INTRODUCTION
Increasing evidence has demonstrated that cell proliferation, differentiation and migration in the intestinal mucosa is dependent on the supply of polyamines to the dividing cells[1-8]. Intracellular polyamine levels are highly regulated[9-15] and completely depend on the activation or inhibition of ornithine decarboxylase (ODC), which is the first rate-limiting step in polyamine biosynthesis[16-22]. In addition to the general effect of metabolic hormones, the amount of ODC in the gastrointestinal mucosa is also regulated by growth-related gut peptides present in the diet or secreted from digestive glands[23-29]. Gastrin is an important gut peptide which stimulates cell proliferation in the mucosa under physiological condition[30]. Administration of gastrin significantly increases ODC activity and intracellular polyamine levels in intestinal epithelial cells[30]. Intestinal epithelial restitution is a complex process.Because of the limitation to study such issues in natural mucosae,cultured rat small intestinal epithelial cell lines(IEC-6) were commonly employed to characterize the physiological events such as growth, differentiation, metabolism and so on during restitution in detail. In the present study, we investigated the influence of gut peptide gastrin on the proliferation, expression of the ODC gene, ODC activity and polyamine biosysthesis in cultured normal rat intestinal crypt cells (IEC-6 cell line).
MATERIALS AND METHODS
Chemicals and supplies
IEC-6 cell line (CRL-1592) was purchased from American Type Culture Collection (ATCC, Rockville, MD) at passage 13. Disposable culture was purchased from Corning Glass Works (Corning, NY). Dulbecco’s modified Eagle’s medium containing 4500 mg·L-1 D-glucose, L-glutamine, 25 mmol·L-1 HEPES buffer and pyridoxine hydrochloride (DMEM), dialyzed fetal bovine serum (dFBS), trypsin-EDTA solution, gentamicin sulfate and insulin, Dulbecco’s PBS(D-PBS), and biochemicals such as pentagastrin, putrescine, sodium dodecyl sulfate(SDS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) were from Sigma (St. Louis, MO). L-[1-14C]ornithine (sp act 1.93TBq·mol-1) was purchased from NEN (Boston, MA).
General experimental protocol
To insure the highest level of viability, the culture was started as soon as possible upon receipt of the vial.Thaw the vial by gentle agitation in 37 °C water bath and decontaminate by dipping in 700 mL·L-1 ethanol, transfer the vial contents to 25 cm2 tissue culture flasks containing 10 mL DMEM supplemented with 50 g·L-1 dFBS, 10 mg·L-1 insulin, and 50 mg·L-1 gentamicin sulfate(cDMEM) then place into the incubator for 15 min prior to addition of the vial contents to allow the medium to reach its normal pH(7.2). Incubate the cells at 37 °C in a humidified atmosphere of 900 mL·L-1 air-100 mL·L-1 CO2. Stock cells were subcultured once a week; and the medium was changed three times weekly. The cells were restarted from frozen stock every five to six passages. Tests for mycoplasma were routinely negative. For cell counting and subculturing, the cells were dispersed with 0.5 g·L-1 trypsin and 0.2 g·L-1 EDTA. Remove the solution and add 1-2 mL of trypsin-EDTA solution. Allow the flask to sit at room temperature until the cells detach. Add fresh culture medium, aspirate and dispense into new culture flasks.
Treatment of pentagastrin on IEC-6 cells
IEC-6 cell numbers were directly measured with the help of a cell counter, and plated in 96-wells with 200 μL cDMEM, or 6-wells with 2000 μL cDMEM. The cells were incubated in a humidified atmosphere at 37 °C in 900 mL·L-1 air-100 mL·L-1 CO2, which was followed by a period of different experimental treatments. Pentagastrin was dissolved in two or three drops of 300 g·L-1 ammonium hydroxide (sterile), adjusted to pH 7.5, and then diluted with medium to the desired concentrations before use. Media pentagastrin were prepared immediately before the experiments.
In the first series of studies, we examined the effect of pentagastrin on cell proliferation in IEC-6 cells. Cells were plated in 96-well microplates at a density of 1 × 104 cells per well with 200 μL cDMEM and grown in incubator under the condition described above. After 24 h, 10 μL media pentagastrin were added at final concentrations of 500 and 250 μg·L-1. Control cells were fed with fresh medium without gastrin as well. To determine the time course of cell proliferation, cell numbers were measured at different time points after exposure of the cells to pentagastrin with MTT assay.
In the second series of studies, we examined the effect of pentagastrin on cellular ODC mRNA levels, ODC enzyme activity and putrescine content in IEC-6 cells. Cells were plated in 6-well microplates at a density of 2 × 106 cells per well with 2000 μL cDMEM and grown under incubator at the condition described above. After 24 h, 100 μL media pentagastrin were added at final concentrations of 250 and 500 μg·L-1, respectively. Control cells were fed with fresh medium without gastrin as well. Cultures were harvested at 12 h after exposure of the cells to pentagastrin. The dishes were placed on ice, the monolayers were washed three times with ice-cold D-PBS, and different solutions were then added according to the assays to be conducted.
Measurement of ODC mRNA level
Total RNA was isolated from IEC-6 cells using RNA TRIzol reagent from Gibco(Gaithersburg, MD). Isolation and extraction were performed according to the manufacturer’s protocol. Briefly, the cells were washed with Dulbecco’s PBS and were lysed with 1.0 mL Trizol/well.
RNA was extracted with 0.2 mL chloroform and precipitated with 0.5 mL isopropanol. The precipitated RNA was washed with 1 mL 700 mL·L-1 ethanol and redissolved in RNase-free water. The concentration of the extracted RNA was calculated by measuring the absorbance at 260 nm.The ratio of the absorbance at 260 nm to that at 280 nm was always > 1.9.
Aliquots of RNA (5 μg) were reverse-transcribed using an RT-PCR kit from Gibco(Gaithersburg, MD).Briefly, 5 μg RNA in 10 μL of diethyl pyrocarbonate-treated water(DEPC-water) was mixed with 1 μL of 50 μmol·L-1 oligo(dT)20, heated at 65 °C for 5 min, and then plased on ice. The following reagents were added to the tubes: 4 μL of 5 × concentrated cDNA synthesis buffer,1 μL of 0.1 mol·L-1 DTT, 1 μL of RNaseOUT(40 MU·L-1), 1 μLof DEPC-water, 2 μL of 10 mmol·L-1 dNTP Mix, and 1 μL of Thermoscript RT(15 MU·L-1). The reaction mixture was incubated for 50 min at 50 °C before the reaction was terminated by incubating the tube at 85 °C for 5 min.The mixture was added with 1 μL RNase H and incubated at 37 °C for 20 min. The tube was stored at -80 °C until PCR was performed using the Platinum Taq DNA polymerase with rat-specific primers prepared on a DNA synthesizer (Seagon, Shanghai, China). The primers were designed according to sequences of rat ornithine decarboxylase gene (EC 4.1.1.17). as follows: upstream primer: 5’>TGGCTGGCGCTGGTCTGTAGT<3’; downstream primer:5’>AGCTCCTGCCTGGGT.CTT.ATGA<3’.
The cDNA amplification products were predicted to be 300 bp in length for ODC. To initiate the PCR, 2 μL RT products were added to the PCR master mix, including 5 μL 10 × PCR reaction buffer, 2 μL 50 mmol·L-1 MgCl2, 0.2 μL Platinum Taq DNA polymerase(5 MU·L-1), 1 μL 10μmol·L-1 each of the primers, 10 mmol·L-1 dNTP Mix and 37.8 μL DEPC-water. Tubes were placed in a programmed tempcontrol system (PE) as follows: 1) incubation at 94 °C for 2 min (initial denaturation); 2) 40 cycles of the following sequential steps: 94 °C for 30 s (denaturation), 60 °C for 30 s(annealing), and 72 °C for 1 min (extension); and 3) incubation at 72 °C for 7 min (final extension). The PCR products were size-fractionated by agarose gel electrophoresis. After electrophoresis and ethidium bromide staining, DNA bands were visualized and the relative levels of mRNA for ODC were corrected for cDNA loading as measured with an ultraviolet transilluminator.
Assay for ODC activity
ODC activity was determined with radiometric technique in which the amount of 14CO2 liberated from DL-[L-14C]ornithine was estimated. Samples were collected as described above and placed in 0.5 mL of 20 mmol·L-1 Tris buffer (pH7.4) containing 0. 05 mmol·L-1 EDTA, 0.05 mmol·L-1 pyrodoxal phosphate, and 5 mmmol·L-1 dithiothreitol. The cells were frozen and thawed three times, scraped, and transferred to Eppendorf tubes. Cells were centrifuged at 12000 g at 4 °C for 15 min. The ODC activity of an aliquot of the supernatant was determined during incubation in stoppered vials in the presence of 7.6 nmol of [14C] ornithine (sp act 1.93TBq·mol-1) for 15 min at 37 °C. The 14CO2 liberated by the decarboxylation of ornithine was trapped on a piece of filter paper impregnated with 20 μL of 2 mol·L-1 NaOH, which was suspended in a center well above the reaction mixture. The reaction was stopped by the addition of trichloroacetic acid to a final concentration of 100 g·L-1. The 14CO2 trapped in the filter paper was measured by liquid scintillation spectroscopy at a counting efficiency of 95%. Aliquots of the 12000 g supernatant were assayed for total protein, by Lowry method. Enzymatic activity was expressed as picomoles of CO2 per milligram of protein per hour.
Analysis of cellular putrescine
The cellular putrescine content was analyzed by HPLC as described previously[5]. In brief, after monolayers were washed three times with ice-cold D-PBS, 0.5 mol·L-1 perchloric acid were added and the monolayers were frozen at -80 °C until ready for extraction, dansylation, and HPLC cells were harveated, and centrifuged at 1600 g for 10 min.This supernatant was collected, neutralized to pH 7.0 with 3 mol·L-1 KOH, and centrifuged to remove the precipitate.A 0.5 mL aliquot of solution was delivered to clean Eppendorf tubes. After addition of 0.25 mL saturated Na2CO3 and 0.5 mL dansyl chloride solution(10 g·L-1 acetone), reaction was allowed to proceed by heating at 70 °C for 30 min and then added to 1.5 mL toluene. After mixing and centrifugation, the organic protein portion was collected and dryed by vacuum centrifugation. To the residue, 300 μL methanol was added and filtered, and an aliquot of 200 μL was used for HPLC analysis. Solvent A and B were composed of acetonitrile, water, glacial acetic acid,and triethylamine in the volume proportions of 80:20: 0.02:0.001 and 95:5:0.02:0.005, respectively.The mobile phases used in this separation consisted of 60% solvent A and 40% solvent B. Each sample was run for 20 min, and the equilibration delay between injections was 2 min. Sufficient mobile phases (A and B) were prepared fresh before starting the automatic injector. The polyamine putrescine was measured by comparing ratios of polyamines to 1, 10-diaminodecane peak areas with a standard curve. Protein was dissolved in 1 mol·L-1 NaOH and determined by the Lowry method. The results were expressed as nanomoles of polyamines per milligram of protein.
Statistics
All data are expressed as -x±s from three-four dishes. The significance of the difference between means was determined by independent t test using SPSS statistical software.
RESULTS
Effect of gastrin on the proliferation Of IEC-6 cells
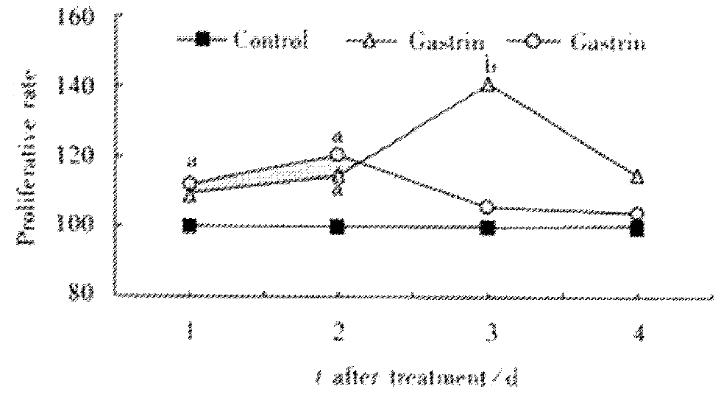
IEC-6 cells were grown in cDMEM in the presence or absence of pentagastrin. Exposure of IEC-6 cells to pentagastrin, the proliferation increased initially on d1 and reached a peak on d3 in 250 μg·L-1 concentration. Pentagastrin 500 μg·L-1 increased cell proliferation on d1 and d2 and then decreased as shown in Figure 1.
Figure 1 Gastrin effect on IEC-6 proliferation by the MTT assay.
aP < 0.05, bP < 0.01 vs control.
ODC mRNA amount in IEC-6 cells treated with gastrin
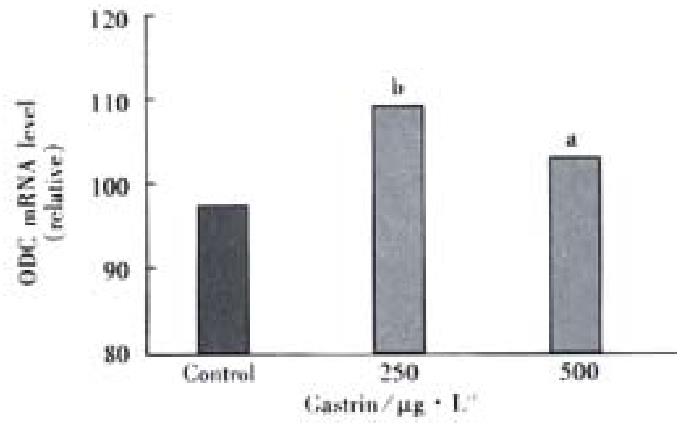
Administration of pentagastrin 250 and 500 μg·L-1 significantly raised the ODC mRN levels by 1.16-fold and 1.09-fold, respectively as compared with control group (Figure 2). But there was no significant difference between the two doses of gastrin.
Figure 2 ODC mRNA levels in IEC-6 cells.
aP < 0.05, bP < 0.01 vs control.
Gastrin induction of ODC activity
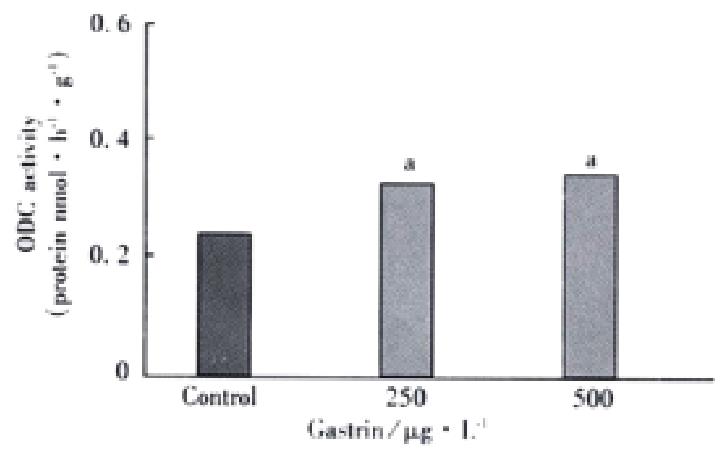
In these studies, IEC-6 cells were grown in cDMEM in the presence or absence of pentagastrin. This exposure of IEC-6 cells to pentagastrin 250 and 500 μg·L-1 caused ODC activity to increase significantly by 1.71-fold and 1.63-fold, respectively at 12 h after treatment as compared with control group. But there was no significant difference between them (Figure 3).
Figure 3 ODC activity in IEC-6 cells exposed to pentagastrin.
aP < 0.05 vs control.
Effect of pentagastrin on putrescine content in IEC-6 cells
Increases in ODC activity in cells exposed to pentagastrin were paralleled with increases in cellular putrescine levels. Compared with a value of 0.46 ± 0.02 μmol·g-1 from 4 cultures in the control group, the cellular putrescine levels treated with pentagastrin 250 and 500 μg·L-1 significantly increased (P < 0.01), the values were 2.44 ± 0.05 μmol·g-1 and 2.03 ± 0.03 mol·g-1 from 4 cultures, which were 5.30-fold and 4.41-fold of control group, respecticely.
DISCUSSION
Numerous studies have demonstrated that polyamine biosynthesis plays a critical role in the control of normal mucosal growth and repair[1-12] and that ODC in small intestinal mucosa has a high basal activity compared with most tissues and significantly increases in response to a variety of chronic and acute mitogenic stimuli[16-29]. The rapid and striking increases in ODC and polyamine levels are absolutely required for the stimulation of intestinal mucosal growth[16-21].
IEC-6 cells which were established by Quaroni et al[41] are derived from neonatal normal rat small intestine and have characteristics of crypt-type epithelial cells as judged by morphological and immunologic criteria which do not exhibit differentiated morphology or specific gene expression. They are nontumorigenic and retain the undifferentiated character of epithelial stem cells. These cells exhibit a number of features of normal cells in culture: i.e. a normal rat diploid karyotype, strong density inhibition of growth, lack of growth in soft agar, and a low plating efficiency when seeded at low density. The establishment of IEC-6 cell lines play an important role in functional researches of small intestine epithelial cells such as growth, differentiation, metabolism, the pharmacological effects, and the pathophysiological changes and the mechanism of intestinal mucosa resulted from various pathogic factors. This cell line also provided an appropriate in vitro model for the study on cell proliferation and mucosal healing. This cell line was broadly used in the studies on cellular, molecular and genetic mechanism of small intestinal mucosal repair since the establishment[31-40].
The gastrointestinal mucosa must maintain a barrier against the harsh luminal contents of acid, enzymes, bacteria, and toxins. Disruption of this barrier is the salient feature of a variety of common and important gastrointestinal disorders, including inflammatory bowel disease and peptic ulcers. The mucosal epithelium of the small intestine has the capacity for rapid renewal and adaptation after injury or resection. Crypt cell proliferation leading to intestinal growth and promoting re-establishment of mucosal integrity after injury are essential processes for the differentiation, maintenance, and repair of the intestinal epithelium and are regulated via a complex interplay of nutrients, pancreatic and biliary secretions, and both locally derived and circulating growth factors[41-51].
It is of interest and important to investigate the effect of growth-related gut peptides on the regulation of ODC activity in intestinal epithelial cells. The current study clearly shows that, in small intestinal crypt cells maintained in cDMEM, gastrin stimulates cell proliferation, increases ODC mRNA levels, ODC activity and polyamine content, in which the effects of pentagastrin 250 μg·L-1 seems to be better than those of 500 μg·L-1. Consistent with the results reported by other authors[30] higher dosage of pentagastrin( > 1000 μg·L-1) inhibited IEC-6 cell proliferation(data not shown).
In summary, our results indicate that the increased ODC activity in IEC-6 cells treated with gastrin is associated with a rise in ODC mRNA levels and an increase of intracellular putrescine resulting in cell proliferation. It suggests that the induction of ODC activity by gastrin plays an important role in the regulation of cell proliferation in the intestinal mucosa under physiological condition.