INTRODUCTION
TRAIL (TNF-related apoptosis inducing ligand, Apo2 ligand, Apo 2 L), which belongs to the tumor necrosis factor (TNF) cytokine family, can induce rapid apoptosis in a wide variety of tumor cell lines. TRAIL protein consists of 281 (human TRAIL) or 291 (mouse TRAIL) amino acids. The human TRAIL gene is located in chromosome 3 (3q26). The mRNA distribution of TRAIL is broad[1,4-8]. Although the in vivo role of the TRAIL is not currently known, in vitro studies have found that TRAIL is capable of inducing apoptosis in a wide range of human tumor cells, but generally not normal cells[9-16]. TRAIL induces apoptosis by binding and cross-linking death-domain containing receptors , TRAIL-R1[22,23] (also known as DR4) and TRAIL-R2[24-26] (DR5). Apoptotic signaling occurs via recruitment of adapter proteins, which results in the activation of caspases[24,27]. Because of its selective killing effect on tumor, TRAIL is likely to be a drug in cancer treatment[28-30]. Therefore, we tested the effect of TRAIL expression in human gastric carcinoma cells, most commonly found in oriental population. In addition, the study may form a basis for the safety and effectiveness of TRAIL expression in gene therapy of cancer.
In gene therapy, it is important to regulate gene expression effectively. The tetracycline-controlled gene expression system (Tet system) is a tight control system[31]. It is based on the Tn10 specified tetracycline resistance operon of E. coli. In the Tet-Off system, the regulator unit, encodes a hybrid tTA (tetracycline-controlled transactivator) protein composed of the tetracycline repressor (tetR) fused to the herpes simplex virus (HSV) transactivator protein, VP16. The response unit, tetracycline-responsive element (TRE), is composed of the tetracycline resistance operon regulatory elements (tetO) embedded within a minimal cytomegalovirus (CMV) promoter. The expression of a gene inserted downstream of the promoter is highly dependent on tTA, which binds tetO sequences. With the addition of tetracycline, tTA protein dissociates from the TRE and the gene expression is inhibited. Conversely, the Tet-On system allows gene expression to be activated by the addition of tetracycline. It is based on the reverse tTA (rtTA) in the regulator unit.
The RevTet-Off/On system uses the retroviral vectors. They have the characteristic of Tet system, and their gene transfer is more rapid and efficient, which can be used in gene therapy. In this study, TRAIL gene was cloned into the RevTet-On system, to achieve tight control of the expression of TRAIL protein in mammalian cells. The gene expression system on gastric carcinoma cell line NCI-N87 was constructed, and the killing effect of controlled-gene expression in the resulting cells was observed.
MATERIALS AND METHODS
Construction of retroviral plasmids
The RevTet-On System, which include pRevTet-On and pRev-TRE vectors and the RetroPack PT67 cell line, were purchased from Clontech, Palo Alto, California, USA. TRAIL gene was obtained by using PCR method from a hu-man placenta cDNA library (Clontech). The PCR primers are: 5’-AAGCTTATGGCTATGATGGAGGTCCAGGGGGG-AC-3’and 5’-AAGCTTTTAGCCAACTAAAAAGGCCCCG-AAAAAACTGGC-3’. The resulting PCR product was cloned into an intermediate vector PCR-2 (Invitrogen, Carlsbad, California, USA) and then cut with Hin dIII, which was cloned into the same site in vector pLNCX, resulting in vector pLNCX-TRAIL. The Tet-regulated vector pRev-TRE-TRAIL was cloned in the same manner.
Cell culture
The retroviral packaging cell line PT67 and the murine fibroblast cell line NIH3T3 were maintained in Dulbecco’s modified essential medium (DMEM) containing 100 mL·L-1 fetal bovine serum. The human gastric carcinoma cell line, NCI-N87, and the human leukemia cell line, Jurkat, were maintained in RPMI 1640 medium containing 100 mL·L-1 or fetal bovine serum respectively.
Transfections, infections and determination of viral titer
The packaging cells PT67 were plated in a 60 mm plate at a density of 50%-80% 24 h before transfection. Cells were washed with DMEM twice and incubated with 2 mL DMEM before transfected with 10 μg of plasmid DNA by lipofectin method (Life Technology, Gibco BRL). Four h-6 h after transfection, 2 mL DMEM (containing 200 mL·L-1 FBS) was added. To obtain stable virus-producing cell lines, the packaging cells transfected with retroviral plasmids were plated in selection medium 48 h later. The regulatory vector Tet-On carries the neomycin gene as a selectable marker. For G418 selection, cells were cultured in the presence of G418 (0.4 g·L-1, Gibco) for two-weeks. The vectors pRev-TRE and pRev-TRE-TRAIL carry the gene for hygromycin selection. These cells were selected in the presence of hygromycin B (0.06 g·L-1, Sigma) for two weeks. For transduction, NCI-N87 cells were plated 24 h before infection. The medium from packaging cells containing virus were collected, filtered through a 0.45 μm filter, and added to the NCI-N87 cells in the presence of Polybrene (4 g·L-1, Sigma). The medium was replaced 4 h later. Three to six serial infections were performed to increase the efficiency of infection. Forty-eight hours after infection, the cells were subjected to G418 or hygromycin selection. For determination of viral titer, NIH3T3 cells were plated 24 h before transduction in 6-well plates (2 × 105 cells per well). The cells were infected with filtered virus-containing medium (six 10-fold serial dilutions, 1-105). The cells were then incubated in G418 or Hygromycin 48 h later, and the selection lasted about weeks until clear colonies appears.
Growth curve
The cells were plated in 96-well plates (5 × 104 cells per well), and counted by Typan blue dye exclusion method over a 24 h period.
Induction of gene expression
The cells were added with the medium containing Dox (0.01-10 mg·L-1, Sigma). After a period of time, the fraction of dead cell was calculated by Typan blue dye counting. The medium after induction was added in Jurkat cells (100 mL·L-1), the fraction of dead cell was calculated by Trypan blue dye.
RESULTS
Construction of retroviral vectors
To clone the TRAIL gene into the Tet system, we inserted the cDNA of TRAIL into the Tet response vector pRev-TRE, which was named pRevTRE-TRAIL. The desired recombinant plasmid orientation was confirmed by Hin dIII and Ssp I digestion. In this plasmid, the TRAIL gene is the downstream of the TRE, which can bind rtTA expressed by pRevTet-On. So, in combination with the pRevTet-On regulatory vector, the TRAIL gene can be inductively expressed at high levels in response to varying concentrations of tetracycline or doxycycline. Because of their retrovirus-mediated gene transfer, we can incorporate the Tet-controlled TRAIL protein expression system into mammalian cells.
Establishment of Tet-controlled TRAIL system
We transfected vectors into packaging cells PT67. Using G418 (pRevTet-On) and hygromycin selection (pRev-TRE and pRevTRE-TRAIL), we obtained the stable virus-producing cell lines, PT67-TetOn, PT67-TRE and PT67-TRE-TRAIL. Viral titers were 1-2 × 108 CFU·L-1, determined by titering NIH3T3 cells. After serial infections of NCI-N87 cells with viral-containing supernatants and antibiotic selections, two kinds of cell lines were established. One is the test group NCI-N87-TetOn-TRE-TRAIL (NN3T, Figure 1A), which is a Tet-controlled TRAIL expression cell line. The second is the control cell line NCI-N87-TetOn-TRE (NN2T, Figure 1B), which is the same with NN3T but without TRAIL expression.
Figure 1 Morphology of cells.
A: NN3T; B: NN2T
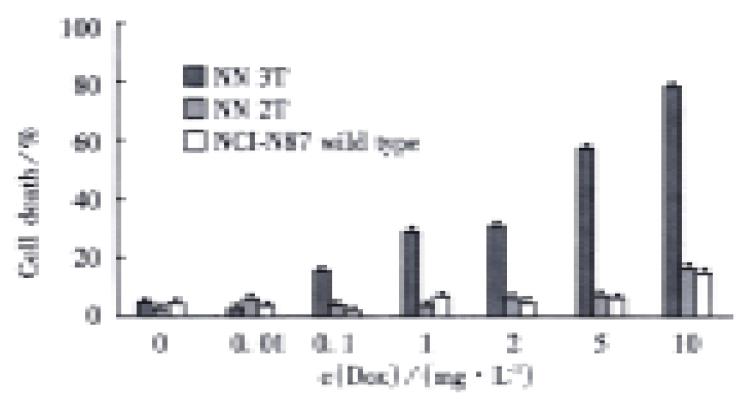
Killing effect of TRAIL induced by doxycycline
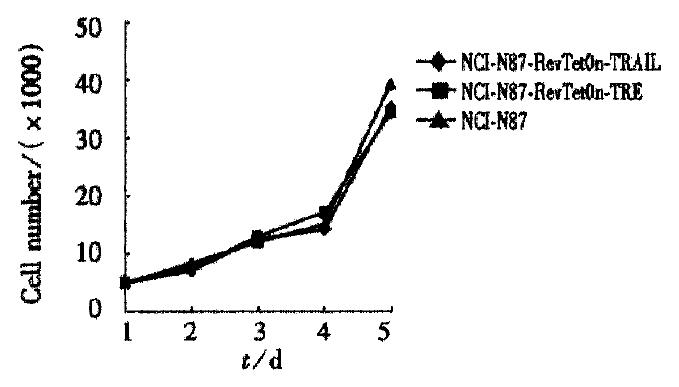
When we added doxcycline to cells, some cells died in the test group NN3T 48 h later (Figure 2), whereas no clear death effect can be detected in cells in the control group (NN2T) and wild type NCI-N87. Under normal conditions without Dox, the growth curves of these three types of cells showed no obvious differences (Figure 3). In order to test the possible secretion of TRAIL protein into the medium, the media from the test group and control group were removed and inoculated into the human leukemia cells Jurkat (sensitive to TRAIL-induced apoptosis). Obvious cell death was observed after one day culturing in the test group as compared with the control and the normal groups. From these results, we concluded that the regulated expression of TRAIL in the Tet system has killing effect on the gastric carcinoma cell NCI-N87.
Figure 2 Killing effect of TRAIL regulated by doxcycline.
Figure 3 Growth curves of cells.
DISCUSSION
Tumor necrosis factor (TNF) is a prototypic member of the family of cytokines that interact with a corresponding set of receptors that form the TNF receptor (TNFR) family. Three of these ligands, CD95L, TNF and LTα, have received particular attention because they can induce apoptosis in transformed cells and activated lymphocytes[32-34]. The potential utility of systemically administered ligands is limited by their acute toxic effects on normal tissues in vivo, thereby limiting their potential widespread use in the treatment of cancer[35]. Previous studies have shown that TRAIL can induce apoptosis in a variety of tumor cell lines. In contrast to other members of TNF family, TRAIL mRNA is expressed constitutively in many tissues including peripheral blood lymphocytes, spleen, thymus, prostate, ovary, small intestine, colon and placenta[1,7,17,36-38], which suggests the existence of physiological mechanisms that can protect many normal cell types from induction of apoptosis specifically by TRAIL. A relatively high proportion (approximately two-thirds) of tumor cell lines tested so far are sensitive to the cytotoxic effects of TRAIL in vitro[1,7,11,39], indicating that TRAIL may prove to be a powerful cancer therapeutic factor.
Although TRAIL potently induces apoptosis in tumor cells and some virally infected cells, it has little or no detectable cytotoxic effects on normal cells, whereas this was first thought to be due to regulated expression of the TRAIL receptors[3,24,40-42], the fact that mRNA for both TRAIL and TRAIL receptors is often expressed in the same cells makes this explanation untenable[17,43]. Indeed, the identification of four distinct TRAIL receptors (TRAIL-R1, R2, R3, R4) has significantly increased the potential complexity of this receptor/ligand system[17,43,44]. Based on current information it seems mikely that multiple factors, both intra and extra-cellular, may function together to protect normal cells from the cytotoxic effects of TRAIL[17,43,45-48], but many questions remain unexplained.
Although the reason for determining the sensitivity of cells to TRAIL-induced apoptosis has not been understood, we still try to use TRAIL in cancer treatment to achieve the effectiveness and the safety of the therapy. In our laboratory, the cDNA sequence of TRAIL has been cloned from human placenta cDNA library. We have expressed the TRAIL in the E. coli by the pET system[49], after purification and refolding the antitumor activity of the protein has been examined[50]. On the other hand, a chief concern with the potential use of TRAIL protein in the treatment of tumors in vivo is the potential undesirable toxicities. Because of the recent report that some normal human cells were sensitive for the apoptosis induced by TRAIL[51], we inserted the gene into a mammalian expression vector to study its effect further in this experiment.
In most inducible mammalian gene expression systems (heavy metals, steroid hormones, or heat shock), induction is nonspecific and expression levels cannot be precisely regulated. In addition, these systems are generally leaky in the “off” state, and the inducing agent itself may be cytotoxic or have pleiotropic effects. In contrast, regulation of gene expression by the Tet system is very specific[31]. Furthermore, the levels of tetracycline or doxycycline required for the full range of gene expression are not cytotoxic and have no significant effect on cell proliferation or animal growth. Using retroviral vectors instead of DNA transfection to transduce the complete inducible system greatly expands the target cell types in which gene functions can be studied. This strategy may be useful for clinical applications in human gene therapy trials.
In this study, the effectiveness of TRAIL in killing human gastric cells was evident. It showed that the gastric carcinoma cell line was sensitized to TRAIL-induced cell death. In addition, the concentrations of doxycyline that constituted the effect were within a range of clinical security. We can believe that this effect will be safe to normal tissues with the specificity of TRAIL and regulation of the Tet system.
At the moment, malignant gastric carcinoma remains one of the difficult types of cancer to treat successfully, and with the incidence of gastric carcinoma increasing at China, it continues to be a leading cause of death in Japan and other countries of Asia. In our experiment, the expression system of TRAIL gene controlled by tetracycline was established in gastric carcinoma cell line NCI-N87, and its killing effect to the tumor cell was observed. This study clearly demonstrates the possible usefulness of Tet-controlled TRAIL expression system in gastric cancer gene therapy, and further studies of the mechanisms of TRAIL-mediated cytotoxicity are required to assess the potential use of TRAIL as an anti-cancer therapeutic in vivo.