Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6277
Peer-review started: April 5, 2021
First decision: July 3, 2021
Revised: July 13, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: October 7, 2021
Little is known about the engagement in hepatitis C virus (HCV) care and completion of HCV treatment in people living with human immunodeficiency virus (HIV) (PLWH) who have HCV coinfection in the Asia-Pacific region. Examining the HCV care cascade can identify barriers to the completion of HCV treatment and facilitate achievement of HCV micro-elimination in PLWH.
To investigate the care cascade of incident HCV infections among PLWH in Taiwan.
PLWH with incident HCV infections, defined as HCV seroconversion, were retrospectively identified by sequential anti-HCV testing of all archived blood samples at National Taiwan University Hospital between 2011 and 2018. All PLWH with incident HCV infections were followed until December 31, 2019. The care cascade of HCV examined included all incident HCV-infected patients, the percentages of anti-HCV antibodies detected by HIV-treating physicians in clinical care, plasma HCV RNA load tested, HCV RNA positivity diagnosed, referral to treatment assessment made, anti-HCV treatment initiated, and sustained virologic response achieved. Those who had HCV seroconversion during the interferon (IFN) era (2011–2016) and the direct-acting antiviral (DAA) era (2017–2018) were analyzed separately. The duration of HCV viremia—from the date of seroconversion to viral clearance by treatments or until the end of observation—and the incidence of sexually transmitted infections (STIs) during the HCV viremic period were estimated.
During the study period, 287 of 3495 (8.2%) PLWH (92.3% being men who have sex with men) who were HCV-seronegative at baseline developed HCV seroconversion by retrospective testing of all archived blood samples. Of the 287 incident HCV infections, 277 (96.5%) had anti-HCV antibodies detected by HIV-treating physicians, 270 (94.1%) had plasma HCV RNA determined and 251 (87.5%) tested positive for HCV RNA. Of those with HCV viremia, 226 (78.7%) were referred to treatment assessment, 215 (74.9%) initiated anti-HCV treatment, and 202 (70.4%) achieved viral clearance. Compared with that in the IFN era, the median interval from HCV seroconversion by retrospective testing to detection of HCV seropositivity by HIV-treating physicians was significantly shorter in the DAA era {179 d [interquartile range (IQR) 87-434] vs 92 d (IQR 57-173); P < 0.001}. The incidence rate of STIs in the DAA vs the IFN era was 50.5 per 100 person-years of follow-up (PYFU) and 38.5 per 100 PYFU, respectively, with an incidence rate ratio of 1.31 (95% confidence interval 0.96-1.77), while the duration of HCV viremia was 380 d (IQR 274-554) and 735 d (IQR 391-1447) (P < 0.001), respectively.
While anti-HCV therapies are effective in achieving viral clearance, our study suggests more efforts are needed to expedite the linkage of PLWH diagnosed with incident HCV infections to HCV treatment.
Core Tip: We examined the hepatitis C virus (HCV) care cascade among people living with human immunodeficiency virus who acquired incident HCV infections at a university hospital in Taiwan between 2011 and 2018. We observed high rates of linkage to HCV care and retention in care in both interferon (IFN, 2011 to 2016) and direct-acting antiviral (DAA, 2017 to 2018) eras. The rate of referral to treatment assessment had increased from the IFN era to the DAA era. Moreover, the duration of HCV viremia was markedly shortened because of early diagnosis and linkage to effective treatment in the DAA era compared to that in the IFN era.
- Citation: Huang MH, Sun HY, Ho SY, Chang SY, Hsieh SM, Sheng WH, Chuang YC, Huang YS, Su LH, Liu WC, Su YC, Hung CC. Recently acquired hepatitis C virus infection among people living with human immunodeficiency virus at a university hospital in Taiwan. World J Gastroenterol 2021; 27(37): 6277-6289
- URL: https://www.wjgnet.com/1007-9327/full/v27/i37/6277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i37.6277
Viral hepatitis is a major public health threat and approximately 2.3 million people living with human immunodeficiency virus (HIV) (PLWH) are coinfected with hepatitis C virus (HCV) globally[1-3]. Injection drug users (IDUs) have been the major contributors to HCV transmission[4]. In Asia-Pacific region, the prevalence of HCV coinfection among PLWH ranges from 3.8% to 42.6%, and may be as high as 80.8% to 98.5% among IDUs[5,6]. Since 2000, incident HCV infections among PLWH who are MSM have continued to increase in the United States, European countries, and Australia[7-9]. Similar increasing trends were observed among PLWH who are MSM in Asia-Pacific region[10,11]. The causes of the increases of HCV transmission among MSM are multifactorial and are related to concurrent sexually transmitted infections (STIs), unprotected anal intercourse, traumatic sexual contacts, and use of recreational drugs by inhalation or injection[12].
The evolution of HCV treatment from interferon (IFN)-based therapy to IFN-free direct-acting antivirals (DAAs) has significantly improved tolerability and effectiveness in achieving viral clearance[13]. The rates of viral clearance with DAAs are similarly high for HCV-monoinfected patients and HCV/HIV-coinfected patients in clinical trials and real-world experience[14-18]. With the introduction of effective DAAs, ending the HCV epidemic is considered an achievable goal by scaling up diagnosis and treatment of HCV infection[1]. Among the interventional strategies proposed to achieve hepatitis elimination, the continuum assessed by the "cascade of care" on the population level is suggested to evaluate the status of hepatitis treatment services and the level of engagement in care[19,20]. The proportion of patients in each step of the cascade—infected, tested, status confirmed, engagement in care, treatment initiated, cured, and followed up for chronic care—reflects the coverage of diagnosis, access to treatment, and quality of care.
In Taiwan, pegylated IFN plus ribavirin (PEG-IFN/RBV) has become the standard anti-HCV treatment regimen since 2004 and has been fully reimbursed by the National Health Insurance (NHI) since 2009[21,22]. DAAs were not available until 2017, when the regimens were restricted to individuals with chronic HCV infection with evidence of significant hepatic fibrosis. Generic versions of patented DAAs could be purchased for individual use from Bangladesh[23]. Before 2019, referral to a hepatologist for hepatitis C evaluation and treatment was required according to NHI regulations. The reimbursement restrictions on the use of DAAs were lifted in January of 2019 by allowing HCV viremic patients to initiate DAAs regardless of liver fibrosis status. Subsequently, PLWH with HCV viremia could be evaluated and DAAs initiated by HIV-treating physicians at infectious disease clinics.
In this study, we aimed to examine the HCV care cascade among PLWH who received a diagnosis of incident HCV infection at a university hospital in Taiwan.
We retrospectively reviewed the medical records of all PLWH aged 18 years or older seeking medical care for HIV at the National Taiwan University Hospital (NTUH), the largest designated hospital for HIV inpatient and outpatient care in Taiwan, from January 2011 to December 2018; and those included were followed until death, loss to follow-up, transfer of care, or the end of the study on December 31, 2019, whichever occurred first. The study period (2011-2018) spanned two different eras of anti-HCV treatment, from the IFN/RBV era (2011-2016) to the DAA era (2017-2018) before restricted access to HCV treatments were completely lifted in early 2019. In this study, the IFN-based regimen was PEG-IFN plus weight-based RBV with response-guided treatment duration. From 2016 to 2018, generic versions of patent IFN-free DAAs (sofosbuvir/velpatasvir) were used for treatment of recently acquired HCV infection.
Anti-HCV antibodies were detected using a fourth-generation enzyme immuno
The date of HCV seroconversion was arbitrarily assigned as the mid-point between the dates of the first positive and the last negative anti-HCV test. Using a standardized case record form, we collected data on patient demographics, HIV transmission risk groups, and laboratory test results, including CD4 lymphocyte count and plasma HIV RNA load (PVL), hepatitis B surface antigen (HBsAg), rapid plasma reagin (RPR) titer, plasma HCV RNA load and genotype when HCV viremia was detected.
The study was approved by the Research Ethics Committee of the hospital (registration number: 201605103RINC and 201605128RINC) and informed consent was obtained from all the participants.
The HCV care cascade comprised the following steps of care: (1) “HCV infected” included all seroconverters in the past 12 mo identified by testing of archived blood samples; (2) “antibody detected” defined as a confirmed anti-HCV-positive test identified by HIV-treating physicians and documented in the medical records; (3) “HCV RNA tested” as the performance of a plasma HCV RNA test either after a positive anti-HCV test or when HCV viremia is detected during an acute infection with a negative anti-HCV test; (4) “HCV RNA positivity” as detectable plasma HCV RNA at the time of testing; (5) “Referred to treatment assessment” as successful referral of HCV-infected individuals to a hepatology clinic for evaluation of liver fibrosis or HCV treatment; or completion of pretreatment blood testing in infectious disease clinics as required by NHI regulation in 2019, including evaluation of liver fibrosis, renal function, HCV genotype, and plasma HCV RNA load[16]; (6) “Treat
The durations between two consecutive steps of the care cascade were recorded. Those who had HCV seroconversion during the IFN era (2011–2016) and the DAA era (2017–2018) were analyzed separately for comparison of the proportion of patients attaining each step and the interval between two consecutive steps of the cascade. The total duration of HCV viremia was defined as the interval between the estimated date of HCV seroconversion to the end of HCV treatment in those with undetectable HCV RNA; those who did not achieve SVR were followed until the completion of re
The number of STIs, including syphilis (defined as a four-fold increase of RPR titers, consistent clinical symptoms, or administration of syphilis treatment), and gonorrhea (defined as culture-confirmed Neisseria gonorrhoeae infection or consistent clinical symptoms and administration of gonorrhea treatment), during the defined intervals of HCV viremia was calculated. We used occurrences of STIs as surrogate markers for unprotected sex contacts of the included PLWH during the HCV viremic period without effective treatment to indicate the risk for onward transmission of HCV.
The cascade of care after diagnosis of recently acquired HCV infection was examined by each step using descriptive analysis. Comparisons of categorical variables were performed by chi-square analysis and those of continuous variables by a Mann-Whitney U Test. The incidence rate of STIs during HCV viremia was calculated as the number of STIs per 100 PYFU. All analyses were two-tailed, and a P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata/SE, version 14.0. The statistical methods of this study were reviewed by the staff of National Taiwan University Hospital–Statistical Consulting Unit (NTUH-SCU).
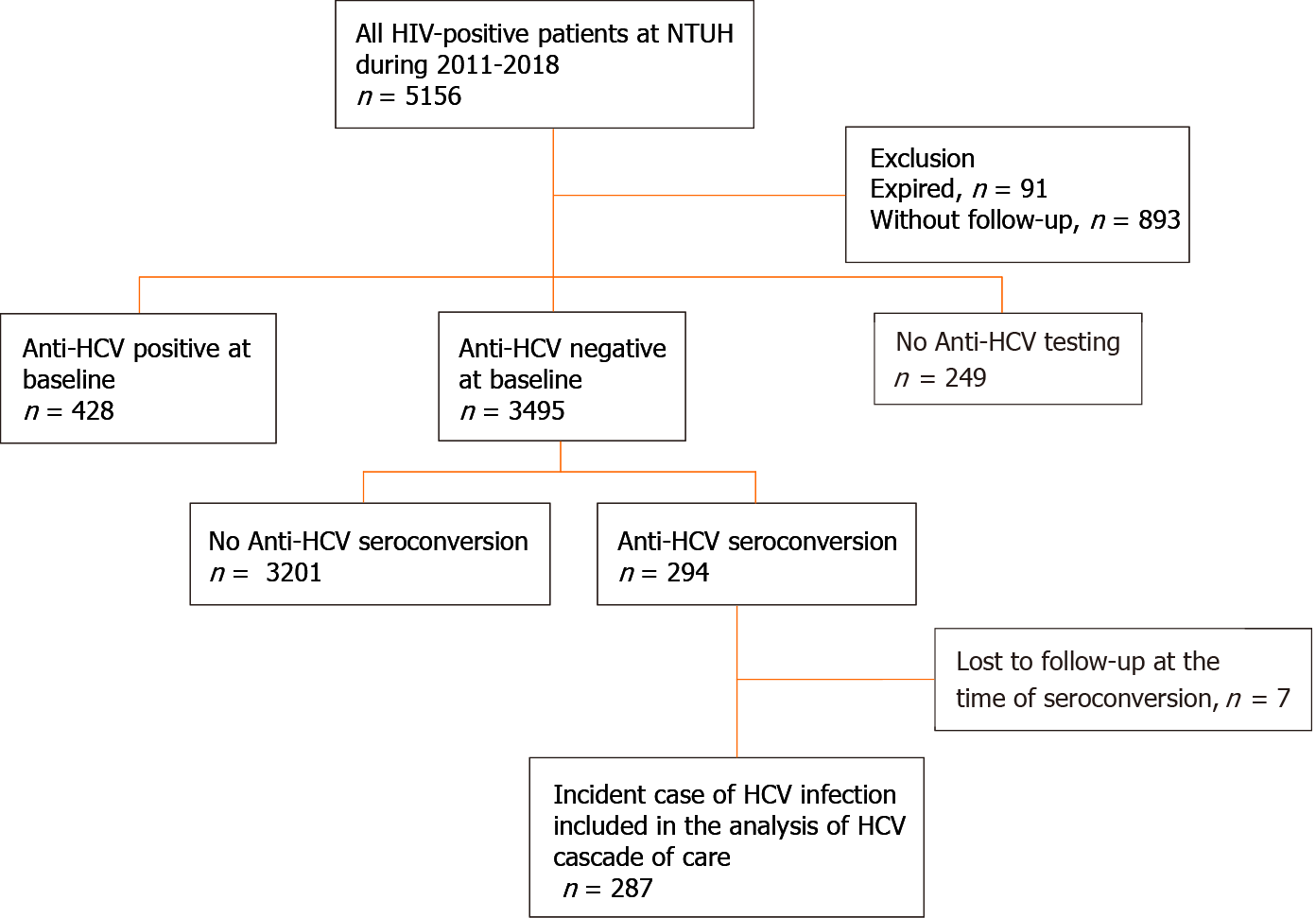
Between January 2011 and December 2018, 3,495 PLWH had a negative anti-HCV antibody test at baseline (Figure 1) and HCV seroconversion was detected in 294 (8.4%) PLWH. After excluding 7 PLWH who were lost to follow-up when seroconversion occurred, a total of 287 PLWH were included in the analysis of the HCV care cascade.
The clinical characteristics of the included 287 PLWH with recently acquired HCV infection are shown in Table 1. All of them were male, with a mean age of 34.5 years [standard deviation (SD) 7.6]. The mean PVL was 1.72 log10 copies/mL (SD 1.09) and mean CD4 count was 597.7 cells/mm3 (SD 251.1) at the time of HCV seroconversion. The HBsAg seropositivity rate was 14.9% and active or previous syphilis was present in 89.2%. The risk groups of HIV acquisition included 265 (92.3%) MSM, 5 (1.8%) heterosexuals, 4 (1.4%) IDUs, and 13 (4.5%) unknown. The mean plasma HCV RNA load was 5.72 log10 copies/mL (SD 1.50). Twenty-two (7.7%), 84 (29.3%), 119 (41.5%), 6 (2.1%), and 34 (11.8%) PLWH had infection with HCV genotypes 1a, 1b, 2a, 3, and 6, respectively, and 3 (1%) had mixed infections of genotypes 1b and 2.
| Clinical characteristics | n = 287 |
| Age, mean ± SD, yr | 34.5 ± 7.6 |
| Male sex, n (%) | 287 (100) |
| Risk group, n (%) | |
| Men who have sex with men | 265 (92.3) |
| Heterosexuals | 5 (1.8) |
| Injecting drug users | 4 (1.4) |
| Unknown | 13 (4.5) |
| HIV PVL, mean ± SD, log10 copies/mL | 1.72 ± 1.09 |
| CD4 count, mean ± SD, cells/mm3 | 597.7 ± 251.1 |
| HBsAg-positive, n (%) | 42 (14.9) |
| Syphilis, n (%) | 256 (89.2) |
| Plasma HCV RNA, mean ± SD, log10 copies/mL | 5.72 ± 1.50) |
| HCV genotype, n (%) | |
| 1a | 22 (7.7) |
| 1b | 84 (29.3) |
| 2a | 119 (41.5) |
| 3 | 6 (2.1) |
| 6 | 34 (11.8) |
| Mixed genotype 1b+2 | 3 (1.0) |
| No data | 19 (6.6) |
| Year of seroconversion, n (%) | |
| 2011 | 23 (8.0) |
| 2012 | 28 (9.8) |
| 2013 | 27 (9.4) |
| 2014 | 24 (8.4) |
| 2015 | 31 (10.8) |
| 2016 | 43 (14.9) |
| 2017 | 54 (18.8) |
| 2018 | 57 (19.9) |
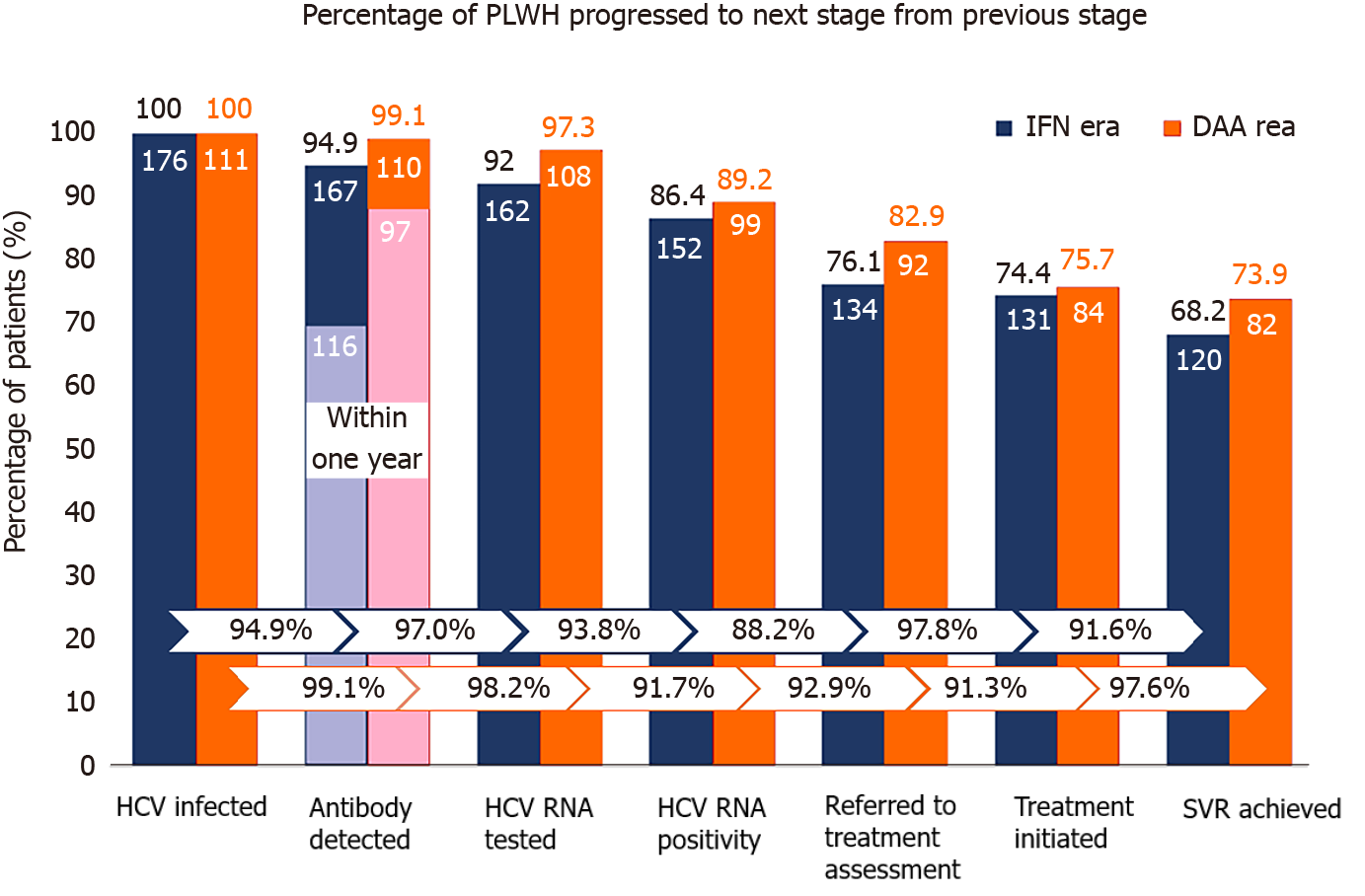
Of the 287 PLWH, 277 (96.5%) were found to have HCV seroconversions by HIV-treating physicians, including 8 diagnosed at the stage of acute infection with positive HCV RNA and negative anti-HCV antibody. Plasma HCV RNA load was determined in 270 (94.1%) PLWH after seroconversion, with 19 (12.5%) clearing viremia spontaneously and 251 (87.5%) remaining viremic (Table 2). Overall, 226 (78.7%) PLWH were referred to hepatology clinics for treatment assessment, and anti-HCV treatments were initiated in 215 (74.9%) PLWH, of whom 202 (70.4%) achieved SVR (Table 2).
| Total 2011-2018, n = 287 | Seroconversion in | P value | ||
| 2011-2016 IFN era, n = 176 | 2017-2018DAA era, n = 111 | |||
| Antibody detected, n (%)1 | 277 (96.5) | 167 (94.9) | 110 (99.1) | 0.09 |
| HCV RNA tested, n (%)1 | 270 (97.5) | 162 (97.0) | 108 (98.2) | 0.08 |
| HCV RNA positivity, n (%)1 | 251 (93.0) | 152 (93.8) | 99 (91.7) | 0.48 |
| Referred to treatment assessment, n (%)1 | 226 (90.0) | 134 (88.2) | 92 (92.9) | 0.17 |
| Treatment initiated, n (%)1 | 215 (95.1) | 131 (97.8) | 84 (91.3) | 0.81 |
| SVR achieved, n (%)1 | 202 (94) | 120 (91.6) | 82 (97.6) | 0.19 |
| Interval between each step, median days (IQR) | ||||
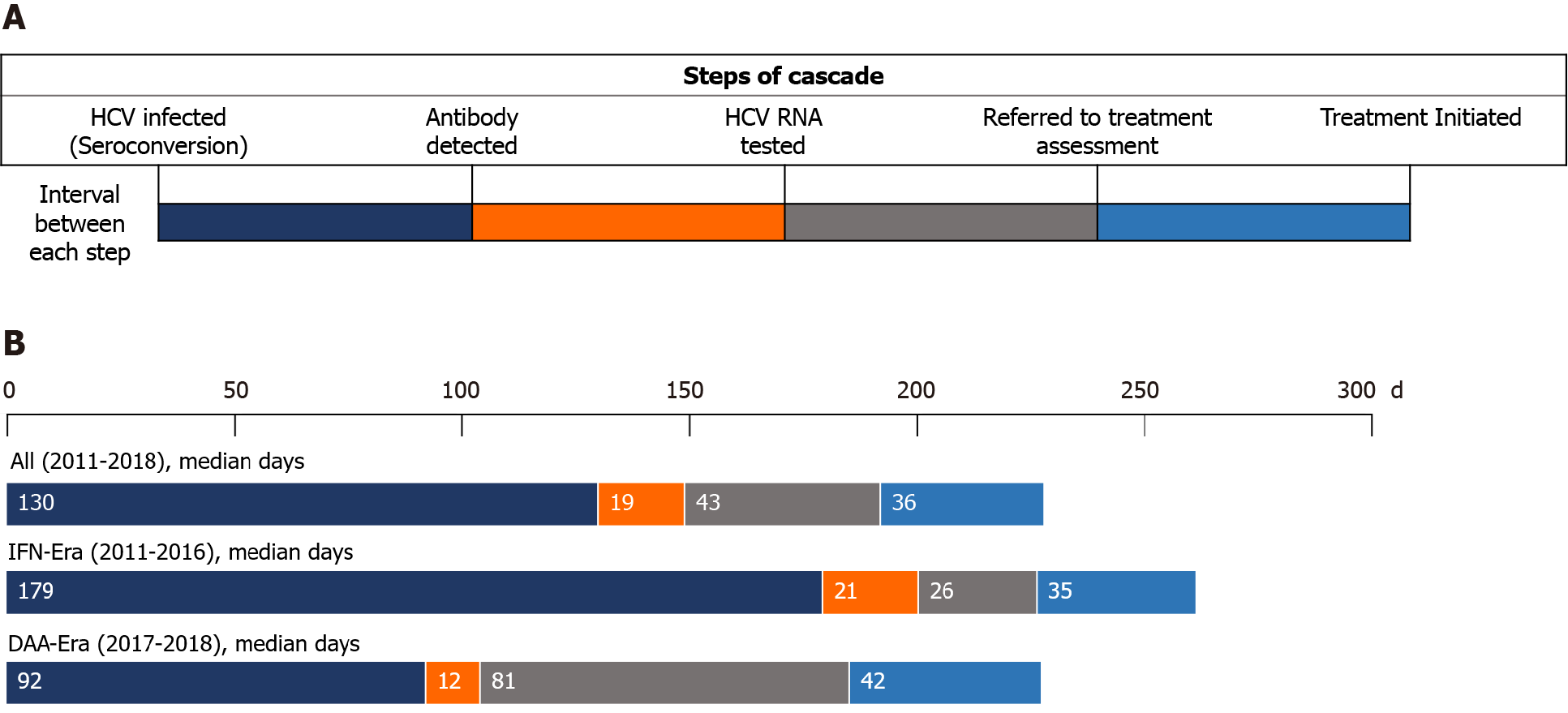
| Seroconversion to antibody detected | 130 (80-295) | 179 (87-434) | 92 (57-173) | < 0.001 |
| Antibody detected to HCV RNA tested | 19 (6-81) | 21 (6-93) | 12 (6-68) | 0.19 |
| HCV RNA tested to treatment assessment | 43 (11-181) | 26 (7-208) | 81 (14-169) | 0.25 |
| Treatment assessment to treatment initiation | 36 (21-90) | 35 (27-90) | 42 (18-84) | 0.55 |
| Duration of viremia2 | 502 (325-945) | 735 (391-1447) | 380 (274-554) | < 0.001 |
| Events of STIs during HCV viremia | 220 | 165 | 55 | < 0.001 |
| Incidence rate of STIs during HCV viremia, (per 100-PYFU) | 41.0 | 38.5 | 50.5 | 0.09 |
Compared with PLWH who had HCV seroconversion in the IFN era (2011–2016), those who seroconverted in the DAA era (2017–2018) had higher achievement rates of all sequential steps of the care cascade (Figure 2), though not reaching statistical significance. The rate of antibody diagnosis within one year of HCV seroconversion was significantly higher in the DAA era than that in the IFN era (88.2% vs 69.5%; P < 0.001) (Figure 2). In the IFN era, the major gap of engagement in care was from HCV RNA positivity to referral for assessment and treatment, which resulted in a loss of 11.8% of those found to be HCV viremic.
The median interval from seroconversion to detection of HCV seropositivity by HIV-treating physicians was 130 d [interquartile range (IQR), 80-295], which was significantly shorter in the DAA era than in the IFN era [92 d (IQR, 57-173) vs 179 d (IQR 87-434); P < 0.001] (Table 2, Figure 3). From detection of HCV seropositivity to HCV RNA testing, the interval was 21 d (IQR 6-39) and 12 d (IQR 6-68) in the IFN and DAA eras, respectively (P = 0.19). The intervals from HCV RNA testing to treatment assessment and from assessment to treatment initiation were longer in the DAA era compared with those in the IFN era, 81 d (IQR 14-169) vs 26 d (IQR 7-208), and 42 d (IQR 18-84) vs 35 d (IQR 27-90), which were not statistically significantly different (P = 0.25 and P = 0.55, respectively). The duration of viremia was 735 d (IQR 391-1447) in the IFN era, which was significantly longer than that in the DAA era (380 d; IQR 274-554; P < 0.001).
Among the HCV seroconverters in the IFN era, a total of 165 episodes of STIs were observed in the entire HCV viremic duration of 428.2 PYFU, resulting in an incidence rate of 38.5 per 100 PYFU. In the DAA era, the HCV seroconverters acquired a total of 55 episodes of STIs during 108.8 PYFU of viremia, leading to an incidence rate of 50.5 per 100 PYFU. The incidence rate ratio (IRR) of STIs in the DAA era vs the IFN era was 1.31 (95%CI 0.96-1.77).
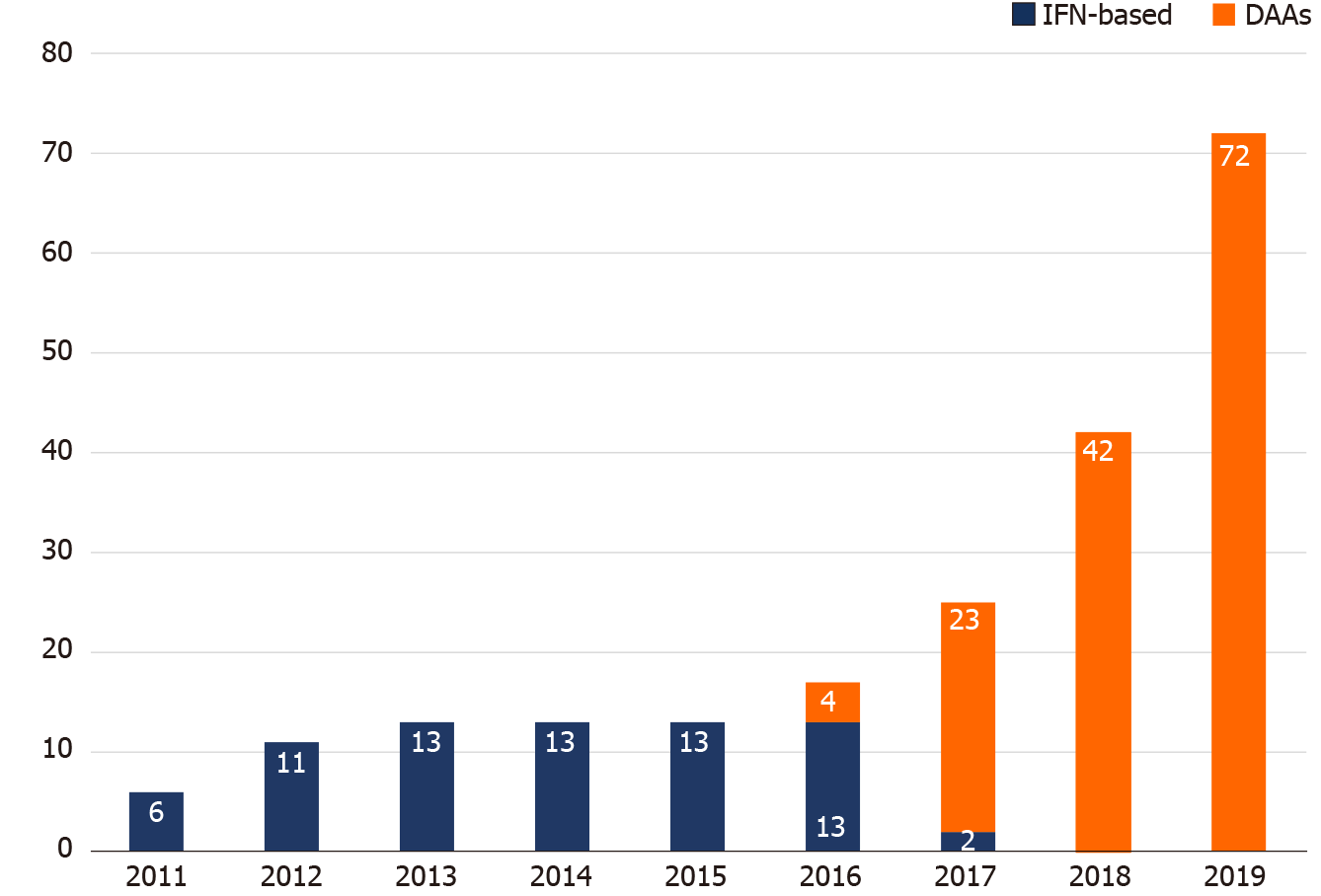
Figure 4 demonstrates the breakdown of HCV treatment uptake by year. The annual number of PLWH with HCV viremia who received IFN-based therapy was unchanged from 2012 to 2016. With the introduction of DAAs in 2016, the number of PLWH receiving DAA treatment increased year by year, especially in 2019, when restrictions on DAA reimbursement were lifted and all HCV viremic patients could be treated with DAAs.
In this study, we observed high rates of linkage to HCV care and retention in the care system among PLWH at our institution, in both the IFN and DAA eras. The retention rates between each step of the care cascade after detection of incident HCV infection primarily exceeded 90% (Figure 2). A major barrier noted in the IFN era was referral to treatment assessment (88.2%) after detection of HCV viremia, while this gap seemed diminished in the DAA era. In addition, the duration of HCV viremia, from seroconversion to completion of HCV treatment, was markedly shortened in the DAA era compared to that in the IFN era (380 d vs 735 d; P < 0.001). The improvement was mainly due to early diagnosis of recently acquired HCV infection by HIV-treating physicians because the diagnostic rate within one year after seroconversion increased from 69.5% (116/167) to 88.2% (97/110), and the median time from seroconversion to antibody diagnosis was shortened from 179 d to 92 d (P < 0.001).
The occurrence of STIs during HCV viremia represented risky sexual behavior, potentially resulting in onward transmission of HCV to their contacts. The incidence rate of STIs observed in the DAA era was higher than that in the IFN era, though not reaching statistical significance (50.5 vs 38.5 per 100 PYFU; IRR 1.31, 95%CI 0.96-1.77). In contrast, the total number of STI episodes was far fewer in the DAA era vs the IFN era (55 episodes among 111 patients vs 165 episodes among 176 patients, respectively), likely due to a shorter duration of HCV viremia. The curtailing of HCV viremia with the initiation of effective DAAs may indicate reduced opportunities to transmit HCV during the viremic period.
There are limited data to describe the care cascade after incident HCV infections were diagnosed both in the general population and in HCV-coinfected PLWH in the Asia-Pacific region. In our study, we tested archived blood samples to include all patients who had recent HCV infections in the previous year and estimated the time of seroconversion. In doing so, we could assess the rate of antibody diagnosis of HCV infection by treating physicians, the interval of delay in clinical diagnosis, and the duration of HCV viremia before effective anti-HCV treatments were initiated. Prior population-level studies of the care cascade either estimated the seroprevalence of HCV[24,25] or described the cascade since the diagnosis of anti-HCV positivity[26]. In single-center studies focusing on HCV-coinfected PLWH, the actual numbers of HCV infections were not shown in the care cascade[27,28].
Previous population-based care cascade assessments for HCV infection in the IFN era revealed low rates of retention in care and treatment initiation, probably related to concerns about inconvenience, lower effectiveness, intolerability, and higher rates of adverse effects with IFN/RBV treatment. The antibody diagnostic rate was 50% to 75%; HCV RNA testing was performed in 30 to 50% of patients; less than 20% of patients received HCV treatment; and the final SVR rate was lower than 10%[24,25]. At our institution during the IFN era, the antibody diagnosis, HCV RNA testing, treatment initiation, and SVR rates were 94.9%, 92.0%, 74.4%, and 68.2%, respectively, much higher than those of previous population-level studies. We believe that regularly scheduled follow-up visits for fully-reimbursed HIV antiretroviral treatments in the majority of PLWH might have provided an opportunity to facilitate HCV testing and increase engagement of HCV care[29,30]. However, mandatory referrals to a hepatologist for HCV pretreatment assessment and management may have raised the barrier to anti-HCV treatment initiation and achievement of viral clearance, as such a requirement negatively impacts convenience and costs, thereby potentially increasing the risk of dropout from HCV care. This barrier has diminished since 2019 when NHI approved HIV-treating physicians to participate in HCV assessment and treatment for PLWH at infectious disease clinics. The rate of treatment assessment after HCV RNA positivity increased from 88.2% (134/152) in the IFN era to 92.9% (92/99) in the DAA era.
The introduction of DAAs has been shown to improve engagement of HCV care and treatment uptake. The British Columbia Hepatitis Tester Cohort demonstrated improvement of the care cascade in the DAA era. Of the patients who tested anti-HCV-positive, 83% had HCV RNA testing done; and, of those who were HCV viremic and were genotyped, 61% received DAA therapy, with 90% achieving SVR[31]. In a report from seven HCV elimination studies among PLWH in the early DAA era, the average treatment uptake increased to 48% and treatment completion rates reached 96%[32]. Our current study showed a significantly higher rate of linkage to care, with > 90% of PLWH consistently advancing from each cascade step to the next in the DAA era, which resulted in a total of 84.8% (84/99) viremic patients initiating anti-HCV treatments, and 97.6% (82/84) achieving SVR. In the Swiss HIV Cohort Study, the treatment incidence increased from 4.5 per 100 PYFU before the availability of DAAs, to 22.4 per 100 PYFU after the introduction of second-generation DAAs[33]. These findings were in line with observations from our study. While the annual number of IFN-based therapy remained unchanged from 2012 to 2016, the number of DAA treatment initiated progressively increased from 2016 to 2018, which further rocketed in 2019, when the restriction on DAA reimbursement was lifted (Figure 4). During the period between 2016 and 2018, when access to DAA through NHI reimbursement remained limited, generic DAAs could be purchased overseas by PLWH themselves; however, accessibility was limited by the cost incurred. During this period, we found that the median intervals from HCV RNA testing to referral to treatment assessment, and those from referral to treatment initiation were longer than those in the IFN era, though not reaching statistical significance (Table 2 and Figure 3).
There are several limitations in this study. First, the cascade of HCV care from this single-center study might not be generalizable to other institutions in Taiwan, given that discrepancies in the patients’ demographic and clinical characteristics and socio-economic status may exist. At our institution, PLWH with recent HCV infection were mainly MSM, accounting for more than 90% of the included patients, while the percentages of heterosexual and IDUs were both lower than 2%. Prior studies suggested from an assessment of the HCV care cascade in IDUs that active drug users had poorer treatment adherence, resulting in lower rates of viral clearance[34-36]. Second, our study did not investigate factors that may be associated with failure of engagement in the HCV care cascade. Third, HCV reinfection, which can occur in this high-risk group[37-39], were not included in the analyses of the HCV care cascade.
In conclusion, we describe the HCV care cascade at a referral hospital in Taiwan from the IFN era to the DAA era. The rates of engagement in each step of the HCV care cascade were high in both eras and the barriers to referral and reimbursement diminished over time. However, given both the longer intervals of HCV viremia observed in the DAA era and a higher incidence of STIs potentially contributing to onward transmission of HCV, more efforts are needed to expedite the linkage of PLWH diagnosed with incident HCV infections to HCV treatment.
Recently acquired hepatitis C virus (HCV) infections are increasingly reported in people living with human immunodeficiency virus (HIV) (PLWH) who are men who have sex with men. With availability of highly effective direct-acting antivirals (DAAs) for the treatment of HCV, microelimination HCV is considered an achievable goal in this at-risk population.
To achieve microelimination of HCV in PLWH, each step of the continuum of HCV care, from diagnosis, linkage to and engagement in care, initiation of anti-HCV treatment, treatment completion, to prevention against reinfection, is crucial. Examining the HCV care cascade can identify barriers to the completion of HCV treatment and facilitate achievement of HCV micro-elimination in PLWH.
The study aimed to evaluate the care cascade of PLWH with recently acquired HCV infections at a university hospital designated for HIV care in Taiwan.
The authors retrospectively reviewed the medical records of all PLWH testing negative for anti-HCV at baseline who developed anti-HCV seroconversion between 2011 to 2018 and were observed till the end of 2019. The number of people in each step of HCV care cascade was assessed and the duration between two sequential steps was estimated.
A total of 287 PLWH recently acquired HCV infections during the study period. High rates of linkage to HCV care and retention in the care were observed in our cohort. Compared with the interferon (IFN, 2011-2016) era, the barrier of referral to anti-HCV treatment assessment was diminished and the total duration of HCV viremia marked decreased in the direct-acting antiviral (DAA, 2017-2018) era.
The achievement rates of engagement in each step of the HCV care cascade were high in both IFN and DAA eras and the barriers to referral and treatment initiation diminished over time.
The impact of scale-up of HCV testing and DAA treatment after lifting the restriction on DAA reimbursement on the trends of incident HCV infections in PLWH warrants more long-term observation.
The authors would like to express their gratitude to the staff of National Taiwan University Hospital-Statistical Consulting Unit (NTUH-SCU) for statistical consultation and analyses.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Zarębska-Michaluk D S-Editor: Ma YJ L-Editor: A P-Editor: Wu RR
| 1. | Thomas DL. Global Elimination of Chronic Hepatitis. N Engl J Med. 2019;380:2041-2050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 2. | Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 3. | Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, Dusheiko G, Feld JJ, Gore C, Griswold MG, Hamid S, Hellard ME, Hou J, Howell J, Jia J, Kravchenko N, Lazarus JV, Lemoine M, Lesi OA, Maistat L, McMahon BJ, Razavi H, Roberts T, Simmons B, Sonderup MW, Spearman CW, Taylor BE, Thomas DL, Waked I, Ward JW, Wiktor SZ; Lancet Gastroenterology & Hepatology Commissioners. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 4. | Trickey A, Fraser H, Lim AG, Peacock A, Colledge S, Walker JG, Leung J, Grebely J, Larney S, Martin NK, Hickman M, Degenhardt L, May MT, Vickerman P. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4:435-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Martinello M, Amin J, Matthews GV, Dore GJ. Prevalence and Disease Burden of HCV Coinfection in HIV Cohorts in the Asia Pacific Region: A Systematic Review and Meta-Analysis. AIDS Rev. 2016;18:68-80. [PubMed] [Cited in This Article: ] |
| 6. | Zhou J, Dore GJ, Zhang F, Lim PL, Chen YM; TREAT Asia HIV Observational Database. Hepatitis B and C virus coinfection in The TREAT Asia HIV Observational Database. J Gastroenterol Hepatol. 2007;22:1510-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, Vogel M, Baumgarten A, Chaix ML, Fisher M, Gotz H, Matthews GV, Neifer S, White P, Rawlinson W, Pol S, Rockstroh J, Coutinho R, Dore GJ, Dusheiko GM, Danta M. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Wandeler G, Gsponer T, Bregenzer A, Günthard HF, Clerc O, Calmy A, Stöckle M, Bernasconi E, Furrer H, Rauch A; Swiss HIV Cohort Study. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55:1408-1416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Boesecke C, Grint D, Soriano V, Lundgren JD, d'Arminio Monforte A, Mitsura VM, Chentsova N, Hadziosmanovic V, Kirk O, Mocroft A, Peters L, Rockstroh JK; EuroSIDA in EuroCoord. Hepatitis C seroconversions in HIV infection across Europe: which regions and patient groups are affected? Liver Int. 2015;35:2384-2391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Sun HY, Uemura H, Wong NS, Chan DP, Wong BC, Lin PH, Su LH, Hung CC, Oka S, Chang SY, Lee SS. Molecular epidemiology of acute HCV infection in HIV-positive patients from Hong Kong, Taipei, Tokyo. Liver Int. 2019;39:1044-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ho SY, Su LH, Sun HY, Huang YS, Chuang YC, Huang MH, Liu WC, Su YC, Lin PH, Chang SY, Hung CC. Trends of recent hepatitis C virus infection among HIV-positive men who have sex with men in Taiwan, 2011-2018. EClinicalMedicine. 2020;24:100441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Chan DP, Sun HY, Wong HT, Lee SS, Hung CC. Sexually acquired hepatitis C virus infection: a review. Int J Infect Dis. 2016;49:47-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Sikavi C, Chen PH, Lee AD, Saab EG, Choi G, Saab S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population. Hepatology. 2018;67:847-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Brown RS Jr, Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, Horváth G, Zuckerman E, Carrion BR, Rodriguez-Perez F, Urbánek P, Abergel A, Cohen E, Lovell SS, Schnell G, Lin CW, Zha J, Wang S, Trinh R, Mensa FJ, Burroughs M, Felizarta F. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J Hepatol. 2020;72:441-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 15. | Rockstroh JK, Lacombe K, Viani RM, Orkin C, Wyles D, Luetkemeyer AF, Soto-Malave R, Flisiak R, Bhagani S, Sherman KE, Shimonova T, Ruane P, Sasadeusz J, Slim J, Zhang Z, Samanta S, Ng TI, Gulati A, Kosloski MP, Shulman NS, Trinh R, Sulkowski M. Efficacy and Safety of Glecaprevir/Pibrentasvir in Patients Coinfected With Hepatitis C Virus and Human Immunodeficiency Virus Type 1: The EXPEDITION-2 Study. Clin Infect Dis. 2018;67:1010-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Wyles D, Bräu N, Kottilil S, Daar ES, Ruane P, Workowski K, Luetkemeyer A, Adeyemi O, Kim AY, Doehle B, Huang KC, Mogalian E, Osinusi A, McNally J, Brainard DM, McHutchison JG, Naggie S, Sulkowski M; ASTRAL-5 Investigators. Sofosbuvir and Velpatasvir for the Treatment of Hepatitis C Virus in Patients Coinfected With Human Immunodeficiency Virus Type 1: An Open-Label, Phase 3 Study. Clin Infect Dis. 2017;65:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Liu CH, Sun HY, Hsieh SM, Liu WC, Sheng WH, Liu CJ, Su TH, Tseng TC, Chen PJ, Hung CC, Kao JH. Evolution of estimated glomerular filtration rate in human immunodeficiency virus and hepatitis C virus-coinfected patients receiving sofosbuvir-based direct-acting antivirals and antiretroviral therapy. J Viral Hepat. 2021;28:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Liu CH, Chen PY, Chen JJ, Lo CC, Su WW, Tseng KC, Liu CJ, Huang CS, Huang KJ, Yang SS, Peng CY, Tsai MC, Kao WY, Chang CY, Shih YL, Fang YJ, Chen CY, Lee PL, Huang JJ, Su PY, Tseng CW, Hung CC, Chang CH, Huang YJ, Lai HC, Chang CC, Lee FJ, Hsieh TY, Kao JH. Sofosbuvir/velpatasvir for patients with chronic hepatitis C virus infection and compensated liver disease: real-world data in Taiwan. Hepatol Int. 2021;15:338-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Rockstroh J, Boesecke C. Treatment of acute hepatitis C in HIV coinfection: Is this a chance for achieving microelimination? United European Gastroenterol J. 2019;7:465-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Heffernan A, Cooke GS, Nayagam S, Thursz M, Hallett TB. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 21. | Kao JH. Hepatitis C virus infection in Taiwan: Past, present, and future. J Formos Med Assoc. 2016;115:65-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Liu CH, Sheng WH, Sun HY, Hsieh SM, Lo YC, Liu CJ, Su TH, Yang HC, Liu WC, Chen PJ, Chen DS, Hung CC, Kao JH. Peginterferon plus Ribavirin for HIV-infected Patients with Treatment-Naïve Acute or Chronic HCV Infection in Taiwan: A Prospective Cohort Study. Sci Rep. 2015;5:17410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Liu CH, Sun HY, Liu CJ, Sheng WH, Hsieh SM, Lo YC, Liu WC, Su TH, Yang HC, Hong CM, Tseng TC, Chen PJ, Chen DS, Hung CC, Kao JH. Generic velpatasvir plus sofosbuvir for hepatitis C virus infection in patients with or without human immunodeficiency virus coinfection. Aliment Pharmacol Ther. 2018;47:1690-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Janjua NZ, Kuo M, Yu A, Alvarez M, Wong S, Cook D, Wong J, Grebely J, Butt ZA, Samji H, Ramji A, Tyndall M, Krajden M. The Population Level Cascade of Care for Hepatitis C in British Columbia, Canada: The BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine. 2016;12:189-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 26. | Simmons R, Ireland G, Irving W, Hickman M, Sabin C, Ijaz S, Ramsay M, Lattimore S, Mandal S. Establishing the cascade of care for hepatitis C in England-benchmarking to monitor impact of direct acting antivirals. J Viral Hepat. 2018;25:482-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Cachay ER, Hill L, Wyles D, Colwell B, Ballard C, Torriani F, Mathews WC. The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS One. 2014;9:e102883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Zuckerman A, Douglas A, Nwosu S, Choi L, Chastain C. Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One. 2018;13:e0199174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Lazarus JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM, Hatzakis A, Jadoul M, Prestileo T, Razavi H, Rockstroh JK, Wiktor SZ, Colombo M. The Micro-Elimination Approach to Eliminating Hepatitis C: Strategic and Operational Considerations. Semin Liver Dis. 2018;38:181-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 30. | Busschots D, Kremer C, Koc ÖM, Heyens L, Bielen R, Apers L, Florence E, Messiaen P, Van Laethem K, Van Wijngaerden E, Nevens F, Hens N, Robaeys G. The hepatitis C cascade of care in the Belgian HIV population: One step closer to elimination. Int J Infect Dis. 2021;105:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Bartlett SR, Yu A, Chapinal N, Rossi C, Butt Z, Wong S, Darvishian M, Gilbert M, Wong J, Binka M, Alvarez M, Tyndall M, Krajden M, Janjua NZ. The population level care cascade for hepatitis C in British Columbia, Canada as of 2018: Impact of direct acting antivirals. Liver Int. 2019;39:2261-2272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Sacks-Davis R, Doyle JS, Rauch A, Beguelin C, Pedrana AE, Matthews GV, Prins M, van der Valk M, Klein MB, Saeed S, Lacombe K, Chkhartishvili N, Altice FL, Hellard ME. Linkage and retention in HCV care for HIV-infected populations: early data from the DAA era. J Int AIDS Soc. 2018;21 Suppl 2:e25051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Béguelin C, Suter A, Bernasconi E, Fehr J, Kovari H, Bucher HC, Stoeckle M, Cavassini M, Rougemont M, Schmid P, Wandeler G, Rauch A; Swiss HIV Cohort Study. Trends in HCV treatment uptake, efficacy and impact on liver fibrosis in the Swiss HIV Cohort Study. Liver Int. 2018;38:424-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Iversen J, Grebely J, Catlett B, Cunningham P, Dore GJ, Maher L. Estimating the cascade of hepatitis C testing, care and treatment among people who inject drugs in Australia. Int J Drug Policy. 2017;47:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Morris MD, Mirzazadeh A, Evans JL, Briceno A, Coffin P, Hahn JA, Page KA. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: Low number treated. Drug Alcohol Depend. 2019;198:133-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Ireland G, Simmons R, Hickman M, Eastwood B, Ramsay M, Mandal S. Mapping the hepatitis C cascade of care in people attending drug treatment services in England: A data linkage study. Int J Drug Policy. 2019;72:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Newsum AM, Matser A, Schinkel J, van der Valk M, Brinkman K, van Eeden A, Lauw FN, Rijnders BJA, van de Laar TJW, van de Kerkhof M, Smit C, Boyd A, Arends JE, Prins M; MSM Observational Study of Acute Infection with hepatitis C (MOSAIC) study group. Incidence of HCV Reinfection Among HIV-Positive MSM and Its Association With Sexual Risk Behavior: A Longitudinal Analysis. Clin Infect Dis. 2021;73:460-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 38. | Huang MH, Chang SY, Liu CH, Cheng A, Su LH, Liu WC, Su YC, Sun HY, Hung CC, Chang SC. HCV reinfections after viral clearance among HIV-positive patients with recent HCV infection in Taiwan. Liver Int. 2019;39:1860-1867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Ingiliz P, Martin TC, Rodger A, Stellbrink HJ, Mauss S, Boesecke C, Mandorfer M, Bottero J, Baumgarten A, Bhagani S, Lacombe K, Nelson M, Rockstroh JK; NEAT study group. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2017;66:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |