Published online Aug 14, 2023. doi: 10.3748/wjg.v29.i30.4616
Peer-review started: May 9, 2023
First decision: June 14, 2023
Revised: July 1, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: August 14, 2023
After being ingested and entering the human stomach, Helicobacter pylori (H.
Core Tip:Helicobacter pylori (H. pylori) adopts several effective strategies to adhere to and colonize the gastric mucosa and move to different regions of the stomach, leading to acute infection and chronic gastritis that can be observed through endoscopy. Herein, we describe the endoscopic manifestations of each stage of H. pylori gastritis and then discuss the potential mechanisms of bacterial intragastric colonization and migration from the perspective of endoscopists to provide direction for future research on the effective therapy and management of H. pylori infection.
- Citation: Mu T, Lu ZM, Wang WW, Feng H, Jin Y, Ding Q, Wang LF. Helicobacter pylori intragastric colonization and migration: Endoscopic manifestations and potential mechanisms. World J Gastroenterol 2023; 29(30): 4616-4627
- URL: https://www.wjgnet.com/1007-9327/full/v29/i30/4616.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i30.4616
More than half of the world’s population is estimated to be infected by the gram-negative, flagellated, spiral-shaped bacterium Helicobacter pylori (H. pylori)[1]. The bacterium has received intensive attention because H. pylori infection is closely associated with the development of peptic ulcers, mucosa-associated lymphoid tissue lymphoma and gastric cancer (GC), resulting in at least 500000 deaths per year[2-4]. The slow carcinogenic process is known as Correa’s cascade[5]: At first, gastritis occurs in all infected individuals[2], and then a series of intermediate stages (characterized by precancerous lesions), including atrophy, intestinal metaplasia (IM) and dysplasia, may slowly develop, and eventually, 1%-3% of infected patients develop gastric malignant tumors[6].
All gastric mucosal lesions that occur after H. pylori infection can be observed by skilled endoscopists through upper gastrointestinal endoscopy. Based on the Kyoto classification of gastritis, endoscopic features, such as nodularity, diffuse redness, spotty redness, mucosal swelling, enlarged folds, xanthoma, atrophy and IM, are helpful in diagnosing H. pylori gastritis[7]. Atrophy can be endoscopically identified with high confidence by applying the Kimura-Takemoto classification[8], while IM and dysplasia can be diagnosed more accurately with advanced image-enhanced endoscopy (IEE)[9].
The highly motile pathogen H. pylori usually infects young children[3] and initiates acute infection that lasts for only a few weeks[10,11] and chronic inflammation that can last for the lifetime of the host[12]. Its ability to swim in the gastric mucus and colonize the stomach enables it to survive in the hostile gastric environment[13] and leads to various endoscopic and histological features as gastric mucosal lesions progress[14]. Many articles and reviews have reported the underlying mechanisms, but few have linked endoscopic features to mechanisms. Therefore, in the following sections, we describe the endoscopic manifestations of each stage of H. pylori gastritis and summarize the process and potential mechanisms of intragastric colonization by H. pylori and its migration.
Acute H. pylori infection only lasts for a few weeks[10,11] and has been rarely observed or reported in recent decades. The endoscopic manifestation of gastric erythema and a gaping pylorus[10,11] is always featureless. Although the gastric mucosa does not appear damaged at this stage, initial colonization of the mucosa is the basis of a series of lesions, such as atrophic gastritis, peptic ulcer and even gastric carcinoma.
The prevalence of H. pylori infection is high, but colonization by this microbe is not easy. Multiple spontaneous eradication events may occur before colonization, leading to acute infection[15]. Sophisticated strategies have been adopted by H. pylori that have enabled it to adapt to and survive in the hostile gastric environment.
When H. pylori is ingested by adults, it is almost completely destroyed in the gastric acid, while it is easier to survive in the stomach of children younger than the age of five, both in developing and developed countries[3]. Bucker et al[3] simulated the pH changes of the postprandial stage in babies, young children and adults and suggested that the bacteria were easiest to reach the mucus layer in young children, whose feature of postprandial gastric condition is moderate food-induced pH elevation and slow reacidification.
During the process of slow reacidification, the urease enzyme is believed to play a key role in bacterial survival and adhesion. Urea is degraded by the urease enzyme, which buffers the cytoplasm and periplasm[16]. This confers many benefits. First, H. pylori prefers to live in an environment with elevated pH. A recent study[17] showed that H. pylori does not escape from phosphate buffer solutions of pH 6.6 and 7.0. Second, intracellular urease could also increase membrane potential, thereby allowing protein synthesis at a low pH[18]. Third, mucosal viscosity highly depends on acidity[19]. At a less acidic pH, the mucus is less gel-like, which enables H. pylori to more easily move through the mucus layer[20]. Fourth, trefoil factor 1 (TFF1) is a member of the trefoil peptide family of proteins and is coexpressed with Mucin-5AC (MUC5AC), a gel-forming mucin that is predominantly secreted and expressed by gastric surface epithelial cells in the stomach[21]. The optimum pH for bacterial binding to TFF1, which thereby promotes colonization, was found to be 5.0-6.0[22]. In addition, urea and bicarbonate were considered to have a chemotactic effect on H. pylori in vitro[23], but research by Schreiber et al[13] shows that neither the urea/ammonium gradient nor the bicarbonate/CO2 gradient are essential for the orientation of H. pylori in vivo.
However, this does not mean that a neutral or alkaline environment is suitable for H. pylori. Previous studies have shown that H. pylori is sensitive to alkaline conditions[24], and its growth is limited at neutral pH[25]. To prevent lethal alkalinization of the cytoplasm, H. pylori utilizes a proton-gated channel, UreI, which regulates the uptake of urea[26] and only functions in conditions of an acidic pH; thus, the transport of urea into the bacterial cell does not occur at a neutral pH[24]. Therefore, H. pylori prefers a weakly acidic environment.
The epithelial surface of the stomach is covered with an approximately 300 μm thick layer of secreted mucus, which mainly consists of mucins Mucin 6 (MUC6) and MUC5AC[27]. MUC6 exists in each layer of the mucus gel, while MUC5AC is mainly present on the surface and bottom. The increase in the viscosity of gastric mucus gel is due to this natural stratification of mucins[28]. While protecting gastric epithelial cells, the mucus layer also plays an important role in the colonization process. The pH is approximately neutral at the epithelium and very acidic (pH 1-2) close to the lumen[21], resulting in a mucus pH gradient that can be used by H. pylori for precise spatial orientation[13]. The membrane-bound chemoreceptor TlpA of H. pylori detects and mediates repulsion from environments with a lower pH, and the cytoplasmic chemoreceptor TlpD mediates both attraction to higher pH environments and repulsion from lower pH environments[17,29]. Under this chemotactic effect, H. pylori penetrates the gastric mucus quickly and reaches the narrow region within 25 μm of the gastric epithelial surface with the help of its two to six sheathed unipolar flagella and helical shape[30,31].
After approaching the lower mucus layer, the majority of H. pylori swim in gastric mucus, while others directly adhere to epithelial cells[13,31]. Although it is considered a noninvasive gastric pathogen to date[19], H. pylori can indeed bind to, invade, be internalized into and proliferate in gastric epithelial cells[27,32]. The invasiveness of H. pylori may partially depend on the strain. Research by Camorlinga-Ponce et al[33] showed that CagA-negative bacteria adhered to the surface of the apical epithelium, while CagA-positive bacteria were identified in the intercellular spaces or the immediate vicinity of epithelial cells. Sigal et al[34] found a subgroup of H. pylori associated with cells deep in the antral glands. These microbes can promote gland hyperplasia by inducing stem cell proliferation and expansion and altering gene expression of stem cells[34].
H. pylori adheres to epithelial cells mainly by outer membrane proteins (OMPs). Blood group antigen-binding adhesion (BabA) and sialic acid-binding adhesion (SabA) are important OMPs[19,27]. Lewis antigens are common in normal, infected and inflamed gastric mucosa[35,36]. BabA can identify and bind to Lewis b antigen[35], while SabA can bind to the antigens Lewis a and Lewis X[36], and its expression can quickly respond to the changes in the stomach or different areas of the stomach, enabling the bacteria to adapt to host’s immune responses and varied microenvironments to maintain long-term colonization and infection[37]. In addition to BabA and SabA, other surface proteins, such as AlpA, AlpB, DupA, outer inflammatory protein A (OipA) and HopZ, are considered related to adhesion, but none of them has been shown to be essential to adhesive mechanisms[38]. After H. pylori adheres to epithelial cells, the Cag type IV secretion system (T4SS) promotes CagA translocation into host cells, resulting in changes in cell shape, disruption of cell‒cell junctions, altered cell polarity and cell adhesion, increased cell motility and cell migration, increased cell proliferation, β-catenin activation, and epithelial-mesenchymal transition[39]. Some bacteria are internalized into the cytoplasm of gastric epithelial cells through endocytosis within 45 minutes of bacterial attachment to the cell surface[32]. H. pylori can replicate and proliferate in epithelial cells[40], escape the immune response, and exit cells to colonize and infect cells again when the external environment is suitable for survival[27].
In an artificial ingestion study[10], histological examination during the acute phase of H. pylori infection showed many polymorphonuclear neutrophil leucocytes (PMNs) in the lamina propria and on the surface of the mucosa and an absence of intracellular mucus. Spiral bacilli adhered to the surface and glandular epithelium as well as among PMNs in the mucus[10]. Zhao et al[41] proposed a novel staging strategy according to the depth and degree of gastric mucosal injury induced by H. pylori infection and the progression of lesions. Stage I means the bacteria were present in the mucus layer, stage IIA refers to the specific adhesion to and selective destruction of gastric epithelial cells, and stage IIB refers to the degeneration and shedding of surface mucus cells[41]. It seems that stages I and II are consistent with the pathological characteristics of acute H. pylori infection.
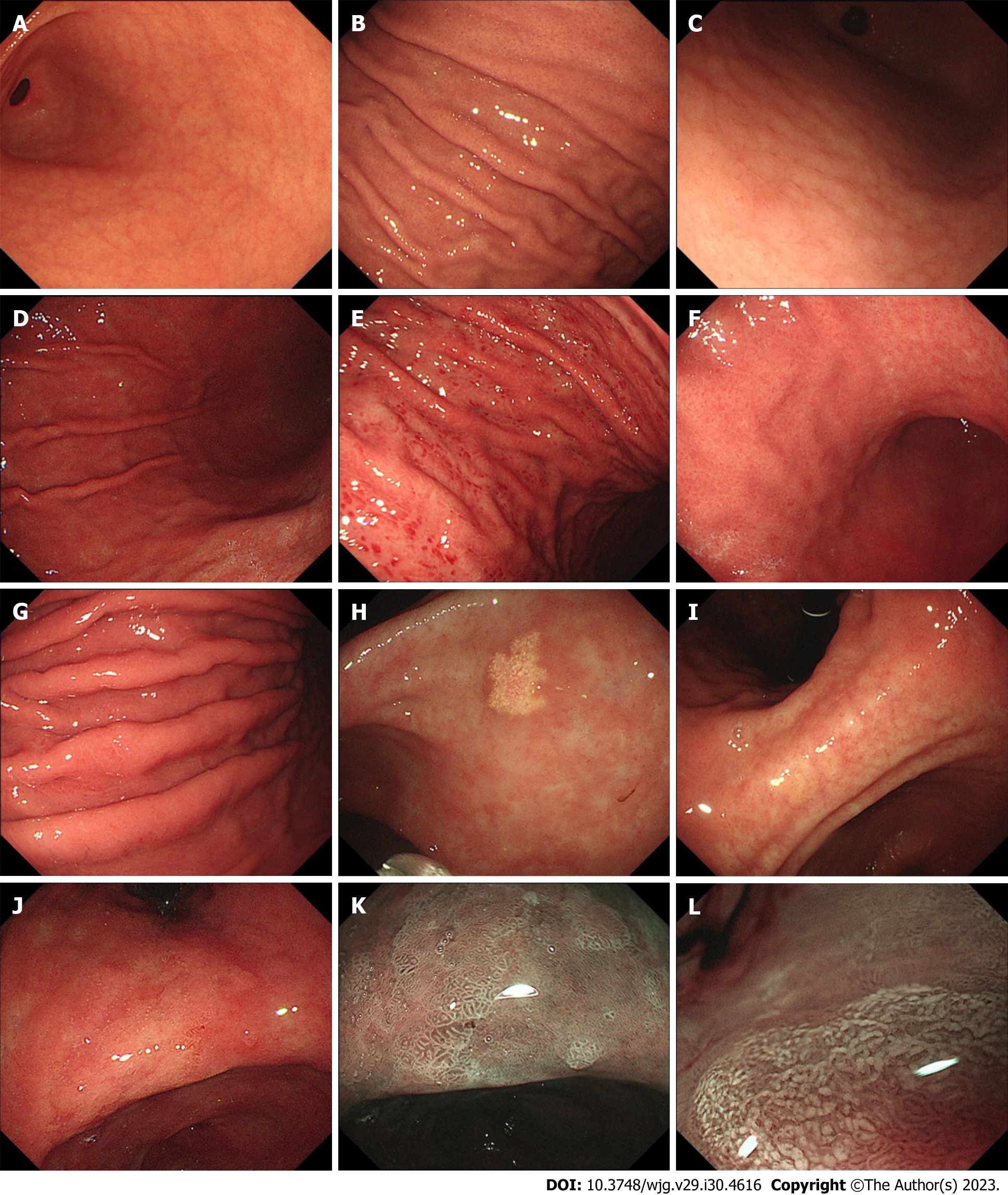
Cases of H. pylori gastritis that are observed by doctors usually involve chronic gastritis that has lasted for years[42]. Chronic gastritis has various endoscopic findings, among which nodularity, diffuse redness, spotty redness, xanthoma, mucosal swelling, enlarged folds, atrophy, and IM are common in H. pylori-infected gastric mucosa[43,44] (Figure 1). Considering the severity and progression of chronic H. pylori gastritis, we discuss endoscopic manifestations and potential mechanisms from the following three aspects: (1) Early stage of H. pylori infection; (2) corpus inflammation; and (3) atrophy and intestinal metaplasia, which are summarized in Table 1.
| Endoscopic features | Mechanisms |
| Nodularity | Follicular lymphoid hyperplasia with intraepithelial lymphocytosis[47]; Superficially located, enlarged hyperplastic lymphoid follicles[48]; Increased numbers of MECA-79 HEV-like vessels[48]; Th2 immune response[49] |
| Diffuse redness | Infiltration of neutrophils and monocytes[44,58] |
| Spotty redness | Unclear |
| Mucosal swelling | Infiltration by neutrophils and monocytes[44] |
| Enlarged folds | Tumor necrosis factor-alpha gene polymorphism[64]; Genome wide hypomethylation and regional hypermethylation[65,66]; Stimulation of epithelial cell proliferation and inhibition of acid secretion induced by interleukin 1 beta and hepatocyte growth factor[61,62]; Inhibition of acid secretion caused by morphological changes in parietal cells[63] |
| Xanthoma | Unclear |
| Atrophy | Cellular injury inflicted by Helicobacter pylori or mediated by inflammation or apoptosis[77]; Th1 immune response[78]; C-X-C motif chemokine receptor 2-mediated cellular senescence[79] |
| Intestinal metaplasia | Death of parietal cells and reprograming of chief cells[82] |
Nodular gastritis is considered a feature of an early stage of H. pylori infection in adults and is more common in children, with an incidence of 32.9% to 85%[45,46]. It appears more frequently in the antral mucosa than in the corpus mucosa[47]. Nodularity is characterized by a miliary pattern resembling “gooseflesh” in the gastric mucosa on endoscopy[46] and follicular lymphoid hyperplasia with intraepithelial lymphocytosis on histological examination[47]. Okamura et al[48] further demonstrated that superficially located, enlarged hyperplastic lymphoid follicles corresponded to nodular and/or granular lesions, and the percentage of MECA-79 high endothelial venule (HEV)-like vessels was greater in areas with gooseflesh-like lesions in nodules than in normal gastric mucosa. The pathogenesis of nodular gastritis may involve a Th2 immune response, which is more likely to occur in children[49,50].
Early colonization usually occurs in the gastric antrum, and early inflammation is always more serious in the gastric antrum, which is consistent with endoscopic findings. Animal research suggested that the wild-type H. pylori strain mostly colonized the antrum and the transition zone between the antrum and corpus rather than the corpus[31,34]. Rolig et al[51] demonstrated that inflammation was worse in the antrum than in the corpus in mice infected with wild-type H. pylori strains. This may be associated with the particularity of antral glands and chemotaxis of the bacterium.
It is well known that the corpus is populated by oxyntic glands containing many acid-secreting parietal cells that promote acidic conditions in the stomach. In contrast, the antrum, which is defined by the presence of gastrin-expressing G cells, mainly comprises the pyloric or antral glands containing MUC6-expressing deep mucous cells, G cells, D cells, enterochromaffin cells and foveolar surface mucous cells[52]. Interestingly, oxyntic glands also exist in the human gastric antrum, but the proportion of parietal cells and chief parietal cells is significantly less than that in corpus glands[53]. The effects of parietal cells in the antrum on H. pylori colonization remains unclear. However, generally, the weaker acidic environment of the antrum provides the bacteria with more opportunities to survive and colonize.
The chemotaxis system of H. pylori includes three membrane-bound chemoreceptors, including TlpA, TlpB, and TlpC; one cytoplasmic chemoreceptor, TlpD[29]; three core signaling complex proteins, including CheW, CheA and CheY[54,55]; and auxiliary chemotaxis proteins containing CheV-type coupling proteins (CheV1, CheV2, and CheV3), CheZ phosphatase and ChePep[56]. The role of pH sensing in chemotaxis has been mentioned above. In addition, a study by Rolig et al[51] shows that chemotaxis is required for H. pylori to swim to and achieve normal bacterial loads in the antrum and transition zone. The number of nonchemotactic mutant (Che-) H. pylori strains at this site was found to increase more slowly than that of the wild-type strains. TlpD plays a major role in this process. Therefore, chemotaxis may be necessary for H. pylori to locate or to maintain colonization of the antrum.
Previous clinical studies focused on the relationship between endoscopic findings and H. pylori infection and demonstrated that diffuse redness, spotty redness, mucosal swelling and enlarged folds under endoscopy are associated with H. pylori infection[14,46]. Diffuse redness, defined as uniform redness with continuous expansion involving the nonatrophic mucosa in the region of fundic gland, and mucosal swelling, defined as swollen gastric mucosa in the region of fundic gland or thick, uneven mucosa in the region of pyloric gland, correlate predominantly with the degree of neutrophilic and mononuclear cell infiltration caused by H. pylori infection[44,57-59]. Spotty redness comprises multiple spotted small flat erythema, commonly observed in the upper corpus and fornix[44], but its mechanism remains unclear. An enlarged fold is defined as a fold with a width of 5 mm or more in the gastric greater curvature, which is not or only partially flattened by air insufflation[60]. Stimulation of epithelial cell proliferation, inhibition of acid secretion, tumor necrosis factor-alpha gene polymorphism, genome-wide hypomethylation and regional hypermethylation may play a role in the generation of enlarged folds caused by bacterial infection[61-66]. We describe another perspective: these endoscopic features that are mainly observed in the corpus indicate the existence of corpus inflammation, the development of gastric mucosal lesions, and a later stage of H. pylori infection that differs from the early stage and mainly manifests as antral inflammation.
H. pylori can survive in and colonize the harsh conditions of the corpus that are promoted by oxyntic glands. This has been indicated by previous studies. H. pylori was identified in the corpus in 83% of patients with a previous diagnosis of intestinal metaplasia and known H. pylori infection[67]. Biopsies taken from the corpus are conducive to an accurate histologic diagnosis and assessment of H. pylori infection[68,69]. Combined antrum and corpus biopsies can lead to a significantly better success rate of H. pylori culture than single antrum biopsy[4].
H. pylori also reaches the corpus under the guidance of chemotaxis, but afterward, chemotaxis is not needed for H. pylori populations to increase[51]. It is likely that the spontaneous eradication of the bacteria is almost impossible at this stage. However, to live, proliferate and induce chronic infection, bacteria need to acquire nutrients and escape immune reactions in addition to adapting to acidic environments, as mentioned above. Due to the low permeability of the mucosal layer, essential nutrients (for example, Fe3+) for ingested microorganisms are scarce in the stomach[70]. Following the successful colonization of gastric epithelial cells, H. pylori induces immune cells that cause cell damage to shed nutrients onto the surface of the gastric mucosa for survival[71]. However, H. pylori needs to take measures to protect itself from host immunity. Sophisticated mechanisms participate in the response to innate immunity; these mechanisms include: (1) The induction of mitochondrial-dependent apoptosis in macrophages; (2) the defense against NO products available in the gastric microniche through production of peroxiredoxin by the AhpC gene; and (3) the reduction of NO or O2- radicals by arginase due to substrate competition; responses to adaptive immunity, which have been elaborated in a previous review, include: (1) The binding of the VacA toxin to an unknown surface ligand in T cells, which results in actin rearrangement and then inhibition of cell proliferation; (2) The promotion of vacuoles in host cells, which leads to apoptosis by an anion-selective channel formed by the VacA toxin; and (3) VacA binding to mitochondria, which activates the associated apoptotic pathway[19]. In addition, H. pylori can be internalized into epithelial cells through endocytosis[32]. Long-term exposure to VacA during chronic infection causes the formation of immature autophagosomes, resulting in a failure to clear the bacteria[72].
In the novel pathological staging strategy mentioned above[41], stage III, the laminar lesion stage, may be consistent with the early stage of gastric antrum and corpus inflammation. Stage III is subdivided into: (1) Stage IIIA: Infiltration of inflammatory cells and vacuolar-like degeneration; (2) stage IIIB: The development of mucous neck cell hyperplasia, glandular hyperplasia and heteroplasia, and serrated structures; (3) stage IIIC: Mucosal ulcers develop; and (4) stage IIID: Histologically diffuse lymphocyte proliferation occurs, and many lymphatic follicles of varying sizes are present.
In the absence of treatment, the inflammation and immune response caused by H. pylori infection may lead to atrophic gastritis[73], which is defined as the loss of gastric glands, with or without metaplasia[74]. This process takes several years in humans[75]. Early H. pylori eradication should be considered for preventing GC development prior to the appearance of atrophy or metaplasia because the benefits of H. pylori eradication diminish after the gastric IM stage is reached, which is referred to as the “point of no return”[76].
Gastric gland replacement by connective tissue or inflammatory cells is referred to as atrophy[73,74]. Previous studies have reported that atrophy may be related to the Th1 immune response and cellular injury, which is directly inflicted by the bacteria or mediated by inflammation or apoptosis[77,78]. A recent study showed a new mechanism of H. pylori–induced atrophy through C-X-C motif chemokine receptor 2 (CXCR2)-mediated cellular senescence[79]. However, in general, the pathogenetic mechanisms that trigger atrophy are still debated.
Color changes (yellowish pale) in the mucosa, mucosal thinning and visible vascular patterns are typical endoscopic atrophic features[80]. In 1966, Kimura and Takemoto described the appearance of an “atrophic transitional zone” in patients with gastritis for the first time, which was subsequently known as the endoscopic atrophic border[8]. The differences in mucosal color and the visibility of capillary networks are remarkable between the two sides of the endoscopic atrophic border[81]. The degree of atrophy can be divided into 6 types based on the location of the endoscopic atrophic border. Endoscopic atrophic findings that are only visible in the antrum are referred to as closed type C-1. In closed types C-2 and C-3, atrophy can be observed in the angulus and the lesser curvature of the corpus. In open type O-1, the atrophic border lies between the lesser curvature and the anterior wall; in type O-2, it lies within the anterior wall; and in type O-3, the endoscopic atrophic area is widely spread within the border between the anterior wall and the greater curvature[81].
When deep damage to the gastric mucosa occurs, acid-secreting parietal cells die, and pepsin-secreting chief cells are reprogrammed into mucin-secreting, wound-healing cells to reduce endogenous production of caustic substances; this response to injury is known as metaplasia[82]. Pathologically, metaplasia refers to gland replacement by a different type of epithelium in a tissue where it is not normally found[74,83]. The characteristics of mucus secretion were used to discriminate metaplastic lineages[83]. Pseudopyloric metaplasia is defined as the presence of MUC6- and trefoil factor 2 (TFF2)-expressing cells at the base of corpus glands with a morphology more characteristic of mucus-producing deep antral glands[84]. IM refers to the presence of Mucin2 (MUC2)/trefoil factor 3 (TFF3)-expressing intestinal-type goblet cells in the stomach[85]. IM can be divided into two types: (1) Incomplete IM, which may be found in either the superficial or foveolar epithelium and in the glands and is characterized by secretive columnar cells that secrete mucin into the apical cytoplasm and the presence of goblet cells; and (2) complete IM, which is characterized by columnar absorptive cells without mucin secretion and the presence of goblet cells[86]. Both incomplete and complete IM can be subdivided into small intestinal type and colonic type (Table 2).
| Cells | Incomplete intestinal metaplasia | Complete intestinal metaplasia | ||
| Small intestinal type | Colonic type | Small intestinal type | Colonic type | |
| Columnar cells | Neutral and scanty sialomucins | Sulpho- and scanty sialomucins | No mucin secretion | No mucin secretion |
| Goblet cells | Sialomucins | sialomucins | Neutral and sialomucins | Sulpho- and sialomucins |
An ash-colored flat nodular change has been considered a typical endoscopic finding of IM since the last century[80]. With the development of endoscopic technology, advanced IEE, including narrow band imaging (NBI) endoscopy, has been used as a more accurate IM diagnostic tool than traditional white light endoscopy[9]. Various markers are related to gastric IM[87]. Light-blue crest (LBC) (Figure 1K), a light blue line observed on the surface of gastric mucosal epithelium, is the earliest mentioned IEE finding[88]. Combining the findings of white opaque substance (WOS) (Figure 1L), white mucosal epithelium observed under IEE, and LBC improves the sensitivity of diagnosing IM[89]. Through systematic review and meta-analysis, the diagnostic sensitivity and specificity of LBC were found to be 0.79 [95% confidence interval (CI): 0.76-0.81] and 0.95 (95%CI: 0.94-0.96), respectively. The sensitivities of the groove type (GT) and marginal turbid band (MTB) were 0.49 (95%CI: 0.43-0.54) and 0.47 (95%CI: 0.40-0.53), respectively, and the specificities were 0.92 (95%CI, 0.89-0.94) and 0.92 (95%CI: 0.89-0.95)[87], respectively. In addition, researchers derived a classification for endoscopic grading of gastric IM (EGGIM) using IEE, which permits immediate grading of intestinal metaplasia without biopsies and is beneficial for GC risk stratification[90].
In addition, gastric xanthoma is a common endoscopic finding in patients with H. pylori infection and may serve as a warning endoscopic sign for advanced atrophic gastritis, intestinal metaplasia and GC[91-93]. It is a small yellowish or yellowish-white plaque-like or nodular lesion characterized by the accumulation of lipids, containing cholesterol, low-density oxidized lipoprotein, low-density lipoprotein and neutral fat, in histiocytic foam cells[93,94]. However, the etiopathogenesis is also unclear.
H. pylori has shared a coevolutionary history with humans for more than 60000 years[41,95]. Human migration has led to the global distribution of the bacterium from East Africa to other continents[19]. In addition to geographical migration, H. pylori has the ability to move between different regions of the stomach.
The motility of H. pylori provided by its flagella and helical shape is the basis of intragastric migration. The bacterium possesses two to six sheathed unipolar flagella[96]. The sheath, which consists of both proteins and lipopolysaccharide, protects the flagellar filaments from gastric acid[97]. Expression of the two major flagellar proteins, FlaA and FlaB, is required for full motility of the bacteria[21]. An efficient screw-like movement resulting from the characteristic helical shape of H. pylori also provides an advantage for penetrating the gastric mucus layer[98]. Any mutation in the genes associated with bacterial morphology, such as Ccrp89, Ccrp58, Ccrp1142 and Ccrp1143, can lead to a deficiency in bacterial shape and motility[99].
The chemotaxis system of H. pylori is necessary for intragastric migration. Chemotactic signals sensed by chemoreceptors are transmitted to the histidine kinase CheA through the coupling protein CheW or CheV1[100]. Repellents activate CheA autophosphorylation, and CheY is subsequently phosphorylated via histidine-to-aspartate phosphorelay[101]. Phosphorylated CheY interacts with the flagellar motor, causing it to rotate clockwise and the bacteria to reverse or change direction[56]. Alternatively, the bacteria swim straight because chemicals perceived as attractants squelch CheA autophosphorylation[56]. As described above, the ability of chemoreceptors to sense pH guides the bacteria to the surface of the gastric epithelium. It has been suggested that different regions of the stomach contain unique chemotactic signals[51]. The gastric antrum is usually the first colonized area because of its weaker acidic environment but not due to chemotaxis. The chemotactic signals produced by the antrum or transition zone play an important role in the increase in H. pylori numbers that occurs from 14 h to 1 wk after colonization[51]. Chemotaxis is also required when H. pylori migrates to the corpus from the antrum but is not needed for the increase in bacterial populations after the initial colonization of the corpus[27]. In addition, H. pylori can swim toward injured epithelia[102].
H. pylori can simultaneously survive in the antrum and the corpus in general. However, when atrophy occurs, an environment that is unfavorable to the growth of H. pylori develops, and the bacteria can only be found in a small percentage of endoscopic biopsy specimens[103]. Research has revealed that atrophy in the corpus manifests as a continuous sheet of pseudopyloric metaplasia and forms an advancing histologically atrophic front, the presence of which is similar to the spread of antral mucosa toward the corpus and is faster in the lesser curvature[104]. This pattern is the same as the endoscopic atrophic border described by Kimura and Takemoto[8]. This may indicate that the suitable region in which H. pylori survives shrinks as the atrophic front advances and is well discriminated by the endoscopic atrophic border.
In addition, H. pylori can migrate to the duodenum and colonize the duodenal gastric metaplasia (DGM) with a bacterial density 100-fold lower than that in the antrum[105,106]. DGM is characterized by the metaplastic replacement of normal duodenal epithelial cells with cells displaying a phenotype similar to that of mucus-secreting cells of the gastric mucosa[107]. It is frequently found in patients with duodenal ulcers with a prevalence of 72 to 90% and is associated with the chronicity and recurrence of duodenal ulcer disease[108-110]. The exact pathogenesis of DGM remains unclear. It is speculated that a high acid burden in the duodenum caused by increased gastrin secretion and the inflammatory damage to duodenal mucosa induced by bacterial cytotoxin may lead to the development of DGM in patients with H. pylori infection[109]. Liu and Wright[111] considered that metaplastic cells originate from Brunner's gland duct epithelium or basal buds growing out of the crypts of Lieberkühn and migrate in straight lines. However, Shaoul et al[112] suggested that DGM develops from goblet cells that simultaneously express gastric antigens, MUC5AC and TFF1, and intestinal antigen, MUC2 core antigen, migrate upward and transform to foveolar-like cells at the site of early metaplastic patches. Published results about the association between H. pylori infection and DGM are also conflicting. Some studies reported that H. pylori infection was one of the independent risk factors for DGM[113], the amount of H. pylori in the duodenal bulb might be related to the extent of gastric metaplasia in the duodenal bulb[114], and the presence of DGM significantly decreased after H. pylori eradication[109]. However, some researchers have suggested that DGM is associated with high acid output in the stomach rather than gastric H. pylori infection[115-117].
The endoscopic characteristics of the cardia have received little attention in previous studies. In recent years, cardiac nodularity, which involves the appearance of miliary nodules or scattered small whitish circular colorations within 2 cm of the esophagogastric junction, has been proposed by researchers[46,118].
Cardia glands lack chief cells and parietal cells, and have similar characteristics to the pyloric glands[53]. The cardiac and pyloric glands secrete mucus and bicarbonate and are involved in the defense of the gastric epithelium[46]. In addition, both of them secrete MUC6 and pepsinogen II rather than pepsinogen I[46]. Unlike the fundic glands, the similarity of the cardiac and pyloric glands may lead to the appearance of cardiac nodularity.
Nodularity can be observed more frequently in the stomach of children and improves gradually with age[119,120]. Reportedly, the eradication of H. pylori in patients with antral nodularity could effectively prevent diffuse-type GC[119]. A study by Nishikawa et al[119] suggested that compared with patients without cardiac nodularity, patients with cardiac nodularity were significantly younger and had lower IM scores. Therefore, cardiac nodularity may also be a feature of the early stage of H. pylori infection, but further research is needed to analyze its clinicopathological importance.
H. pylori infection has received worldwide attention for decades. In this review, we described the process of intragastric colonization by H. pylori and its migration and tried to identify a link between endoscopic manifestations and potential mechanisms. Upper gastrointestinal endoscopy and pathological examination of biopsy specimens are useful tools for diagnosing H. pylori-induced gastritis and estimating the risk of H. pylori-induced GC. In addition to animal models, exploring the mechanisms of H. pylori infection requires biopsy sampling. However, extensive study is needed to evaluate the association between endoscopic manifestations and mechanisms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miklos BG, Hungary; Sano W, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1816] [Article Influence: 82.5] [Reference Citation Analysis (3)] |
| 2. | Buck GE, Gourley WK, Lee WK, Subramanyam K, Latimer JM, DiNuzzo AR. Relation of Campylobacter pyloridis to gastritis and peptic ulcer. J Infect Dis. 1986;153:664-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 186] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Bücker R, Azevedo-Vethacke M, Groll C, Garten D, Josenhans C, Suerbaum S, Schreiber S. Helicobacter pylori colonization critically depends on postprandial gastric conditions. Sci Rep. 2012;2:994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Brennan DE, O'Morain C, McNamara D, Smith SM. Combined antrum and corpus biopsy protocol improves Helicobacter pylori culture success. World J Gastrointest Pathophysiol. 2022;13:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] [Cited in This Article: ] |
| 6. | Cirak MY, Akyön Y, Mégraud F. Diagnosis of Helicobacter pylori. Helicobacter. 2007;12 Suppl 1:4-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Quach DT, Hiyama T. Assessment of Endoscopic Gastric Atrophy according to the Kimura-Takemoto Classification and Its Potential Application in Daily Practice. Clin Endosc. 2019;52:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, Marcos-Pinto R, Santos C, Rolanda C, Bastos RP, Areia M, Afonso L, Bergman J, Sharma P, Gotoda T, Henrique R, Moreira-Dias L. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012;44:236-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985;142:436-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 856] [Cited by in F6Publishing: 727] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 11. | Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Thorell K, Lehours P, Vale FF. Genomics of Helicobacter pylori. Helicobacter. 2017;22:Suppl 1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Schreiber S, Konradt M, Groll C, Scheid P, Hanauer G, Werling HO, Josenhans C, Suerbaum S. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci U S A. 2004;101:5024-5029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Kato T, Yagi N, Kamada T, Shimbo T, Watanabe H, Ida K; Study Group for Establishing Endoscopic Diagnosis of Chronic Gastritis. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: a multicenter prospective study. Dig Endosc. 2013;25:508-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Perri F, Pastore M, Clemente R, Festa V, Quitadamo M, Niro G, Conoscitore P, Rutgeerts P, Andriulli A. Helicobacter pylori infection may undergo spontaneous eradication in children: a 2-year follow-up study. J Pediatr Gastroenterol Nutr. 1998;27:181-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Scott DR, Weeks D, Hong C, Postius S, Melchers K, Sachs G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology. 1998;114:58-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 217] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Huang JY, Goers Sweeney E, Guillemin K, Amieva MR. Multiple Acid Sensors Control Helicobacter pylori Colonization of the Stomach. PLoS Pathog. 2017;13:e1006118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Stingl K, Altendorf K, Bakker EP. Acid survival of Helicobacter pylori: how does urease activity trigger cytoplasmic pH homeostasis? Trends Microbiol. 2002;10:70-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Abadi ATB. Strategies used by helicobacter pylori to establish persistent infection. World J Gastroenterol. 2017;23:2870-2882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 64] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, Bansil R. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci U S A. 2009;106:14321-14326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Dunne C, Dolan B, Clyne M. Factors that mediate colonization of the human stomach by Helicobacter pylori. World J Gastroenterol. 2014;20:5610-5624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 74] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 22. | Reeves EP, Ali T, Leonard P, Hearty S, O'Kennedy R, May FE, Westley BR, Josenhans C, Rust M, Suerbaum S, Smith A, Drumm B, Clyne M. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology. 2008;135:2043-2054, 2054.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Nakamura H, Yoshiyama H, Takeuchi H, Mizote T, Okita K, Nakazawa T. Urease plays an important role in the chemotactic motility of Helicobacter pylori in a viscous environment. Infect Immun. 1998;66:4832-4837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Clyne M, Labigne A, Drumm B. Helicobacter pylori requires an acidic environment to survive in the presence of urea. Infect Immun. 1995;63:1669-1673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 144] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | van Vliet AH. Use of pan-genome analysis for the identification of lineage-specific genes of Helicobacter pylori. FEMS Microbiol Lett. 2017;364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 312] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 27. | Huang Y, Wang QL, Cheng DD, Xu WT, Lu NH. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front Cell Infect Microbiol. 2016;6:159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Lertsethtakarn P, Ottemann KM, Hendrixson DR. Motility and chemotaxis in Campylobacter and Helicobacter . Annu Rev Microbiol. 2011;65:389-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 30. | Ottemann KM, Lowenthal AC. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect Immun. 2002;70:1984-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 219] [Article Influence: 10.0] [Reference Citation Analysis (3)] |
| 31. | Howitt MR, Lee JY, Lertsethtakarn P, Vogelmann R, Joubert LM, Ottemann KM, Amieva MR. ChePep controls Helicobacter pylori Infection of the gastric glands and chemotaxis in the Epsilonproteobacteria. mBio. 2011;2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Ozbek A, Ozbek E, Dursun H, Kalkan Y, Demirci T. Can Helicobacter pylori invade human gastric mucosa?: an in vivo study using electron microscopy, immunohistochemical methods, and real-time polymerase chain reaction. J Clin Gastroenterol. 2010;44:416-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Camorlinga-Ponce M, Romo C, González-Valencia G, Muñoz O, Torres J. Topographical localisation of cagA positive and cagA negative Helicobacter pylori strains in the gastric mucosa; an in situ hybridisation study. J Clin Pathol. 2004;57:822-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Sigal M, Rothenberg ME, Logan CY, Lee JY, Honaker RW, Cooper RL, Passarelli B, Camorlinga M, Bouley DM, Alvarez G, Nusse R, Torres J, Amieva MR. Helicobacter pylori Activates and Expands Lgr5(+) Stem Cells Through Direct Colonization of the Gastric Glands. Gastroenterology. 2015;148:1392-404.e21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 35. | Van de Bovenkamp JH, Mahdavi J, Korteland-Van Male AM, Büller HA, Einerhand AW, Borén T, Dekker J. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter. 2003;8:521-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Lindén S, Mahdavi J, Semino-Mora C, Olsen C, Carlstedt I, Borén T, Dubois A. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog. 2008;4:e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Goodwin AC, Weinberger DM, Ford CB, Nelson JC, Snider JD, Hall JD, Paules CI, Peek RM, Forsyth MH. Expression of the Helicobacter pylori adhesin SabA is controlled via phase variation and the ArsRS signal transduction system. Microbiology (Reading). 2008;154:2231-2240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Oleastro M, Ménard A. The Role of Helicobacter pylori Outer Membrane Proteins in Adherence and Pathogenesis. Biology (Basel). 2013;2:1110-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Cover TL, Lacy DB, Ohi MD. The Helicobacter pylori Cag Type IV Secretion System. Trends Microbiol. 2020;28:682-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 40. | Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78:4157-4165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Zhao G, Zhang Z, Li B, Huang S, Li W, Zhu C, Jiang B, He S, Wang Y, Wang S. Follow-up analysis and histopathological study of gastric mucosa in patients with Helicobacter pylori infection. J Int Med Res. 2021;49:3000605211055397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 42. | Andersen LP. Colonization and infection by Helicobacter pylori in humans. Helicobacter. 2007;12 Suppl 2:12-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Takahashi Y, Yamamichi N, Hata K, Seto Y, Koike K, Watanabe H, Suzuki H. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: A cross-sectional study. World J Gastroenterol. 2018;24:4061-4068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Nomura S, Terao S, Adachi K, Kato T, Ida K, Watanabe H, Shimbo T; Research Group for Establishment of Endoscopic Diagnosis of Chronic Gastritis. Endoscopic diagnosis of gastric mucosal activity and inflammation. Dig Endosc. 2013;25:136-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Miyamoto M, Haruma K, Yoshihara M, Hiyama T, Sumioka M, Nishisaka T, Tanaka S, Chayama K. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci. 2003;48:968-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Watanabe H, Yoshida S, Nakai Y, Hata K, Ebinuma H, Suzuki H, Koike K. Nodularity-like appearance in the cardia: novel endoscopic findings for Helicobacter pylori infection. Endosc Int Open. 2020;8:E770-E774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Hayashi S, Imamura J, Kimura K, Saeki S, Hishima T. Endoscopic features of lymphoid follicles in Helicobacter pylori-associated chronic gastritis. Dig Endosc. 2015;27:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Okamura T, Sakai Y, Hoshino H, Iwaya Y, Tanaka E, Kobayashi M. Superficially located enlarged lymphoid follicles characterise nodular gastritis. Pathology. 2015;47:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Uchida K, Okazaki K, Debrecceni A, Nishi T, Iwano H, Inai M, Uose S, Nakase H, Ohana M, Oshima C, Matsushima Y, Kawanami C, Hiai H, Masuda T, Chiba T. Analysis of cytokines in the early development of gastric secondary lymphoid follicles in Helicobacter pylori-infected BALB/c mice with neonatal thymectomy. Infect Immun. 2001;69:6749-6754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Kako S, Iwaya Y, Nagaya T, Hara D, Okamura T, Iwaya M, Kurasawa S, Kato S, Nakayama Y, Akamatsu T, Umemura T. Clinicopathological features of nodular gastritis in three classes of age. Helicobacter. 2021;26:e12845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Rolig AS, Shanks J, Carter JE, Ottemann KM. Helicobacter pylori requires TlpD-driven chemotaxis to proliferate in the antrum. Infect Immun. 2012;80:3713-3720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Choi E, Roland JT, Barlow BJ, O'Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Engevik AC, Kaji I, Goldenring JR. The Physiology of the Gastric Parietal Cell. Physiol Rev. 2020;100:573-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 54. | Foynes S, Dorrell N, Ward SJ, Stabler RA, McColm AA, Rycroft AN, Wren BW. Helicobacter pylori possesses two CheY response regulators and a histidine kinase sensor, CheA, which are essential for chemotaxis and colonization of the gastric mucosa. Infect Immun. 2000;68:2016-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Pittman MS, Goodwin M, Kelly DJ. Chemotaxis in the human gastric pathogen Helicobacter pylori: different roles for CheW and the three CheV paralogues, and evidence for CheV2 phosphorylation. Microbiology (Reading). 2001;147:2493-2504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Johnson KS, Ottemann KM. Colonization, localization, and inflammation: the roles of H. pylori chemotaxis in vivo. Curr Opin Microbiol. 2018;41:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 58. | Uchiyama K, Ida K, Okuda J, Asai Y, Ohyama Y, Kuroda M, Matsumoto N, Takami T, Ogawa T, Takaori K. Correlations of hemoglobin index (IHb) of gastric mucosa with Helicobacter pylori (H. pylori) infection and inflammation of gastric mucosa. Scand J Gastroenterol. 2004;39:1054-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Hojo M, Nagahara A, Kudo T, Takeda T, Ikuse T, Matsumoto K, Ueda K, Ueyama H, Asaoka D, Shimizu T. Endoscopic findings of Helicobacter pylori gastritis in children and young adults based on the Kyoto classification of gastritis and age-associated changes. JGH Open. 2021;5:1197-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Toyoshima O, Yoshida S, Nishizawa T, Toyoshima A, Sakitani K, Matsuno T, Yamada T, Matsuo T, Nakagawa H, Koike K. Enlarged folds on endoscopic gastritis as a predictor for submucosal invasion of gastric cancers. World J Gastrointest Endosc. 2021;13:426-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Yasunaga Y, Shinomura Y, Kanayama S, Higashimoto Y, Yabu M, Miyazaki Y, Kondo S, Murayama Y, Nishibayashi H, Kitamura S, Matsuzawa Y. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996;39:787-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Yasunaga Y, Shinomura Y, Kanayama S, Higashimoto Y, Yabu M, Miyazaki Y, Murayama Y, Nishibayashi H, Kitamura S, Matsuzawa Y. Mucosal interleukin-1 beta production and acid secretion in enlarged fold gastritis. Aliment Pharmacol Ther. 1997;11:801-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Murayama Y, Miyagawa J, Shinomura Y, Kanayama S, Yasunaga Y, Nishibayashi H, Yamamori K, Higashimoto Y, Matsuzawa Y. Morphological and functional restoration of parietal cells in helicobacter pylori associated enlarged fold gastritis after eradication. Gut. 1999;45:653-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Ohyama I, Ohmiya N, Niwa Y, Shirai K, Taguchi A, Itoh A, Hirooka Y, Wakai K, Hamajima N, Mori N, Goto H. The association between tumour necrosis factor-alpha gene polymorphism and the susceptibility to rugal hyperplastic gastritis and gastric carcinoma. Eur J Gastroenterol Hepatol. 2004;16:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Yamamoto E, Toyota M, Suzuki H, Kondo Y, Sanomura T, Murayama Y, Ohe-Toyota M, Maruyama R, Nojima M, Ashida M, Fujii K, Sasaki Y, Hayashi N, Mori M, Imai K, Tokino T, Shinomura Y. LINE-1 hypomethylation is associated with increased CpG island methylation in Helicobacter pylori-related enlarged-fold gastritis. Cancer Epidemiol Biomarkers Prev. 2008;17:2555-2564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Tahara T, Tahara S, Horiguchi N, Kato T, Shinkai Y, Okubo M, Terada T, Yoshida D, Funasaka K, Nagasaka M, Nakagawa Y, Kurahashi H, Shibata T, Tsukamoto T, Ohmiya N. Prostate Stem Cell Antigen Gene Polymorphism Is Associated with H. pylori-related Promoter DNA Methylation in Nonneoplastic Gastric Epithelium. Cancer Prev Res (Phila). 2019;12:579-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | el-Zimaity HM, al-Assi MT, Genta RM, Graham DY. Confirmation of successful therapy of Helicobacter pylori infection: number and site of biopsies or a rapid urease test. Am J Gastroenterol. 1995;90:1962-1964. [PubMed] [Cited in This Article: ] |
| 68. | Satoh K, Kimura K, Taniguchi Y, Kihira K, Takimoto T, Saifuku K, Kawata H, Tokumaru K, Kojima T, Seki M, Ido K, Fujioka T. Biopsy sites suitable for the diagnosis of Helicobacter pylori infection and the assessment of the extent of atrophic gastritis. Am J Gastroenterol. 1998;93:569-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney System. Hum Pathol. 1999;30:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Basso D, Plebani M, Kusters JG. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2010;15 Suppl 1:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 71. | Talebi Bezmin Abadi A. Helicobacter pylori: Emergence of a Superbug. Front Med (Lausanne). 2014;1:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Raju D, Hussey S, Ang M, Terebiznik MR, Sibony M, Galindo-Mata E, Gupta V, Blanke SR, Delgado A, Romero-Gallo J, Ramjeet MS, Mascarenhas H, Peek RM, Correa P, Streutker C, Hold G, Kunstmann E, Yoshimori T, Silverberg MS, Girardin SE, Philpott DJ, El Omar E, Jones NL. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 73. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 113] [Cited by in F6Publishing: 114] [Article Influence: 22.8] [Reference Citation Analysis (8)] |
| 74. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 75. | Burkitt MD, Duckworth CA, Williams JM, Pritchard DM. Helicobacter pylori-induced gastric pathology: insights from in vivo and ex vivo models. Dis Model Mech. 2017;10:89-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 76. | Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461-5473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 167] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (3)] |
| 77. | Genta RM. Review article: Gastric atrophy and atrophic gastritis--nebulous concepts in search of a definition. Aliment Pharmacol Ther. 1998;12 Suppl 1:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Freire de Melo F, Rocha GA, Rocha AM, Teixeira KN, Pedroso SH, Pereira Junior JB, Fonseca de Castro LP, Cabral MM, Carvalho SD, Bittencourt PF, de Oliveira CA, Queiroz DM. Th1 immune response to H. pylori infection varies according to the age of the patients and influences the gastric inflammatory patterns. Int J Med Microbiol. 2014;304:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Cai Q, Shi P, Yuan Y, Peng J, Ou X, Zhou W, Li J, Su T, Lin L, Cai S, He Y, Xu J. Inflammation-Associated Senescence Promotes Helicobacter pylori-Induced Atrophic Gastritis. Cell Mol Gastroenterol Hepatol. 2021;11:857-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 80. | Kaminishi M, Yamaguchi H, Nomura S, Oohara T, Sakita T. Endoscopic classification of chronic gastritis based on a pilot study by the Research Society for Gastritis. Digest Endosc. 2002;14:138-151. [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Kimura K, Takemoto T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy. 1969;1:87-97. [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 314] [Article Influence: 19.6] [Reference Citation Analysis (3)] |
| 82. | Goldenring JR, Mills JC. Cellular Plasticity, Reprogramming, and Regeneration: Metaplasia in the Stomach and Beyond. Gastroenterology. 2022;162:415-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 83. | Petersen CP, Mills JC, Goldenring JR. Murine Models of Gastric Corpus Preneoplasia. Cell Mol Gastroenterol Hepatol. 2017;3:11-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 84. | Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 85. | Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 86. | Baracchini P, Fulcheri E, Lapertosa G. Patterns of intestinal metaplasia in gastric biopsies. A comparison of different histochemical classifications. Histochem J. 1991;23:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Wei N, Mulmi Shrestha S, Shi RH. Markers of gastric intestinal metaplasia under digital chromoendoscopy: systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:470-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S, Tatsuta M. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 89. | Kanemitsu T, Yao K, Nagahama T, Imamura K, Fujiwara S, Ueki T, Chuman K, Tanabe H, Atsuko O, Iwashita A, Shimokawa T, Uchita K, Kanesaka T. Extending magnifying NBI diagnosis of intestinal metaplasia in the stomach: the white opaque substance marker. Endoscopy. 2017;49:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Pimentel-Nunes P, Libânio D, Lage J, Abrantes D, Coimbra M, Esposito G, Hormozdi D, Pepper M, Drasovean S, White JR, Dobru D, Buxbaum J, Ragunath K, Annibale B, Dinis-Ribeiro M. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy. 2016;48:723-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 91. | Isomoto H, Mizuta Y, Inoue K, Matsuo T, Hayakawa T, Miyazaki M, Onita K, Takeshima F, Murase K, Shimokawa I, Kohno S. A close relationship between Helicobacter pylori infection and gastric xanthoma. Scand J Gastroenterol. 1999;34:346-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Köksal AŞ, Suna N, Kalkan İH, Eminler AT, Sakaoğulları ŞZ, Turhan N, Saygılı F, Kuzu UB, Öztaş E, Parlak E. Is Gastric Xanthelasma an Alarming Endoscopic Marker for Advanced Atrophic Gastritis and Intestinal Metaplasia? Dig Dis Sci. 2016;61:2949-2955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Sekikawa A, Fukui H, Maruo T, Tsumura T, Kanesaka T, Okabe Y, Osaki Y. Gastric xanthelasma may be a warning sign for the presence of early gastric cancer. J Gastroenterol Hepatol. 2014;29:951-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Moumin FA, Mohamed AA, Osman AA, Cai J. Gastric Xanthoma Associated with Gastric Cancer Development: An Updated Review. Can J Gastroenterol Hepatol. 2020;2020:3578927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Moodley Y, Brunelli A, Ghirotto S, Klyubin A, Maady AS, Tyne W, Muñoz-Ramirez ZY, Zhou Z, Manica A, Linz B, Achtman M. Helicobacter pylori's historical journey through Siberia and the Americas. Proc Natl Acad Sci U S A. 2021;118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Geis G, Leying H, Suerbaum S, Mai U, Opferkuch W. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J Clin Microbiol. 1989;27:436-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Geis G, Suerbaum S, Forsthoff B, Leying H, Opferkuch W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J Med Microbiol. 1993;38:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Sycuro LK, Wyckoff TJ, Biboy J, Born P, Pincus Z, Vollmer W, Salama NR. Multiple peptidoglycan modification networks modulate Helicobacter pylori's cell shape, motility, and colonization potential. PLoS Pathog. 2012;8:e1002603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 99. | Sycuro LK, Pincus Z, Gutierrez KD, Biboy J, Stern CA, Vollmer W, Salama NR. Peptidoglycan crosslinking relaxation promotes Helicobacter pylori's helical shape and stomach colonization. Cell. 2010;141:822-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 100. | Abedrabbo S, Castellon J, Collins KD, Johnson KS, Ottemann KM. Cooperation of two distinct coupling proteins creates chemosensory network connections. Proc Natl Acad Sci U S A. 2017;114:2970-2975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Jiménez-Pearson MA, Delany I, Scarlato V, Beier D. Phosphate flow in the chemotactic response system of Helicobacter pylori. Microbiology (Reading). 2005;151:3299-3311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Aihara E, Closson C, Matthis AL, Schumacher MA, Engevik AC, Zavros Y, Ottemann KM, Montrose MH. Motility and chemotaxis mediate the preferential colonization of gastric injury sites by Helicobacter pylori. PLoS Pathog. 2014;10:e1004275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | Perez-Perez GI. Accurate diagnosis of Helicobacter pylori. Culture, including transport. Gastroenterol Clin North Am. 2000;29:879-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | El-Zimaity HM, Ota H, Graham DY, Akamatsu T, Katsuyama T. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Hamlet A, Thoreson AC, Nilsson O, Svennerholm AM, Olbe L. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology. 1999;116:259-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 106. | Cui R, Zhou L, Yan X, Jin Z, Zhang H. Clinicopathological features of duodenal bulb biopsies and their relationship with upper gastrointestinal diseases. Ann Diagn Pathol. 2019;40:40-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Carrick J, Lee A, Hazell S, Ralston M, Daskalopoulos G. Campylobacter pylori, duodenal ulcer, and gastric metaplasia: possible role of functional heterotopic tissue in ulcerogenesis. Gut. 1989;30:790-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 131] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Kawaguchi M, Saito T. Incidence of Gastric Metaplasia and Helicobacter pylori Infection in Duodenal Bulb - With Specific Reference to Patients With Duodenal Ulcers. Diagn Ther Endosc. 1999;6:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Li XB, Ge ZZ, Chen XY, Liu WZ. Duodenal gastric metaplasia and Helicobacter pylori infection in patients with diffuse nodular duodenitis. Braz J Med Biol Res. 2007;40:897-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Gisbert JP, Blanco M, Cruzado AI, Pajares JM. Helicobacter pylori infection, gastric metaplasia in the duodenum and the relationship with ulcer recurrence. Eur J Gastroenterol Hepatol. 2000;12:1295-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Liu KC, Wright NA. The migration pathway of epithelial cells on human duodenal villi: the origin and fate of 'gastric metaplastic' cells in duodenal mucosa. Epithelial Cell Biol. 1992;1:53-58. [PubMed] [Cited in This Article: ] |
| 112. | Shaoul R, Marcon P, Okada Y, Cutz E, Forstner G. The pathogenesis of duodenal gastric metaplasia: the role of local goblet cell transformation. Gut. 2000;46:632-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Voutilainen M, Juhola M, Färkkilä M, Sipponen P. Gastric metaplasia and chronic inflammation at the duodenal bulb mucosa. Dig Liver Dis. 2003;35:94-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Futami H, Takashima M, Furuta T, Hanai H, Kaneko E. Relationship between Helicobacter pylori infection and gastric metaplasia in the duodenal bulb in the pathogenesis of duodenal ulcer. J Gastroenterol Hepatol. 1999;14:114-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 115. | Veijola L, Sankila A, Rautelin H, Kosunen TU, Sipponen P, Hyvärinen H, Tilvis R, Sarna S, Arkkila PE, Seppälä K. Clinical significance of widespread gastric metaplasia in the duodenal bulb. J Clin Gastroenterol. 2006;40:510-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Seo JH, Do HJ, Park CH, Woo HO, Youn HS, Ko GH, Baik SC, Lee WK, Cho MJ, Rhee KH, Lee JH. Helicobacter pylori infection and duodenal gastric metaplasia in healthy young adults. Korean J Gastroenterol. 2013;61:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Harris AW, Gummett PA, Walker MM, Misiewicz JJ, Baron JH. Relation between gastric acid output, Helicobacter pylori, and gastric metaplasia in the duodenal bulb. Gut. 1996;39:513-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Nishizawa T, Sakitani K, Suzuki H, Yoshida S, Kataoka Y, Nakai Y, Ebinuma H, Kanai T, Toyoshima O, Koike K. Clinical features of cardiac nodularity-like appearance induced by Helicobacter pylori infection. World J Gastroenterol. 2020;26:5354-5361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Nishikawa I, Kato J, Terasoma S, Matsutani H, Tamaki H, Tamaki T, Kuwashima F, Nakata H, Tomeki T, Matsunaka H, Ibata Y, Yamashita Y, Maekita T, Higashi K, Ichinose M. Nodular gastritis in association with gastric cancer development before and after Helicobacter pylori eradication. JGH Open. 2018;2:80-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Shiotani A, Kamada T, Kumamoto M, Nakae Y, Nakamura Y, Kakudo K, Haruma K. Nodular gastritis in Japanese young adults: endoscopic and histological observations. J Gastroenterol. 2007;42:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |