels of furan[74].
Hydroxymethylfurfural (HMF) is produced by the 1,2-enolation reaction in a mild alkaline medium, and HMF (6-carbon hetrocyclic aldehyde) is the main intermediate product of the Amadori rearrangement[77]. HMF is created via a variety of processes, including the thermal breakdown of sugars and interactions with other intermediates[78]. Under acidic conditions, disaccharide (sucrose) mostly degrades to glucose and fructose, which are then enolized and dried out to produce fructofuranosyl. Furthermore, at high temperatures, this cation changes to HMF[79]. Alternatively, the carbonyl group of reducing sugars such as maltose or glucose can join with lysine or another amino acid as a precursor. As a result, under controlled heat conditions, a sugar pyrolysis reaction occurs, forming browning and HMF[80]. Within a pH-controlled environment, the polymerization of HMF with a food product containing nitrogen results in melanoidins, which give the surface a brown color[81]. The presence of HMF in honey is utilized as a quality and freshness index indication. In honey and fruit juices, the level of thermal breakdown of sugar that results in the production of hazardous metabolites may also be clearly seen. Samples of honey from 29 different nations were shown to be directly correlated with storage temperature, time, and HMF content levels. According to country-specific honey samples, Malaysian honey had a value of 1132 mg/kg after more than 2 years of storage at 30 °C, Turkey’s honey had a value of 0.0-11.5 mg/kg, and India’s honey had a value of 0.15-1.70 mg/kg[82]. Other parameters that affect HMF levels, such as pH, water activity, kind of sugar, mineral content, and its origin, were explored by Kamboj et al[83]. Fructose-rich high fructose corn syrup (HFCS) is used in a variety of beverages and drinks. According to a study, fresh HFCS syrup has modest levels of HMF, which rise with temperature and storage time[84]. Similar findings have revealed that HMF level increases eight times in highly acidic media heated to 110 °C for 40 min[85] HMF levels in apple juice range from 0.06 mg/L to 18.12 mg/L as a result of heat exposure[86]. Date Syrup: Fresh 1000-2675 mg/kg and Industrial 12-456 mg/kg[87], Malaysian tropical fruit juices range 0.08-91.50 mg/L[88]. Towards the end of the baking process, volatile chemicals are formed. Longer periods of higher temperatures can lead to increased HMF production[89] and in biscuits and cookies, many aromas such as “bready,” ”almond,” “pungent,” and “sweet” form[90]. A recent study found that breakfast cereal has an HMF content of 13.3 mg/kg, but ultra-processed cereal has a content of 32.1 mg/kg. The HMF level of all cereals ranges from 0.3 mg/kg to 159.6 mg/kg. Due to the addition of sugar, refined wheat flakes have an elevated level of 159.6 mg/kg[91].
While ketose (fructose) creates an equivalent Heyns compound, simple sugars such as glucose form an amadori intermediate (1-amino-1-deoxi-2-ketose) by losing a water molecule. Amadouri or Heyns compound breakdown then produces dicarbonyl intermediates[92]. Reactive dicarbonyl structures are created as intermediates during the MR as a result of a series of chemical events including isomerization, dehydration, fragmentation, and redox reactions. These compounds have an affinity to react with the side chains of the amino groups’ lysine and arginine, producing stable protein adducts. Recent molecular structure investigations have shown that the amino acids arginine and lysine react with the molecules GO, methyl-GO (MGO), and 3-DG to form a number of crosslinkages[93]. Dehydration of hexose sugar produces 3-DG, and fragmentation of intermediate MR products produces 2,3-butanedione, GO, and MGO. On the other hand, these chemicals have also developed as a byproduct of the breakdown of lipids[94]. Carbonyl synthesis by lipid oxidation is supported by the advanced lipoxidation end products (ALE) process[95]. Group I chemicals as a result of lipid peroxidation include the following: acrolein, 4-hydroxy-2-nonenal, 4-hydroxy-hexenal, and 4-hydroxy-nonenal are examples of unsaturated aldehydes. Group (1): di-aldehydes include malondialdehyde and GO compounds. Group (3): cheto-aldehydes include MGO, 4-oxo-nonenal, and levuglandins[92]. By using a lipidomic technique, 35 aldehydes and ketones have so far been isolated from various fatty acid-rich sources. The researcher also highlighted how the oxidation of oleic acid and eicosapentaenoic acid helps to produce GO[96]. Depending on the manner of cooking and the type of processing used, these MR products alter the texture and flavor of food[97]. The production of MR intermediates is affected by caramelization and heat processing. Dicarbonyl concentration rises during baking in foods high in sugar and low in moisture. Cookies have been shown to have varying concentrations of 3-DG, GO, and MGO[98]. Early on, coffee roasting rises[99]. Due to nonenzymatic browning and fermentation, GO is primarily found in soybean paste, soy sauce, alcoholic beverages, and fermented coffee[100]. However, in both vegetarian and nonvegetarian food preparation, MGO production occurs during glycolysis[101].
Early-stage MR products such as furosine bind to proteins that contain N-substituted 1-amino-1-deoxy-2-ketose, including fructose-lysine, lactulose-lysine, and maltose-lysine[102]. The N-substituted 1-amino-1-deoxy-2-ketose found in proteins such as fructose-lysine, lactulose-lysine, and maltose-lysine is bound by the early-stage MR product furosine. Degradation of the Amodori product results in formation of the dicarbonyl molecule, which either interacts with free amino acids to produce Strecker aldehydes or with amino groups of amino acids, peptides, and proteins to rearrange and produce AGEs. An amine group on a protein or peptide combines with a reducing sugar to produce various aromatic compounds and melanoidins, which are crosslinked proteins. This process is known as the dehydroalanine route[103]. Lactulosyl-lysine, a protein-bound AP, is the first stable chemical created during milk’s MR process, and furosine is created following acid digestion. The primary causes of lysine blockage are temperature, time, and length of storage. Ten percent in ultra-high temperature (UHT) milk, 15% in sterilized container milk, and 25%-30% in newborn formula make up the percentage of lysine that is unavailable[104]. Dairy products’ nutritional value is evaluated by their low furosin content. In their analysis of the furosine content of several heat-treated milk samples, Shi et al[51] found that charcoal-flavored fermented milk had the highest concentration, followed by flavored fermented milk, and low temperature (LT) pasteurized fresh milk had the lowest concentration of furosine. In hydrolyzed dairy samples, Montilla et al[105] estimated that furosine level ranged from 235-820 mg/100 g protein and increased by up to 90% after 4 mo of storage at 20 °C. Boitz and Mayer[106] calculated the amount of furosine in whipping cream for retailed pasteurized, extended shelf life, and UHT cream samples were 47.8 mg ± 14.0 mg, 72.2 mg ± 36.6 mg, and 172.5 mg ± 17.7 mg in 100g-1 protein. The amounts of furosine in soy and whey hydrolyzed protein-based infant formula were 379 mg/100 g and 1459 mg/100 g, respectively. Similar to the subsequent formula and other partially hydrolyzed milk formulas, casein makes up 945 mg/100 g of the protein in the latter[107]. Due to its higher lactose content than other dairy foods, infant food is more likely to include furosin. According to Lund et al[107], whey protein concentrate (WPC) underwent a number of alterations to the protein as a result of thermal treatment. The time of storage also enhances the quantity of furosin in both types of (DI-IF and IN-IF) processing, and recently, the role of WPC has created more furosine than other whey protein ingredients[107]. Other authors have noted that various newborn formulas contain furosin concentrations ranging from 471.9 mg/100 g to 639.5 mg/100 g[108]. Another investigation examined the impact of drying heat on various pasta samples. Artisanal pasta had the lowest furosine level, ranging from 107 to 186 mg/100 g protein, as a result of the LT drying method[109]. Due to the usage of durum wheat flour and other chemical components, whole grain pasta has a furosine concentration ranging from 229 to 836 mg/100 g protein[110]. Gluten-free spaghetti contains lower furosin 19-134 mg/100 g protein in another study by Gasparre et al[111] than durum wheat pasta.
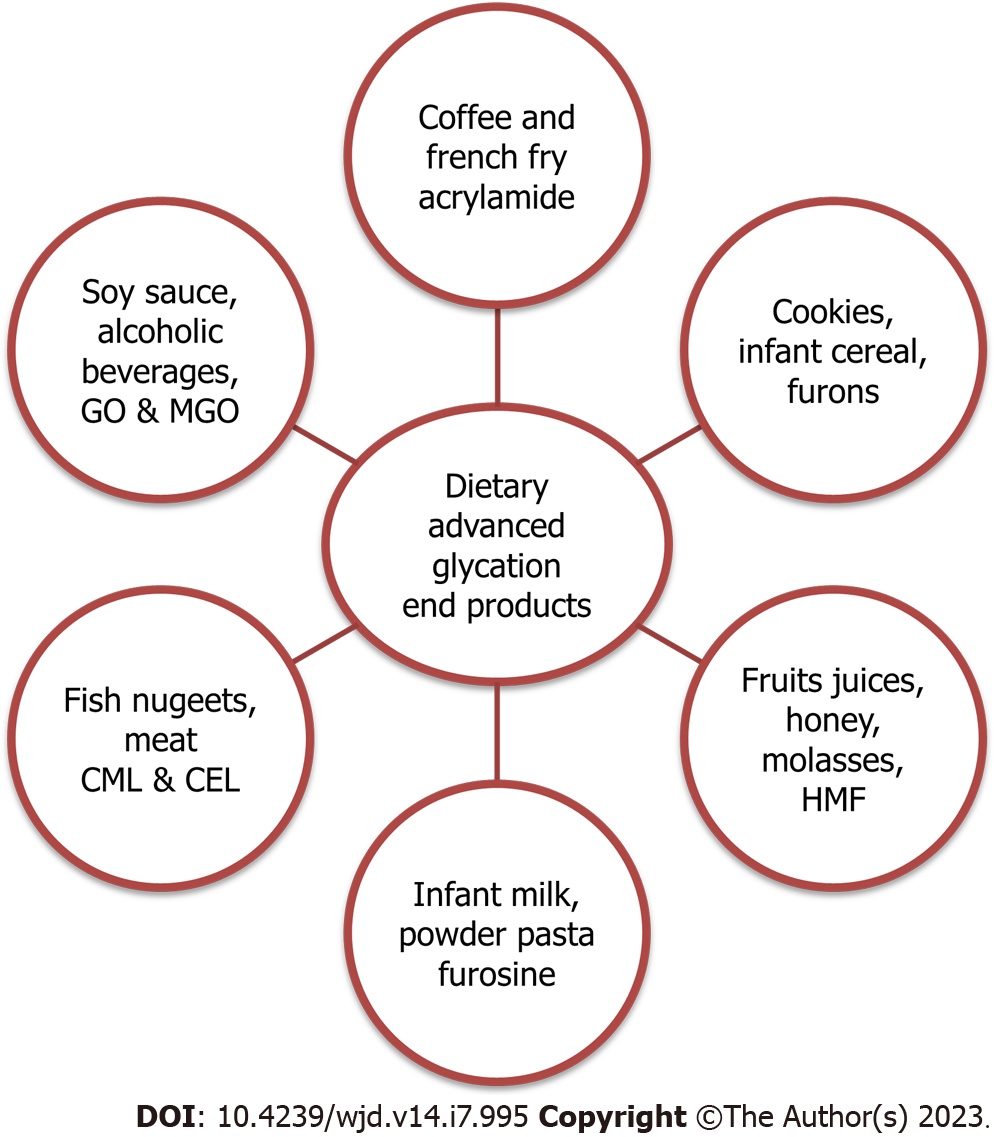
Animal proteins and processed plant foods contain furosine, ACR, heterocyclic amines (HCAs), and HMF[112]. Specifically cooked beef products and other processed foods include the amino acid furosine (2-furoil methyl lysine)[113]. High concentrations of CML, CEL, and MG-O are found in heat-treated nonvegetarian foods, peanut butter, and cereal items[114]. Infant milk formula contains CML as well because of the milk proteins in it. Lysines and other amino acids are released more freely after hydrolysis[115]. According to the AGE database, processed canned meats and nuts have the highest AGE levels, whereas fruits, vegetables, and butter have the lowest levels[116] (Figure 2).
Several endogenous factors may hasten the generation of AGE in the body.
AGE production and the stimulation of oxidative damage (OX) are two of hyperglycemia’s main side effects [117]. Obese but healthy individuals could avoid the formation of AGEs and OX during metabolic stress by increasing the fractional excretion of AGEs via renal clearance. In particular, hyperglycemia induces excessive reactive oxygen species (ROS) production and OS, which in turn promotes the formation of AGEs, events eventually resulting in the development of insulin resistance, impaired insulin secretion, and endothelial dysfunction[118].
It is still unclear whether AGEs cause the aging process or aging process speeds up the buildup of AGEs[119]. In the natural progression of the aging process, some researchers have hypothesized that AGE production plays a critical role[117]. AGEs generate oxidative stress, and as a result, inflammatory and thrombogenic reactions via contact with RAGE, as well as metabolic changes[119]. Son et al[120] concluded that circulating glycotoxins are undoubtedly linked to oxidative stress and an inflammatory response that cause cell malfunction. They concluded that visceral fat was involved in the pathogenesis of inflammatory problems in the elderly. Biological aging, neuron related inflammatory illnesses, DM and its complications, bone-degenerative diseases, and renal disorders are all examples of AGE-related diseases[121]. The authors came to the conclusion that the common contributing factors to the inflammatory state in these noncommunicable chronic inflammatory disorders were AGE-RAGE signaling abnormalities.
Obesity is typically linked to a higher risk of metabolic syndrome, which includes insulin-resistant type 2 DM, hypertension, fatty liver, and vascular problems due to the unnecessary production of adipokines by fat cells. Gaens et al[122] reported that obesity was associated with higher plasma and tissue levels of MGO, AGEs, and ALE surrogated by CML. Brix et al[123] showed that in patients with MO, soluble-form RAGE (sRAGE) levels were significantly lower than those in the nonobese group. But following bariatric surgery to lose weight, which stopped the AGE-mediated inflammatory process, sRAGE levels rose. Similarly, Sanchez et al[124] with an AGE reader, and skin autofluorescence (SAF) in the forearm to measure AGE buildup. It was found that SAF levels were higher in metabolic syndrome-affected MO patients than in nonobese people. SAF remained high following bariatric surgery until glycemic memory failed. Deo et al[125] examined how weight loss in overweight participants without diabetes affected their CML levels. After losing weight, CML readings dropped by 17%, but this was less beneficial in people with diabetes or prediabetes who were not overweight. These findings might imply that AGE formation and tissue accumulation in the body are influenced by both obesity and hyperglycemia.
Patients with uremia, whether or not they had diabetes, had significantly higher amounts of AGEs in their plasma[126]. Miyata et al[127] investigated the destiny of AGEs by administering pentosidine, a synthetic AGE, intravenously to rats. Pentosidine was found to be eliminated in urine after being filtered by the renal glomeruli, reabsorbed in the proximal renal tubules, and subjected to catabolic or metabolic changes. Later, Asano et al[128] studied the metabolism of protein-linked pentosidine using three cell lines: proximal tubular, distal tubular, and nonrenal, in contrast to the distal tubular and nonrenal cell lines, they showed that pentosidine was quickly found in the cytoplasm of the proximal renal tubular cell line. They came to the conclusion that renal proximal tubular cells were crucial for the elimination of plasma pentosidine. Adriamycin-induced chronic nephropathy in nondiabetic rats was directly associated with renal pentosidine buildup[129]. Chronic heart failure, cardiovascular illnesses, diabetes, neurological diseases, osteoarthritis, and nondiabetic atherosclerosis all developed together with AGE accumulation in chronic kidney disease[130]. A high-AGE diet may also increase the chance of developing chronic illnesses, including chronic kidney disease[131]. According to Inagi, this alleged “glycation stress” was discovered to be directly related to kidney aging[132].
Reactive carbonyl compounds, which are pentosidine’s precursors, are detoxicated by glyoxalase in a hemodialysis patient with uremia. By chance, the authors discovered that this patient’s renal blood vessels (RBVs) had far higher plasma levels of pentosidine and CML than those of hemodialysis patients. Further analysis revealed that this patient’s RBVs had very low glyoxalase activity. They came to the conclusion that high AGE levels in uremia patients were largely caused by glyoxalase I deficiency (GLO-I), which was unable to detoxify AGEs[133]. In addition, Shinohara et al[134] reported that the bovine endothelial cells that overexpress GLO-I reduce intracellular AGE production and stop hyperglycemia from causing an increase in macromolecular endocytosis in the circulation. Similarly, Brouwers et al[135] revealed that in mesangial cells taken from diabetic rats and mice, overexpression of GLO-I decreased hyperglycemia-induced AGE formation and oxidative stress. Furthermore, Kurz et al[136] demonstrated that glycation stress may be prevented from causing cell damage by reducing the hazardous levels of MGO, GO, and other AGEs. Xue et al[137] explored the molecular underpinnings of erythroid 2-related factor 2’s transcriptional regulation of GLO-I. The team identified a defense mechanism against stress caused by decarbonyl glycation (MGO) in high glucose concentration, inflammation, cell aging, and senescence as a result. Recently, Garrido et al[138] reported that MGO-derived AGE buildup might be prevented by fatty acid production working with GLO-I to protect against glycation damage.
The fast rise in the consumption of foods and beverages with added sugar during the past three decades, in both industrialized and developing nations, has been linked to an increase in metabolic illnesses. The function of advanced glycation end products in the pathophysiology of metabolic illnesses associated with modern nutrition is a new area of research (AGEs) (Figure 3)[139].
In vivo AGE formation is dependent on particular intracellular and extracellular circumstances. The rate at which proteins are turned over, oxidative stress in the intra- or extracellular environment, and the degree of hyperglycemia are some of the elements that have been explored as contributing to the creation of AGEs[140]. After 1 wk of hyperglycemia, endothelial cells have been shown to produce considerably more intracellular AGEs. Additionally, the type of reducing sugar has an impact on how quickly AGEs form when combined with intracellular proteins, with glucose having the slowest reaction when compared to fructose, glyceraldehyde-3-phosphate, and glucose-6-phosphate[140]. Healthy aging individuals have been shown to accumulate AGEs in their blood and tissues, and this buildup is greater when their blood glucose levels are high. In addition, In situations of metabolic and vascular illnesses such DM, atherosclerosis, and renal disease, AGEs have been observed to be raised in human tissues, plasma, and urine[141]. Semba et al[142] showed that higher circulating AGEs were a reliable indicator of renal function in an older group. Study found that after 3 years and 6 years of follow-up, the estimated glomerular filtration rate (a measure of kidney function) at baseline and chronic kidney disease were independently related with a higher plasma content of CML[143] and results indicate that the overall population of older community-dwelling persons may be affected by the potential negative effects of AGEs on the kidney[143]. In a different investigation, 51.6% of the 548 women from the Women’s Health and Aging Study I in Baltimore had worse glomerular filtration rates, which were linked to higher serum levels of CML and sRAGE (the soluble form of RAGE)[143]. Normal renal function involves the kidneys clearing circulating AGEs, although elevated AGE levels have been seen in individuals with uremia and diabetic nephropathy, likely due to insufficient renal clearance[144].
Additionally, individuals with DM have peripheral nerves with high amounts of AGEs[145]. Ahmed conducted a recent study and discovered that diabetes has been linked to increased myelin glycation in in vitro investigations. By phagocytosing the glycated myelin, macrophages could explain the nerve demyelination found in diabetic neuropathy. When AGEs are injected into peripheral nerves in animal tests, blood flow, nerve action potentials, and sensory motor conduction velocities all decrease[146].
In terms of developmental diseases, AGE accumulation and obesity interact with health risk factors, as a result, the development of glucose levels is influenced and said that AGEs, glycated hemoglobin, and obesity are all linked to glucose levels, and obesity may be one of the health risk factors pathophysiological mechanisms leading to increased glucose level due to AGE accumulation; thus, obesity could be health risk factors leading to increased glucose level in AGE accumulation[147]. Local inflammatory response is linked to elevated systemic inflammatory cytokines, which are responsible for impaired glucose regulation[148]. It is now well established that external AGEs considerably contribute to the body’s AGE pool[149]. The increased inflammation further triggers the activation of additional mediators which increases inflammation, as well as induces insulin resistance in muscles[150]. Under identical settings of hyperglycemia and AGE accumulation, the interaction of RAGE-induced cellular dysfunction, protein kinases, and inflammation leads to a reduction in insulin sensitivity in target cells[151]. Hofmann and colleagues demonstrated in a RAGE knock-out mouse model that both AGEs and RAGE are implicated in aortic leaflet calcification and consequent aortic stenosis[152]. Reduced sRAGE and endogenous secretory receptor for RAGE (esRAGE), both of which are assumed to be protective against AGEs, have been identified as an early indicator of first target organ damage in moderate hypertensives[153] or diabetics have negative coronary artery remodeling[154]. In DM patients, AGEs and RAGE build within stenotic aortic valves, and the extent of this accumulation is related to the severity of the aortic stenosis and plasma AGE and sRAGE levels were linked to aortic valve area, they may be regarded novel biomarkers of the aortic stenosis course in patients with type 2 diabetes[155], dual character of RAGE, combined with increased AGE consumption by sRAGE in people with poor glucose metabolism, may disrupt direct correlations between RAGE and markers reflecting the degree of aortic stenosis[156].
Insulin resistance and hyperglycemia can impact numerous human tissues and organs, leading to long-term difficulties in a number of systems and organs, including the cardiovascular system[157,158]. Left ventricular concentric hypertrophy, perivascular fibrosis, and interstitial fibrosis are signs of pathological remodeling of the heart, which results in diastolic dysfunctions[159]. Cardiovascular illness affects the former more severely and extensively than the latter, has a worse prognosis, and manifests earlier in the former. Heart failure is 2-4 times more likely to occur in people with type 2 diabetes than in people without the disease[160]. About 70%-80% of diabetics pass away from cardiovascular issues at the end[161]. Additionally, almost 3/4 of people with type 2 diabetes also have a number of cardiovascular risk factors, including obesity, dyslipidemia, and hypertension. The accumulation of these risk factors may directly encourage the development of diabetic cardiovascular problems[162]. The primary fundamental mechanism thought to be responsible for diabetic cardiovascular disorders is increased oxidative stress[163]. In diabetic cardiovascular problems, hyper-glycemia causes NADPH oxidase to become active[164], oxidative stress causes myocardial fibrosis, endothelial dysfunction, hypertrophy and apoptosis of cardiomyocytes, inflammation, endothelial dysfunction, decreased left ventricular compliance, diastolic dysfunction, and ultimately heart failure, arrhythmia, and/or sudden cardiac death[165]. Nin et al[166] confirmed that plasma AGE levels in fatal or nonfatal coronary artery disease are related to all-cause mortality. Steine et al[167] found that Plasma AGE levels are related to left ventricular dysfunction in people with type 1 diabetes. Jia et al[159] also found that the plasma AGE levels are related to left ventricular dysfunction in people with type 1 diabetes. Type IV collagen and laminin, two extracellular matrix proteins of endothelial cells, can be directly modified by AGEs[168]. This mechanism accelerates cardiac fibrosis and damages the natural structure and function of blood vessels[169]. In addition to harming endothelium cells, AGEs also cause endothelial progenitor cells to die and become dysfunctional[170]. Atherosclerosis can be accelerated by circulating AGEs, which can increase lipid oxidation and deposition in atherosclerotic plaques and encourage macrophage infiltration, T cell migration, and proliferation[171]. Additionally, recent research has demonstrated that AGE binding to the platelet membrane receptor cluster of differentiation 36 results in the production of thrombi, which may be a key mechanism by which AGEs encourage myocardial ischemia episodes in diabetes patients[172]. As a result of the AGE-RAGE interaction, numerous signal transduction cascades and downstream pathways are activated, including mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2, p38, and nuclear factor kappa B. This causes oxidative stress to increase, ROS to be produced, and the development of cardiovascular problems in diabetes[173]. Additionally, it was discovered that AGEs increased endothelial cells’ NADPH oxidase production and activity, which is a significant source of oxidative stress in diabetic cardiovascular problems[174,175]. Currently, it is accepted that diabetic patients who take metformin regularly can lower their chance of developing cardiovascular disease[176]. Its antioxidant qualities that lower OX activity and lipid peroxidation in type 2 diabetic patients are responsible for its cardiovascular protective benefit [177]. Metformin treatment reduced AGE plasma levels in diabetic rats, decreasing AGE-induced heart remodeling and oxidative stress[178].
Interestingly, sustained high dietary AGEs have been shown to cause increased arterial stiffness, which leads to an increase in systolic blood pressure and inflammatory activation, leading to vascular issues in type 2 diabetes[179]. Regardless of aortic diameter, elevated circulating sRAGE levels have been connected to the presence of bicuspid aortic valves and linked aortopathies[180]. The AGEs/sRAGE ratio has been recommended as a more effective biomarker of organ damage than either AGEs or sRAGE variants alone[181] Furthermore, differing prediction abilities of esRAGE and cRAGE as cardiovascular risk factor markers have recently been demonstrated[182]. AGEs can also glycate and crosslink basement membrane protein, changing cell-matrix interactions and reducing endothelial cell adhesion leading microvascular and macrovascular problems[183]. AGEs cause oxidative stress, as well as inflammatory and fibrotic reactions, all of which contribute to the development and progression of life-threatening cardiovascular illnesses[184]. AGEs mainly induce arterial damage and exacerbate the development of atherosclerotic plaques by triggering cell receptor-dependent signal resulting in arterial wall injury and plaque formation[185].
The worldwide increase in consumption of highly processed, calorie-dense food is fueling an obesity, diabetic, kidney, and cardiometabolic disease crisis. Focusing on the effects of dietary AGEs has been shown to increase circulating AGEs, accumulate in tissues, to affect endothelial function, increase pro-inflammatory cytokines and oxidation markers, and to act as a ligand for the advanced glycation end products receptor (RAGE). AGEs intake was higher in participants with obesity, diabetes, cardiovascular disease complications when compared with those without complications. AGEs have been found in dietary items, human blood, and tissues such as pyrraline, CML, CEL, pentosidine, and MOLD. In both industrialized and developing countries over the past three decades, consumption of AGE-containing foods and beverages has been associated with an increase in metabolic diseases. Cardiovascular disorders are made worse by diabetes, and patients with diabetic cardiovascular problems have worse clinical outcomes. Since AGEs not only influence oxidative stress but also are impacted by it, it is well known that AGEs and oxidative stress play a central role in the cardiovascular problems associated with diabetes. However, many of these mechanisms are still unclear and require more explanation. Beyond blood glucose control in this population, it has been discovered that glucose-lowering medications have a protective effect on the cardiovascular system.
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia for funding this research work through the project number (QU-IF-2-2-1-27012). The authors also thank to Qassim University for technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Kyrgyzstan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dziegielewska-Gesiak S, Poland; Fatemi A, Iran; Liu D, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YX
| 1. | Kim CS, Park S, Kim J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J Exerc Nutrition Biochem. 2017;21:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Monnier VM, Taniguchi N. Advanced glycation in diabetes, aging and age-related diseases: editorial and dedication. Glycoconj J. 2016;33:483-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Vlassara H, Striker GE. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol Metab Clin North Am. 2013;42:697-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Nowak A, Przywara-Chowaniec B, TYRPIEń-Golder K, Nowalany-Kozielska E. Systemic lupus erythematosus and glycation process. Cent Eur J Immunol. 2020;45:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Johnson EM, Christian MS, Dansky L, Gabel BE. Use of the adult developmental relationship in prescreening for developmental hazards. Teratog Carcinog Mutagen. 1987;7:273-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Dadoniene J, Cypiene A, Ryliskyte L, Rugiene R, Ryliškiene K, Laucevičius A. Skin Autofluorescence in Systemic Sclerosis Is Related to the Disease and Vascular Damage: A Cross-Sectional Analytic Study of Comparative Groups. Dis Markers. 2015;2015:837470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kopeć-Pyciarz K, Makulska I, Zwolińska D, Łaczmański Ł, Baran W. Skin Autofluorescence, as a Measure of AGE Accumulation in Individuals Suffering from Chronic Plaque Psoriasis. Mediators Inflamm. 2018;2018:4016939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation End-products (AGEs): an emerging concern for processed food industries. J Food Sci Technol. 2015;52:7561-7576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 917] [Cited by in F6Publishing: 863] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2:1247-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Vlassara H, Uribarri J, Cai W, Striker G. Advanced glycation end product homeostasis: exogenous oxidants and innate defenses. Ann N Y Acad Sci. 2008;1126:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Del Turco S, Basta G. An update on advanced glycation endproducts and atherosclerosis. Biofactors. 2012;38:266-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Perrone A, Giovino A, Benny J, Martinelli F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid Med Cell Longev. 2020;2020:3818196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 14. | Xie Y, van der Fels-Klerx HJ, van Leeuwen SPJ, Fogliano V. Occurrence of dietary advanced glycation end-products in commercial cow, goat and soy protein based infant formulas. Food Chem. 2023;411:135424. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 15. | Boz H. N(ϵ) -(carboxymethyl)lysine in bakery products: A review. J Food Sci. 2023;88:901-908. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (1)] |
| 16. | Gill V, Kumar V, Singh K, Kumar A, Kim JJ. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules. 2019;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 17. | Boyer F, Vidot JB, Dubourg AG, Rondeau P, Essop MF, Bourdon E. Oxidative stress and adipocyte biology: focus on the role of AGEs. Oxid Med Cell Longev. 2015;2015:534873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond). 2011;121:43-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, Yang P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol. 2017;454:103-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Reynaert NL, Gopal P, Rutten EPA, Wouters EFM, Schalkwijk CG. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int J Biochem Cell Biol. 2016;81:403-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013;25:2185-2197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 347] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 22. | Peppa M, Vlassara H. Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens). 2005;4:28-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Bonnefont-Rousselot D. Resveratrol and Cardiovascular Diseases. Nutrients. 2016;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 24. | Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Pt 1:109-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 664] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 25. | Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 369] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 26. | Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-16.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 703] [Cited by in F6Publishing: 768] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 27. | Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 489] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 28. | Story M, Hayes M, Kalina B. Availability of foods in high schools: is there cause for concern? J Am Diet Assoc. 1996;96:123-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Mojska H, Gielecińska I, Szponar L, Ołtarzewski M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem Toxicol. 2010;48:2090-2096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Abt E, Robin LP, McGrath S, Srinivasan J, DiNovi M, Adachi Y, Chirtel S. Acrylamide levels and dietary exposure from foods in the United States, an update based on 2011-2015 data. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2019;36:1475-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Shamla L, Nisha P. Acrylamide in deep-fried snacks of India. Food Addit Contam Part B Surveill. 2014;7:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Verma V, Yadav N. Acrylamide content in starch based commercial foods by using high performance liquid chromatography and its association with browning index. Curr Res Food Sci. 2022;5:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | El Tawila MM, Al-Ansari AM, Alrasheedi AA, Neamatallah AA. Dietary exposure to acrylamide from cafeteria foods in Jeddah schools and associated risk assessment. J Sci Food Agric. 2017;97:4494-4500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Arisseto AP, Vicente E, Furlani RP, Ueno MS, Pereira AL, Toledo MC. Occurrence of furan in commercial processed foods in Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:1832-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Alsafra Z, Scholl G, De Meulenaer B, Eppe G, Saegerman C. Hazard Ratio and Hazard Index as Preliminary Estimators Associated to the Presence of Furans and Alkylfurans in Belgian Foodstuffs. Foods. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 36. | Mesías M, Guerra-Hernández E, García-Villanova B. Furan content in Spanish baby foods and its relation with potential precursors. CyTA-J Food. 2013;11:1-6. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Waizenegger J, Winkler G, Kuballa T, Ruge W, Kersting M, Alexy U, Lachenmeier DW. Analysis and risk assessment of furan in coffee products targeted to adolescents. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Fromberg A, Mariotti MS, Pedreschi Plasencia F, Fagt S, Granby K. Furan and alkylated furans in heat processed food, including home cooked products. Czech J Food Sci. 2014;32:442-448. [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Khalil MI, Sulaiman SA, Gan SH. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem Toxicol. 2010;48:2388-2392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Islam A, Khalil I, Islam N, Moniruzzaman M, Mottalib A, Sulaiman SA, Gan SH. Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. BMC Complement Altern Med. 2012;12:177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Mortas M, Gul O, Yazici F, Dervisoğlu M. Effect of brewing process and sugar content on 5-hydroxymethylfurfural and related substances from Turkish coffee. Int J Food Pro. 2017;20:1866-75. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | de Andrade JK, Komatsu E, Perreault H, Torres YR, da Rosa MR, Felsner ML. In house validation from direct determination of 5-hydroxymethyl-2-furfural (HMF) in Brazilian corn and cane syrups samples by HPLC-UV. Food Chem. 2016;190:481-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Czerwonka M, Opiłka J, Tokarz A. Evaluation of 5-hydroxymethylfurfural content in non-alcoholic drinks. Eur Food Res Technol. 2018;244:11-8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Alsubot S, Aldiab D. 5-hydroxymethylfurfural Levels in Coffee and Study of some effecting factors. Res J Pharm and Tech. 2019;12:4263-8. [DOI] [Cited in This Article: ] |
| 45. | Fallico B, Grasso A, Arena E. Hazardous Chemical Compounds in Cookies: The Role of Sugars and the Kinetics of Their Formation during Baking. Foods. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 46. | Arribas-Lorenzo G, Morales FJ. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J Agric Food Chem. 2010;58:2966-2972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Maasen K, Scheijen JLJM, Opperhuizen A, Stehouwer CDA, Van Greevenbroek MM, Schalkwijk CG. Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem. 2021;339:128063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 48. | Catak J, Yaman M, Ugur H, Servi EY, Faruk Mizrak Öm. Investigation of the advanced glycation end products precursors in dried fruits and nuts by HPLC using pre-column derivatization. J Food Nutr Res. 2022;61:1-8. [Cited in This Article: ] |
| 49. | Chen Z, Kondrashina A, Greco I, Gamon LF, Lund MN, Giblin L, Davies MJ. Effects of Protein-Derived Amino Acid Modification Products Present in Infant Formula on Metabolic Function, Oxidative Stress, and Intestinal Permeability in Cell Models. J Agric Food Chem. 2019;67:5634-5646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Troise AD, Fiore A, Wiltafsky M, Fogliano V. Quantification of Nε-(2-Furoylmethyl)-L-lysine (furosine), Nε-(Carboxymethyl)-L-lysine (CML), Nε-(Carboxyethyl)-L-lysine (CEL) and total lysine through stable isotope dilution assay and tandem mass spectrometry. Food Chem. 2015;188:357-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Shi X, Wu Q, Ren D, Wang S, Xie Y. Research of the determination method of furfurals and furosine in milk and the application in the quality evaluation of milk. Quality Assurance and Safety of Crops & Foods. 2022;14:12-23. [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 52. | Tekliye M, Pei X, Dong M. RP-HPLC determination of Furosine in fermented milk of different brands retailed in China. Int J Agric Sc Food Technol. 2019;5:064-067. [DOI] [Cited in This Article: ] |
| 53. | Li Y, Wu Y, Quan W, Jia X, He Z, Wang Z, Adhikari B, Chen J, Zeng M. Quantitation of furosine, furfurals, and advanced glycation end products in milk treated with pasteurization and sterilization methods applicable in China. Food Res Int. 2021;140:110088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Chen G, Smith JS. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015;168:190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Sun X, Tang J, Wang J, Rasco BA, Lai K, Huang Y. Formation of advanced glycation endproducts in ground beef under pasteurisation conditions. Food Chem. 2015;172:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Niu L, Sun X, Tang J, Wang J, Rasco BA, Lai K, Huang Y. Free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine in fish muscle: Biological variation and effects of heat treatment. J Food Compo and Ana. 2017;57:56-63. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Wang J, Li Z, Pavase RT, Lin H, Zou L, Wen J, Lv L. Advanced glycation endproducts in 35 types of seafood products consumed in eastern China. J Ocean Univ China. 2016;15:690-6. [DOI] [Cited in This Article: ] |
| 58. | Zhao S, Guan Y. Formation of Advanced Glycation End Products in Simulate Canned Saury Fish Models: Effects of Process Methods, Formulations and Correlation Analysis with Nutritive Substances. J Food Nutr Res. 2022;10:425-436. [DOI] [Cited in This Article: ] |
| 59. | Camire ME, Kubow S, Donnelly DJ. Potatoes and human health. Crit Rev Food Sci Nutr. 2009;49:823-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 60. | Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;419:449-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1195] [Cited by in F6Publishing: 1032] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 61. | Rannou C, Laroque D, Renault E, Prost C, Sérot T. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res Int. 2016;90:154-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 62. | Krishnakumar T, Visvanathan R. Acrylamide in food products: a review. J Food Process Technol. 5:7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Yang Y, Achaerandio I, Pujolà M. Influence of the frying process and potato cultivar on acrylamide formation in French fries. Food Control. 2016;62:216-223. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Granvogl M, Schieberle P. Quantification of 3-aminopropionamide in cocoa, coffee and cereal products. Eur Food Res Technol. 2007;225:857-863. [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Schieberle P, Köhler P, Granvog M. New aspects on the formation and analysis of acrylamide. Adv Exp Med Biol. 2005;561:205-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 66. | Parker JK, Balagiannis DP, Higley J, Smith G, Wedzicha BL, Mottram DS. Kinetic model for the formation of acrylamide during the finish-frying of commercial french fries. J Agric Food Chem. 2012;60:9321-9331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Pedreschi F, Mariotti MS, Granby K. Current issues in dietary acrylamide: formation, mitigation and risk assessment. J Sci Food Agric. 2014;94:9-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects-A review. J func Foods. 2015;18:820-897. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1410] [Cited by in F6Publishing: 1440] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 69. | Seok YJ, Her JY, Kim YG, Kim MY, Jeong SY, Kim MK, Lee JY, Kim CI, Yoon HJ, Lee KG. Furan in Thermally Processed Foods - A Review. Toxicol Res. 2015;31:241-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Moro S, Chipman JK, Wegener JW, Hamberger C, Dekant W, Mally A. Furan in heat-treated foods: formation, exposure, toxicity, and aspects of risk assessment. Mol Nutr Food Res. 2012;56:1197-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Kettlitz B, Scholz G, Theurillat V, Cselovszky J, Buck NR, O' Hagan S, Mavromichali E, Ahrens K, Kraehenbuehl K, Scozzi G, Weck M, Vinci C, Sobieraj M, Stadler RH. Furan and Methylfurans in Foods: An Update on Occurrence, Mitigation, and Risk Assessment. Compr Rev Food Sci Food Saf. 2019;18:738-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 72. | Santonicola S, Mercogliano R. Occurrence and production of furan in commercial foods. Ital J Food Sci. 2016;28:155. [Cited in This Article: ] |
| 73. | Fromberg A, Fagt S, Granby K. Furan in heat processed food products including home cooked food products and ready-to-eat products. EFSA Sup Pub. 2009;6:1E. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Condurso C, Cincotta F, Verzera A. Determination of furan and furan derivatives in baby food. Food Chem. 2018;250:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Mesías M, Morales FJ. Corrigendum to "Reliable estimation of dietary exposure to furan from coffee: An automatic vending machine as a case study" [Food Research International 61 (2014) 257-263]. Food Res Int. 2015;74:338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 76. | Minorczyk M, Góralczyk K, Struciński P, Hernik A, Czaja K, Łyczewska M, Korcz W, Starski A, Ludwicki JK. Risk assessment for infants exposed to furan from ready-to-eat thermally processed food products in Poland. Rocz Panstw Zakl Hig. 2012;63:403-410. [PubMed] [Cited in This Article: ] |
| 77. | Ren GR, Zhao LJ, Sun Q, Xie HJ, Lei QF, Fang WJ. Explore the reaction mechanism of the Maillard reaction: a density functional theory study. J Mol Model. 2015;21:132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Contreras-Calderón J, Guerra-Hernández E, García-Villanova B, Gómez-Narváez F, Zapata-Betancur A. Effect of ingredients on non-enzymatic browning, nutritional value and furanic compounds in Spanish infant formulas. J Food and Nutri Res. 2017;5:243-252. [DOI] [Cited in This Article: ] |
| 79. | Nguyen HT, van der Fels-Klerx HJI, van Boekel MAJS. Acrylamide and 5-hydroxymethylfurfural formation during biscuit baking. Part II: Effect of the ratio of reducing sugars and asparagine. Food Chem. 2017;230:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Agila A, Barringer S. Effect of roasting conditions on color and volatile profile including HMF level in sweet almonds (Prunus dulcis). J Food Sci. 2012;77:C461-C468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Jaeger H, Janositz A, Knorr D. The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol Biol (Paris). 2010;58:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 82. | Shapla UM, Solayman M, Alam N, Khalil MI, Gan SH. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: effects on bees and human health. Chem Cent J. 2018;12:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 83. | Kamboj R, Sandhu RS, Kaler RSS, Bera MB, Nanda V. Optimization of process parameters on hydroxymethylfurfural content, diastase and invertase activity of coriander honey. J Food Sci Technol. 2019;56:3205-3214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | LeBlanc BW, Eggleston G, Sammataro D, Cornett C, Dufault R, Deeby T, St Cyr E. Formation of hydroxymethylfurfural in domestic high-fructose corn syrup and its toxicity to the honey bee (Apis mellifera). J Agric Food Chem. 2009;57:7369-7376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Frizzera D, Del Fabbro S, Ortis G, Zanni V, Bortolomeazzi R, Nazzi F, Annoscia D. Possible side effects of sugar supplementary nutrition on honey bee health. Apidologie. 2020;51:594-608. [DOI] [Cited in This Article: ] |
| 86. | Santini A, Romano R, Meca G, Raiola A, Ritieni A. Antioxidant activity and quality of apple juices and puree after in vitro digestion. J Food Res. 2014;3:41. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Jafarnia A, Soodi M, Shekarchi M. Determination and comparision of hydroxymethylfurfural in industrial and traditional date syrup products. Iran J Toxic. 2016;10:11-16. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Lee TP, Sakai R, Manaf NA, Rodhi AM, Saad B. High performance liquid chromatography method for the determination of patulin and 5-hydroxymethylfurfural in fruit juices marketed in Malaysia. Food Control. 2014;38:142-149. [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 89. | Zhang YY, Song Y, Hu XS, Liao XJ, Ni YY, Li QH. Effects of sugars in batter formula and baking conditions on 5-hydroxymethylfurfural and furfural formation in sponge cake models. Food Res Inter. 2012;49:439-445. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Pasqualone A, Bianco AM, Paradiso VM, Summo C, Gambacorta G, Caponio F, Blanco A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015;180:64-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Morales FJ, Mesías M, Delgado-Andrade C. Association between Heat-Induced Chemical Markers and Ultra-Processed Foods: A Case Study on Breakfast Cereals. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47 Suppl 1:3-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 509] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 93. | Henning C, Glomb MA. Pathways of the Maillard reaction under physiological conditions. Glycoconj J. 2016;33:499-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 94. | Wang Y, Ho CT. Flavour chemistry of methylglyoxal and glyoxal. Chem Soc Rev. 2012;41:4140-4149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Delgado-Andrade C, Fogliano V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu Rev Food Sci Technol. 2018;9:271-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 96. | Suh JH, Niu YS, Hung WL, Ho CT, Wang Y. Lipidomic analysis for carbonyl species derived from fish oil using liquid chromatography-tandem mass spectrometry. Talanta. 2017;168:31-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Hellwig M, Henle T. Baking, ageing, diabetes: a short history of the Maillard reaction. Angew Chem Int Ed Engl. 2014;53:10316-10329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 98. | Hellwig M, Gensberger-Reigl S, Henle T, Pischetsrieder M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin Cancer Biol. 2018;49:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 99. | Daglia M, Papetti A, Aceti C, Sordelli B, Spini V, Gazzani G. Isolation and determination of alpha-dicarbonyl compounds by RP-HPLC-DAD in green and roasted coffee. J Agric Food Chem. 2007;55:8877-8882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Lee YY, Shibamoto T, Ha SD, Ha J, Lee J, Jang HW. Determination of glyoxal, methylglyoxal, and diacetyl in red ginseng products using dispersive liquid-liquid microextraction coupled with GC-MS. J Sep Sci. 2019;42:1230-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Pastor-Belda M, Fernández-García AJ, Campillo N, Pérez-Cárceles MD, Motas M, Hernández-Córdoba M, Viñas P. Glyoxal and methylglyoxal as urinary markers of diabetes. Determination using a dispersive liquid-liquid microextraction procedure combined with gas chromatography-mass spectrometry. J Chromatogr A. 2017;1509:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Sakkas L, Moutafi A, Moschopoulou E, Moatsou G. Assessment of heat treatment of various types of milk. Food Chem. 2014;159:293-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | Sunds AV, Rauh VM, Sørensen J, Larsen LB. Maillard reaction progress in UHT milk during storage at different temperature levels and cycles. Inter Dairy J. 2018;77:56-64. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 104. | Mehta BM, Deeth HC. Blocked Lysine in Dairy Products: Formation, Occurrence, Analysis, and Nutritional Implications. Compr Rev Food Sci Food Saf. 2016;15:206-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 105. | Montilla A, Megías-Pérez R, Olano A, Villamiel M. Presence of galactooligosaccharides and furosine in special dairy products designed for elderly people. Food Chem. 2015;172:481-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Boitz LI, Mayer HK. Evaluation of furosine, lactulose and acid-soluble β-lactoglobulin as time temperature integrators for whipping cream samples at retail in Austria. Inter Dairy J. 2015;50:24-31. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Lund P, Bechshøft MR, Ray CA, Lund MN. Effect of Processing of Whey Protein Ingredient on Maillard Reactions and Protein Structural Changes in Powdered Infant Formula. J Agric Food Chem. 2022;70:319-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Li HY, Xing L, Wang JQ, Zheng N. Toxicology studies of furosine in vitro/in vivo and exploration of the related mechanism. Toxicol Lett. 2018;291:101-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Giannetti V, Mariani MB, Mannino P. Furosine as a pasta quality marker: evaluation by an innovative and fast chromatographic approach. J Food Sci. 2013;78:C994-C999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Marti A, Pagani MA. What can play the role of gluten in gluten free pasta? Trends Food Sci Tech. 2013;31:63-71. [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 111. | Gasparre N, Betoret E, Rosell CM. Quality Indicators and Heat Damage of Dried and Cooked Gluten Free Spaghetti. Plant Foods Hum Nutr. 2019;74:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | ALjahdali N, Carbonero F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit Rev Food Sci Nutr. 2019;59:474-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 113. | Trevisan AJ, de Almeida Lima D, Sampaio GR, Soares RA, Markowicz Bastos DH. Influence of home cooking conditions on Maillard reaction products in beef. Food Chem. 2016;196:161-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Scheijen JLJM, Clevers E, Engelen L, Dagnelie PC, Brouns F, Stehouwer CDA, Schalkwijk CG. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016;190:1145-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 115. | Assar SH, Moloney C, Lima M, Magee R, Ames JM. Determination of Nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids. 2009;36:317-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 116. | Scheijen JLJM, Hanssen NMJ, van Greevenbroek MM, Van der Kallen CJ, Feskens EJM, Stehouwer CDA, Schalkwijk CG. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin Nutr. 2018;37:919-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 117. | Perkins RK, Miranda ER, Karstoft K, Beisswenger PJ, Solomon TPJ, Haus JM. Experimental Hyperglycemia Alters Circulating Concentrations and Renal Clearance of Oxidative and Advanced Glycation End Products in Healthy Obese Humans. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J Diabetes Res. 2020;2020:7489795. [PubMed] [Cited in This Article: ] |
| 119. | Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 120. | Son KH, Son M, Ahn H, Oh S, Yum Y, Choi CH, Park KY, Byun K. Age-related accumulation of advanced glycation end-products-albumin, S100β, and the expressions of advanced glycation end product receptor differ in visceral and subcutaneous fat. Biochem Biophys Res Commun. 2016;477:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 121. | Shen CY, Lu CH, Wu CH, Li KJ, Kuo YM, Hsieh SC, Yu CL. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules. 2020;25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 122. | Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol. 2013;24:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 123. | Brix JM, Höllerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes (Lond). 2012;36:1412-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 124. | Sánchez E, Baena-Fustegueras JA, de la Fuente MC, Gutiérrez L, Bueno M, Ros S, Lecube A. Advanced glycation end-products in morbid obesity and after bariatric surgery: When glycemic memory starts to fail. Endocrinol Diabetes Nutr. 2017;64:4-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 125. | Deo P, Keogh JB, Price NJ, Clifton PM. Effects of Weight Loss on Advanced Glycation End Products in Subjects with and without Diabetes: A Preliminary Report. Int J Environ Res Public Health. 2017;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 126. | Miyata T, Ueda Y, Yoshida A, Sugiyama S, Iida Y, Jadoul M, Maeda K, Kurokawa K, van Ypersele de Strihou C. Clearance of pentosidine, an advanced glycation end product, by different modalities of renal replacement therapy. Kidney Int. 1997;51:880-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Miyata T, Ueda Y, Horie K, Nangaku M, Tanaka S, van Ypersele de Strihou C, Kurokawa K. Renal catabolism of advanced glycation end products: the fate of pentosidine. Kidney Int. 1998;53:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 128. | Asano M, Fujita Y, Ueda Y, Suzuki D, Miyata T, Sakai H, Saito A. Renal proximal tubular metabolism of protein-linked pentosidine, an advanced glycation end product. Nephron. 2002;91:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Waanders F, Greven WL, Baynes JW, Thorpe SR, Kramer AB, Nagai R, Sakata N, van Goor H, Navis G. Renal accumulation of pentosidine in non-diabetic proteinuria-induced renal damage in rats. Nephrol Dial Transplant. 2005;20:2060-2070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | Oleniuc M, Secara I, Onofriescu M, Hogas S, Voroneanu L, Siriopol D, Covic A. Consequences of Advanced Glycation End Products Accumulation in Chronic Kidney Disease and Clinical Usefulness of Their Assessment Using a Non-invasive Technique - Skin Autofluorescence. Maedica (Bucur). 2011;6:298-307. [PubMed] [Cited in This Article: ] |
| 131. | Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary Advanced Glycation End Products and Risk Factors for Chronic Disease: A Systematic Review of Randomised Controlled Trials. Nutrients. 2016;8:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 132. | Inagi R. RAGE and glyoxalase in kidney disease. Glycoconj J. 2016;33:619-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Miyata T, van Ypersele de Strihou C, Imasawa T, Yoshino A, Ueda Y, Ogura H, Kominami K, Onogi H, Inagi R, Nangaku M, Kurokawa K. Glyoxalase I deficiency is associated with an unusual level of advanced glycation end products in a hemodialysis patient. Kidney Int. 2001;60:2351-2359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 134. | Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 380] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 135. | Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, Schrauwen P, Brownlee M, Stehouwer CD, Schalkwijk CG. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286:1374-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 136. | Kurz A, Rabbani N, Walter M, Bonin M, Thornalley P, Auburger G, Gispert S. Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol Life Sci. 2011;68:721-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 137. | Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, Thornalley PJ. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J. 2012;443:213-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 138. | Garrido D, Rubin T, Poidevin M, Maroni B, Le Rouzic A, Parvy JP, Montagne J. Fatty acid synthase cooperates with glyoxalase 1 to protect against sugar toxicity. PLoS Genet. 2015;11:e1004995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 139. | Aragno M, Mastrocola R. Dietary Sugars and Endogenous Formation of Advanced Glycation Endproducts: Emerging Mechanisms of Disease. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 140. | Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1436] [Cited by in F6Publishing: 1496] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 141. | Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 142. | Semba RD, Ferrucci L, Fink JC, Sun K, Beck J, Dalal M, Guralnik JM, Fried LP. Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis. 2009;53:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 143. | Semba RD, Fink JC, Sun K, Bandinelli S, Guralnik JM, Ferrucci L. Carboxymethyl-lysine, an advanced glycation end product, and decline of renal function in older community-dwelling adults. Eur J Nutr. 2009;48:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Dawnay A. Renal clearance of glycation adducts: anti-glycation defence in uraemia and dialysis. Biochem Soc Trans. 2003;31:1386-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 145. | Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 676] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 146. | Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in F6Publishing: 973] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 147. | Baye E, Mark AB, Poulsen MW, Andersen JM, Dragsted LO, Bügel SG, de Courten B. Associations between Urinary Advanced Glycation End Products and Cardiometabolic Parameters in Metabolically Healthy Obese Women. J Clin Med. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 148. | Garay-Sevilla ME, Rojas A, Portero-Otin M, Uribarri J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 149. | Mastrocola R, Collotta D, Gaudioso G, Le Berre M, Cento AS, Ferreira Alves G, Chiazza F, Verta R, Bertocchi I, Manig F, Hellwig M, Fava F, Cifani C, Aragno M, Henle T, Joshi L, Tuohy K, Collino M. Effects of Exogenous Dietary Advanced Glycation End Products on the Cross-Talk Mechanisms Linking Microbiota to Metabolic Inflammation. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 150. | Nandipati KC, Subramanian S, Agrawal DK. Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol Cell Biochem. 2017;426:27-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 151. | Gabryelska A, Karuga FF, Szmyd B, Białasiewicz P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links With Obstructive Sleep Apnea. Front Physiol. 2020;11:1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 152. | Hofmann B, Yakobus Y, Indrasari M, Nass N, Santos AN, Kraus FB, Silber RE, Simm A. RAGE influences the development of aortic valve stenosis in mice on a high fat diet. Exp Gerontol. 2014;59:13-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Maresca AM, Guasti L, Bozzini S, Mongiardi C, Tandurella N, Corso R, Zerba FG, Squizzato A, Campiotti L, Dentali F, Klersy C, Grandi AM, Falcone C. sRAGE and early signs of cardiac target organ damage in mild hypertensives. Cardiovasc Diabetol. 2019;18:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 154. | Du R, Zhang RY, Lu L, Shen Y, Pu LJ, Zhu ZB, Zhang Q, Hu J, Yang ZK, Ding FH, Zhang JS, Shen WF. Increased glycated albumin and decreased esRAGE levels in serum are related to negative coronary artery remodeling in patients with type 2 diabetes: an Intravascular ultrasound study. Cardiovasc Diabetol. 2018;17:149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 155. | Kopytek M, Ząbczyk M, Mazur P, Undas A, Natorska J. Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes. Cardiovasc Diabetol. 2020;19:92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 156. | Miranda ER, Somal VS, Mey JT, Blackburn BK, Wang E, Farabi S, Karstoft K, Fealy CE, Kashyap S, Kirwan JP, Quinn L, Solomon TPJ, Haus JM. Circulating soluble RAGE isoforms are attenuated in obese, impaired-glucose-tolerant individuals and are associated with the development of type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313:E631-E640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 157. | Paneni F, Lüscher TF. Cardiovascular Protection in the Treatment of Type 2 Diabetes: A Review of Clinical Trial Results Across Drug Classes. Am J Cardiol. 2017;120:S17-S27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 158. | Sardu C, De Lucia C, Wallner M, Santulli G. Diabetes Mellitus and Its Cardiovascular Complications: New Insights into an Old Disease. J Diabetes Res. 2019;2019:1905194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 159. | Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 446] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 160. | Hangaard MH, Rossing P, Jensen JS, Jensen MT. [Heart failure often accompanies diabetes mellitus]. Ugeskr Laeger. 2018;180. [PubMed] [Cited in This Article: ] |
| 161. | Umamahesh K, Vigneswari A, Surya Thejaswi G, Satyavani K, Viswanathan V. Incidence of cardiovascular diseases and associated risk factors among subjects with type 2 diabetes - an 11-year follow up study. Indian Heart J. 2014;66:5-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 162. | Mostaza-Prieto JM, Martín-Jadraque L, López I, Tranche S, Lahoz C, Taboada M, Mantilla T, Soler B, Monteiro B, Sanchez-Zamorano MA. Evidence-based cardiovascular therapies and achievement of therapeutic goals in diabetic patients with coronary heart disease attended in primary care. Am Heart J. 2006;152:1064-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 163. | Faria A, Persaud SJ. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol Ther. 2017;172:50-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 164. | Peng JJ, Xiong SQ, Ding LX, Peng J, Xia XB. Diabetic retinopathy: Focus on NADPH oxidase and its potential as therapeutic target. Eur J Pharmacol. 2019;853:381-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 165. | Yang P, Feng J, Peng Q, Liu X, Fan Z. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxid Med Cell Longev. 2019;2019:9570616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 166. | Nin JW, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care. 2011;34:442-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 167. | Steine K, Larsen JR, Stugaard M, Berg TJ, Brekke M, Dahl-Jørgensen K. LV systolic impairment in patients with asymptomatic coronary heart disease and type 1 diabetes is related to coronary atherosclerosis, glycaemic control and advanced glycation endproducts. Eur J Heart Fail. 2007;9:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Simó-Servat O, Simó R, Hernández C. Circulating Biomarkers of Diabetic Retinopathy: An Overview Based on Physiopathology. J Diabetes Res. 2016;2016:5263798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 169. | Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes. 2014;5:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 84] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 170. | Kim JH, Kim KA, Shin YJ, Kim H, Majid A, Bae ON. Methylglyoxal induced advanced glycation end products (AGE)/receptor for AGE (RAGE)-mediated angiogenic impairment in bone marrow-derived endothelial progenitor cells. J Toxicol Environ Health A. 2018;81:266-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 171. | Garg G, Singh S, Singh AK, Rizvi SI. Metformin Alleviates Altered Erythrocyte Redox Status During Aging in Rats. Rejuvenation Res. 2017;20:15-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 172. | Zhu W, Li W, Silverstein RL. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood. 2012;119:6136-6144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 173. | Zhou Q, Cheng KW, Gong J, Li ETS, Wang M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem Pharmacol. 2019;166:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 174. | Chen YH, Chen ZW, Li HM, Yan XF, Feng B. AGE/RAGE-Induced EMP Release via the NOX-Derived ROS Pathway. J Diabetes Res. 2018;2018:6823058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 175. | Ren X, Ren L, Wei Q, Shao H, Chen L, Liu N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc Diabetol. 2017;16:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 176. | Xie X, Vondeling H. Cost-utility analysis of intensive blood glucose control with metformin versus usual care in overweight type 2 diabetes mellitus patients in Beijing, P.R. China. Value Health. 2008;11 Suppl 1:S23-S32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 177. | Ansari G, Mojtahedzadeh M, Kajbaf F, Najafi A, Khajavi MR, Khalili H, Rouini MR, Ahmadi H, Abdollahi M. How does blood glucose control with metformin influence intensive insulin protocols? Evidence for involvement of oxidative stress and inflammatory cytokines. Adv Ther. 2008;25:681-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 178. | Haddad M, Knani I, Bouzidi H, Berriche O, Hammami M, Kerkeni M. Plasma Levels of Pentosidine, Carboxymethyl-Lysine, Soluble Receptor for Advanced Glycation End Products, and Metabolic Syndrome: The Metformin Effect. Dis Markers. 2016;2016:6248264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 179. | Di Pino A, Currenti W, Urbano F, Scicali R, Piro S, Purrello F, Rabuazzo AM. High intake of dietary advanced glycation end-products is associated with increased arterial stiffness and inflammation in subjects with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:978-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 180. | Branchetti E, Bavaria JE, Grau JB, Shaw RE, Poggio P, Lai EK, Desai ND, Gorman JH, Gorman RC, Ferrari G. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler Thromb Vasc Biol. 2014;34:2349-2357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 181. | Scavello F, Tedesco CC, Castiglione S, Maciag A, Sangalli E, Veglia F, Spinetti G, Puca AA, Raucci A. Modulation of soluble receptor for advanced glycation end products isoforms and advanced glycation end products in long-living individuals. Biomark Med. 2021;15:785-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 182. | Scavello F, Zeni F, Tedesco CC, Mensà E, Veglia F, Procopio AD, Bonfigli AR, Olivieri F, Raucci A. Modulation of soluble receptor for advanced glycation end-products (RAGE) isoforms and their ligands in healthy aging. Aging (Albany NY). 2019;11:1648-1663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 183. | Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24:59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 184. | Wasim R, Mahmood T, Siddiqui MH, Ahsan F, Shamim A, Singh A, Shariq M, Parveen S. Aftermath of AGE-RAGE Cascade in the pathophysiology of cardiovascular ailments. Life Sci. 2022;307:120860. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 185. | Singh S, Siva BV, Ravichandiran V. Advanced Glycation End Products: key player of the pathogenesis of atherosclerosis. Glycoconj J. 2022;39:547-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |