Published online Jan 21, 2020. doi: 10.3748/wjg.v26.i3.366
Peer-review started: October 13, 2019
First decision: December 5, 2019
Revised: December 13, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 21, 2020
Despite an expanding number of studies on intraductal papillary neoplasm of the bile duct (IPNB), distant metastasis remains unexplained especially in cases of carcinoma in situ. In the present study, we report a rare and interesting case of IPNB without invasive components that later metastasized to lungs and brain.
A 69-year-old male was referred to our hospital due to suspected cholangiocarcinoma. Laboratory tests on admission reported a mild elevation of alkaline phosphatase, γ-glutamyl transpeptidase, and total bilirubin in serum. Endoscopic retrograde cholangiography revealed a filling defect in the common bile duct (CBD) extending to the left hepatic duct. Peroral cholangioscopy delineated a tumor in the CBD that had a papillary pattern. Multidetector computed tomography and magnetic resonance cholangiopancreatography detected partial blockage ot interlude in the CBD leading to cholestasis without evidence of metastasis. Therefore, a diagnosis of IPNB cT1N0M0 was established. Left hepatectomy with bile duct reconstruction was performed. Pathological examination confirmed an intraepithelial neoplasia pattern without an invasive component and an R0 resection achievement. The patient was monitored carefully by regular examinations. However, at 32 mo after the operation, a 26 mm tumor in the lungs and a 12 mm lesion in the brain were detected following a suspicious elevated CA 19-9 level. Video-assisted thoracoscopic surgery of left upper lobectomy and stereotactic radiotherapy are indicated. In addition to histopathological results, a genomic profiling analysis using whole exome sequencing subsequently confirmed lung metastasis originating from bile duct cancer.
This case highlights the important role of genomic profiling analysis using whole exome sequencing in identifying the origin of metastasis in patients with IPNB.
Core tip: An intraductal papillary neoplasm of the bile duct without an invasive component has unexpectedly metastasized to the brain and lungs. In addition to histopathological results, a genomic profiling analysis using whole exome sequencing subsequently confirmed lung metastasis originating from bile duct cancer by detecting 100 single nucleotide variants and 168 insertion/deletions.
- Citation: Nam NH, Taura K, Kanai M, Fukuyama K, Uza N, Maeda H, Yutaka Y, Chen-Yoshikawa TF, Muto M, Uemoto S. Unexpected metastasis of intraductal papillary neoplasm of the bile duct without an invasive component to the brain and lungs: A case report. World J Gastroenterol 2020; 26(3): 366-374
- URL: https://www.wjgnet.com/1007-9327/full/v26/i3/366.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i3.366
Intraductal papillary neoplasm of the bile duct (IPNB) has recently obtained interest in the field of hepato-biliary-pancreatic surgery. This rare lesion is predominant in Asian countries, such as Japan, Taiwan and Korea, where hepatolithiasis and clonorchiasis are endemic[1]. Since IPNB was recognized as a distinct pathological entity by the World Health Organization in the 2010 Classification of Tumors of the Digestive System[2], this condition has been identified by the predominance of biliary neoplasms accompanied by papillary or villous proliferation inside a dilated bile duct, with or without macroscopically visible mucin secretion. In reference to histomorphology and the immunophenotypical profile, four phenotypes of epithelium have been categorized, including pancreaticobiliary, intestinal, gastric and oncocytic types[3]. From a systematic review and meta-analysis, Gordon et al[4] identified an invasive component in 43% of 476 patients, suggesting a high potential for malignancy. Despite an expanding number of studies on IPNB, distant metastasis remains a mysterious question, especially in cases of carcinoma in situ. In the present study, we report a rare and interesting case of IPNB without invasive components that metastasized to the lungs and brain.
A 69-year-old male with a body mass index of 21.5 kg/m2 was referred to our hospital due to jaundice for a suspected cholangiocarcinoma.
His past medical and surgical history was unremarkable, except for diabetes type 2 and hypertension; both conditions were controlled with medications, including metformin 500 mg twice a day, glimepiride 1 mg twice a day, nifedipine CR 20 mg daily, valsartan 80 mg daily and enalapril 5 mg daily.
His social history was significant for smoking 20 cigarettes per day for 46 years and the frequent intake of alcohol at approximately 360-540 mL a day.
Family history was notable for a father diagnosed with esophageal cancer, a mother with uterine cancer and an elderly brother with duodenal cancer and leukemia.
On examination, the patient had icteric sclera and pale conjunctiva. His vital signs were stable, and physical examination did not reveal any extraordinary signs.
Laboratory tests on admission were within the normal range, except for a mild elevation in the levels of alkaline phosphatase (507 IU/L; normal range, < 359 IU/L), γ-glutamyl transpeptidase (199 IU/L; normal range, < 47 IU/L) and total serum bilirubin (1.4 mg/dL normal range, < 1 mg/dL). Carcinoembryonic antigen and CA 19-9 were within the normal range (2.7 ng/mL, 13.6 U/mL, respectively). Serology was negative for hepatitis B and C infections.
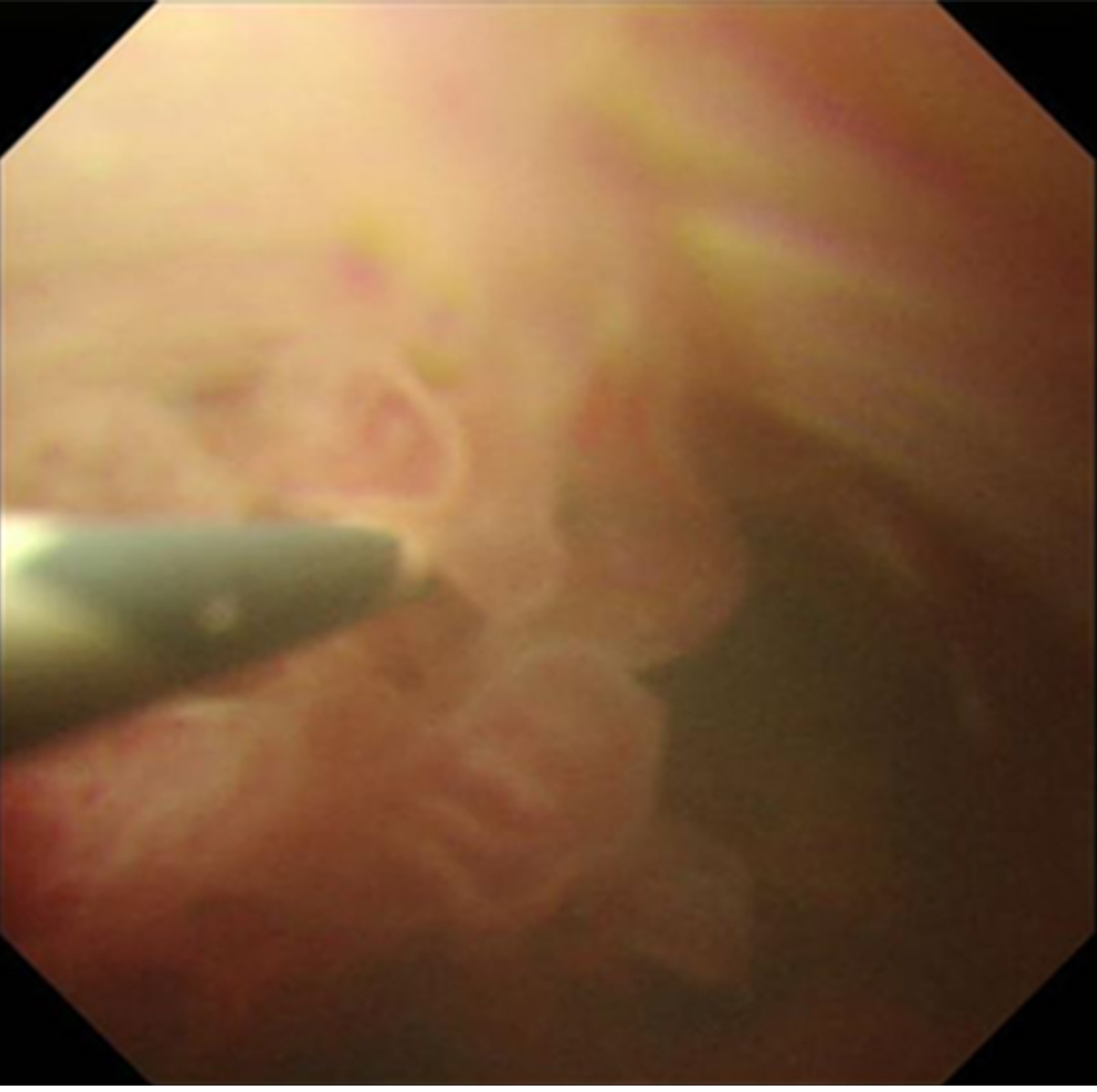
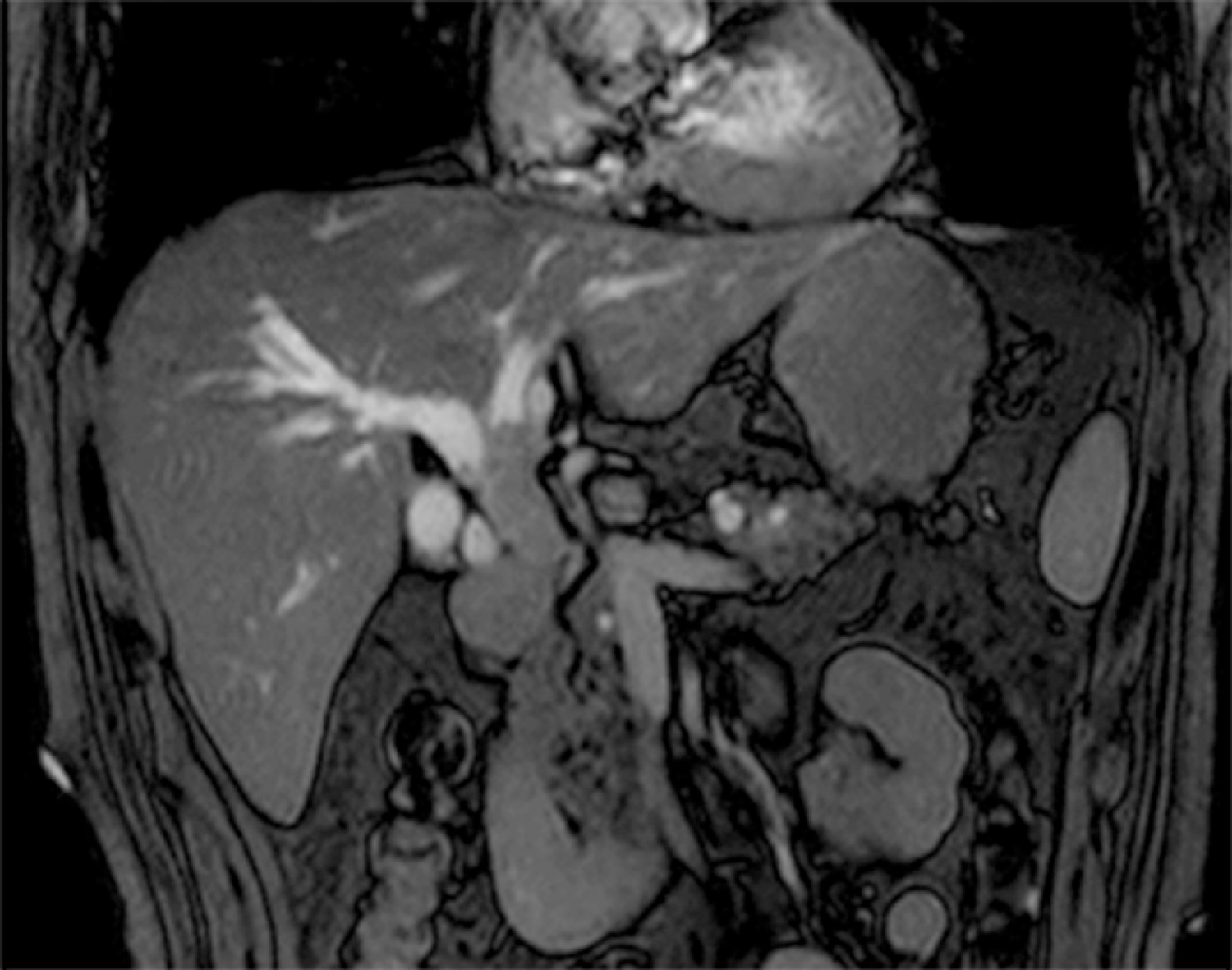
Endoscopic ultrasonography detected a solid lesion filling the intrapancreatic CBD to the main biliary. There was no evidence of extension outside of the bile duct wall, and invasion into the right hepatic artery was not observed. In addition, multilocular and unilateral cystic lesions of the pancreas, which had a maximum diameter of 13.1 mm, were found. Endoscopic retrograde cholangiography revealed bilateral intrahepatic biliary tree dilatation with a filling defect in the upper and middle bile duct, which extended partly to the left hepatic duct. Intraductal ultrasonography confirmed that there was no extended lesion in the right bile duct. Tumor and adjacent tissue biopsies were performed, and a 5 Fr endoscopic nasobiliary drainage ENBD was placed to facilitate biliary drainage. Pathological results endorsed IPNB with high grade and intestinal type neoplasia. In addition, images obtained from peroral cholangioscopy POCS delineated a tumor with a papillary pattern located at the CBD to the left hepatic duct (Figure 1). Multidetector computed tomography detected an interruption for a long segment of CBD causing biliary occlusion, which resulted in bilateral intrahepatic biliary tree dilatation with no intrahepatic lesion. The tumor was predominantly extended into the left hepatic duct. An enlargement of the pancreatic duct suggested the extension of injury into the intrapancreatic CBD. Traces of metastasis, such as lymph node growth, ascites, lung mass or pleural effusion, have not been reported. Three-dimensional liver surgery simulation demonstrated that the right hepatic artery and right portal vein were not in contact with the tumor. Mmagnetic resonance cholangiopancreatography confirmed these findings (Figure 2).
Therefore, an initial diagnosis of IPNB cT1N0M0 was established with good prognosis.
The final diagnosis of the presented case is metastasis of IPNB without an invasive component to the brain and lungs.
The remnant liver was estimated at 63.1% of its original size after left hemihepatectomy, including the caudate lobe, extrahepatic bile duct resection with Roux-en-Y hepaticojejunostomy and regional lymph node dissection. During the operation, the tumor was not exposed to the serosa, and severe diffuse liver steatosis, including a small amount of ascites, was reported. Rapid ascites cytology, para aortic lymph node, and stumps of bile duct in frozen section were intraoperatively negative for cancer. The duration of the operation was 647 min, with blood loss amounting to 360 g without blood transfusion. Intraoperative cholangiography confirmed no evidence of bile leakage.
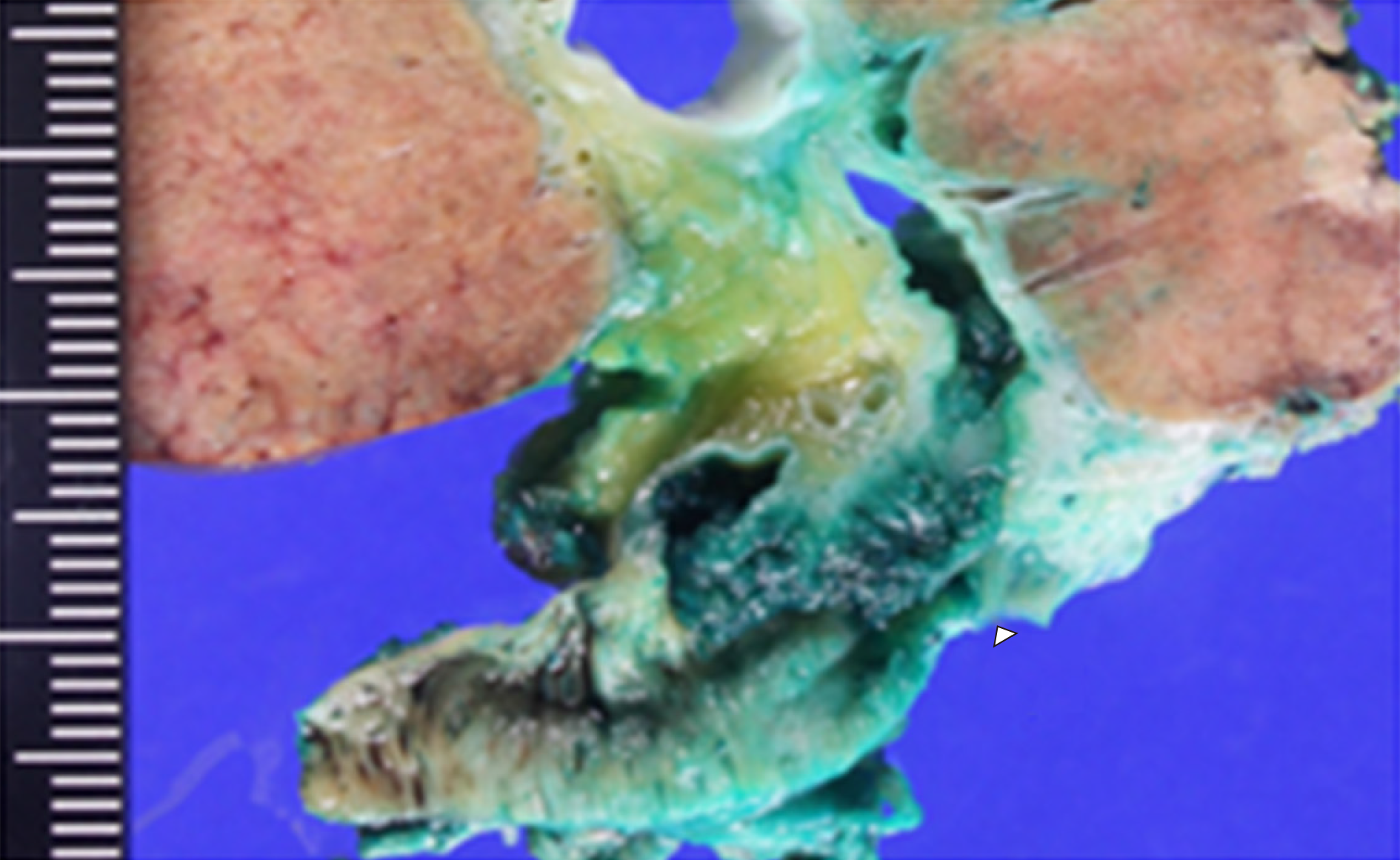
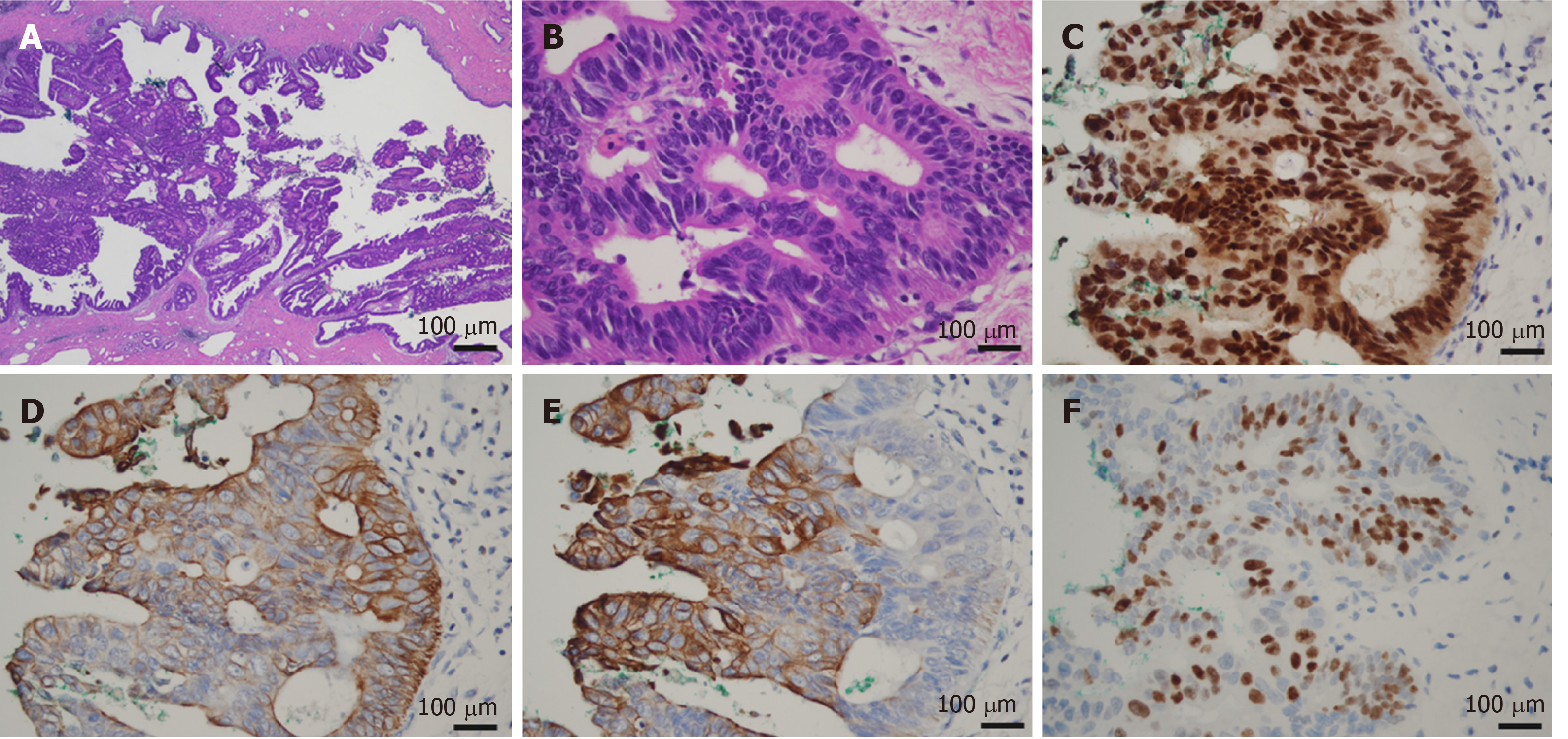
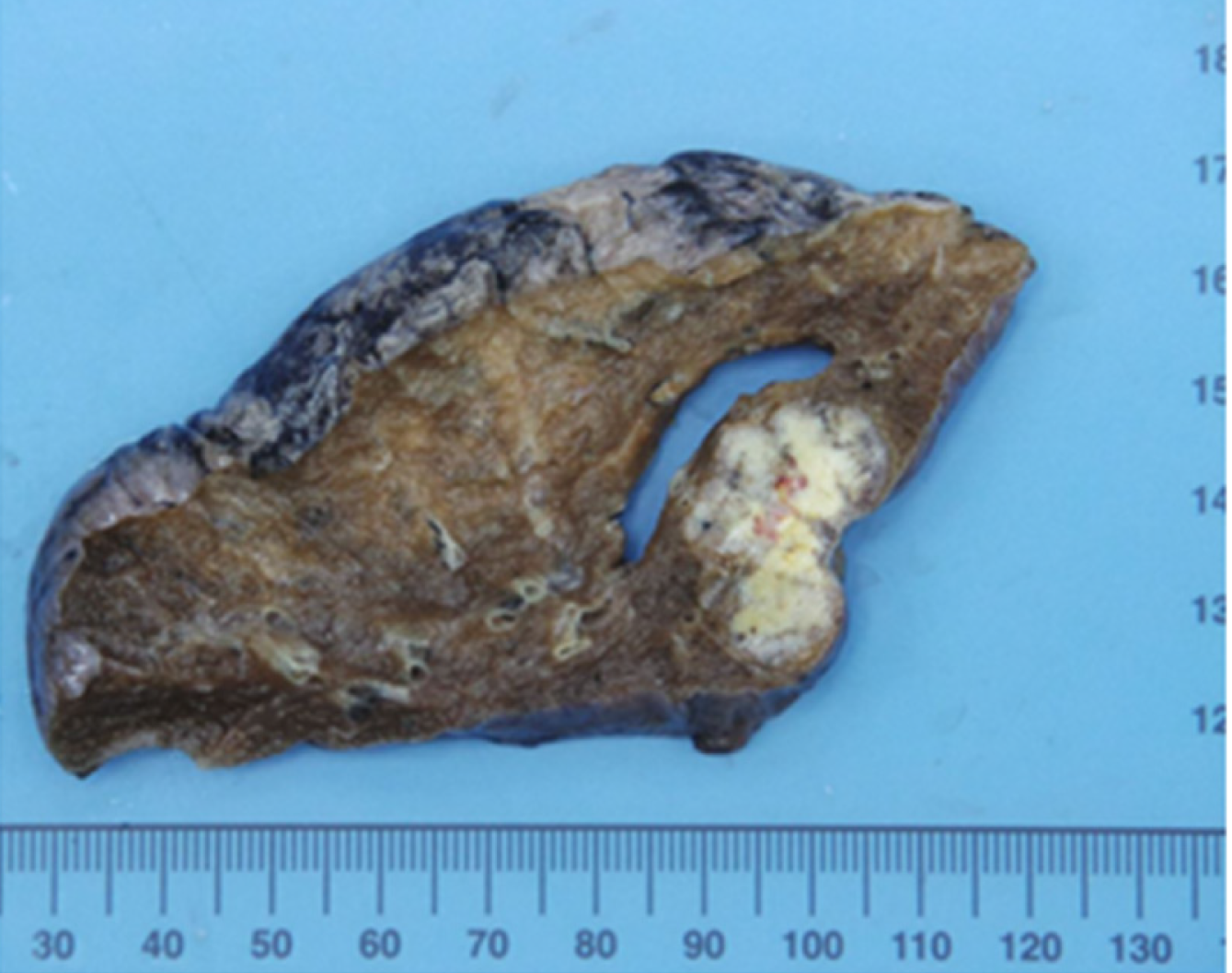
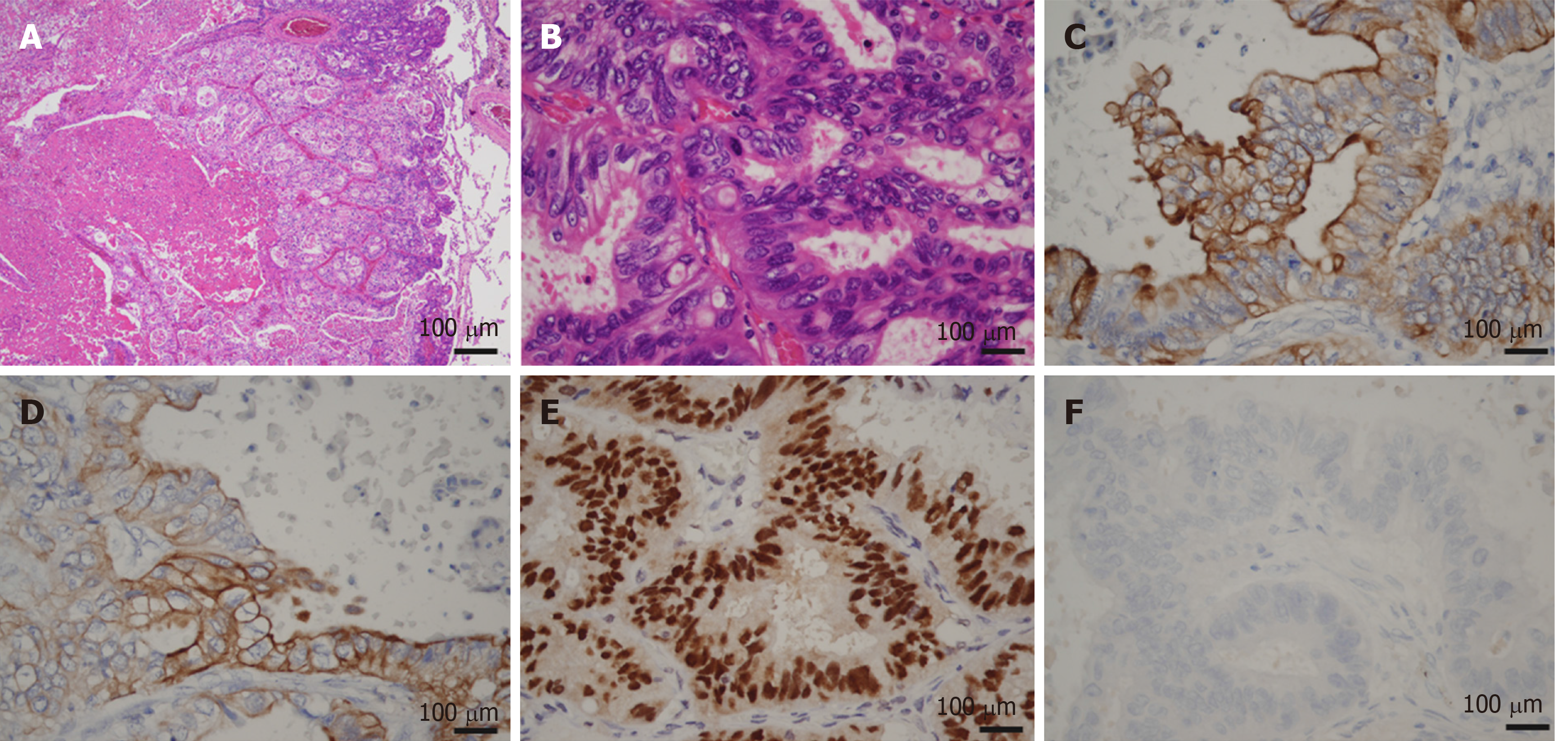
Macroscopic examination of the resected specimen revealed a papillary tumor measuring 22 mm × 10 mm expanding the CBD and the left hepatic bile duct (Figure 3). Microscopic findings showed the papillary neoplasm confined within the bile duct. The intraductal neoplastic growth was a cribriform and tubular pattern and showed no infiltration into the basal ductal wall. The neoplastic epithelial cells revealed nuclear atypia and stratification. Histological examination confirmed the diagnosis of IPNB with high-grade intraepithelial neoplasia (carcinoma in situ) and no regional lymph node metastases (pTis pN0) (Figure 4). Immunohistochemical staining showed that the tumor cells were positive for CK7, CK20 and CDX2. The surgical margin was negative, and no malignant findings were observed in the gallbladder.
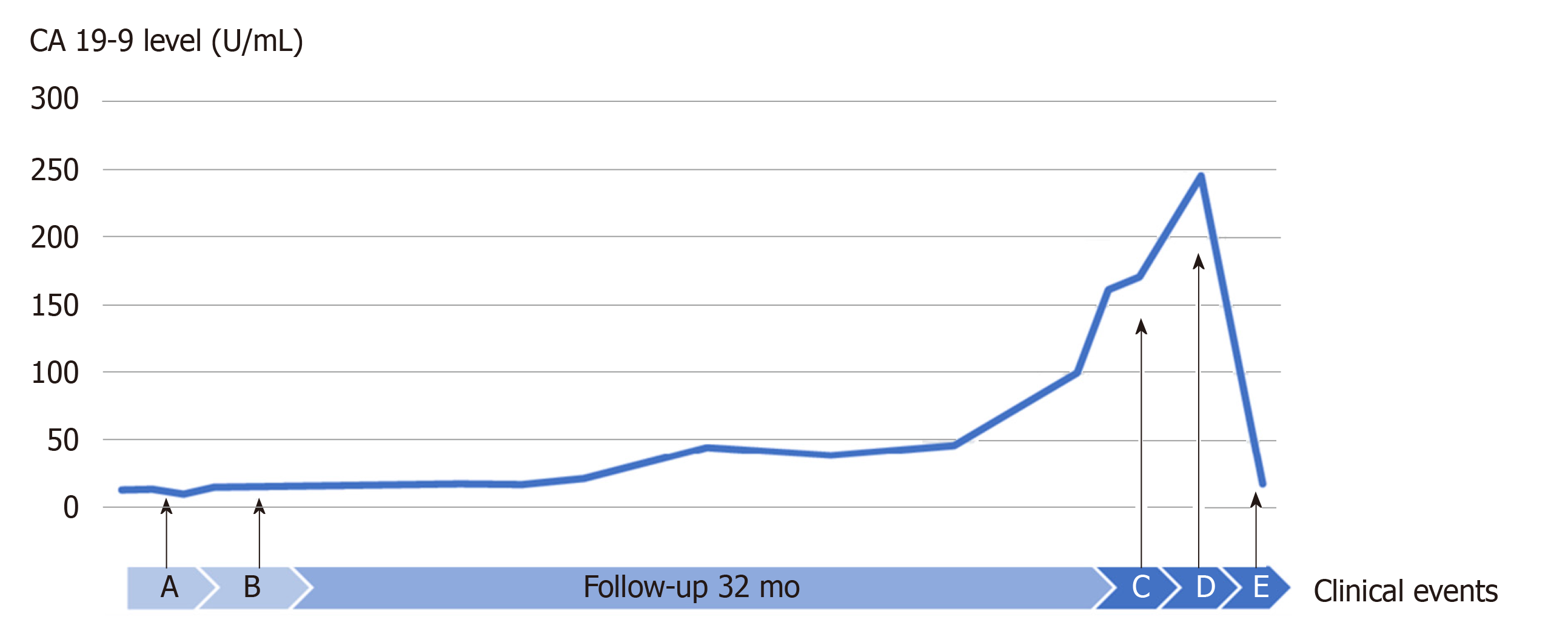
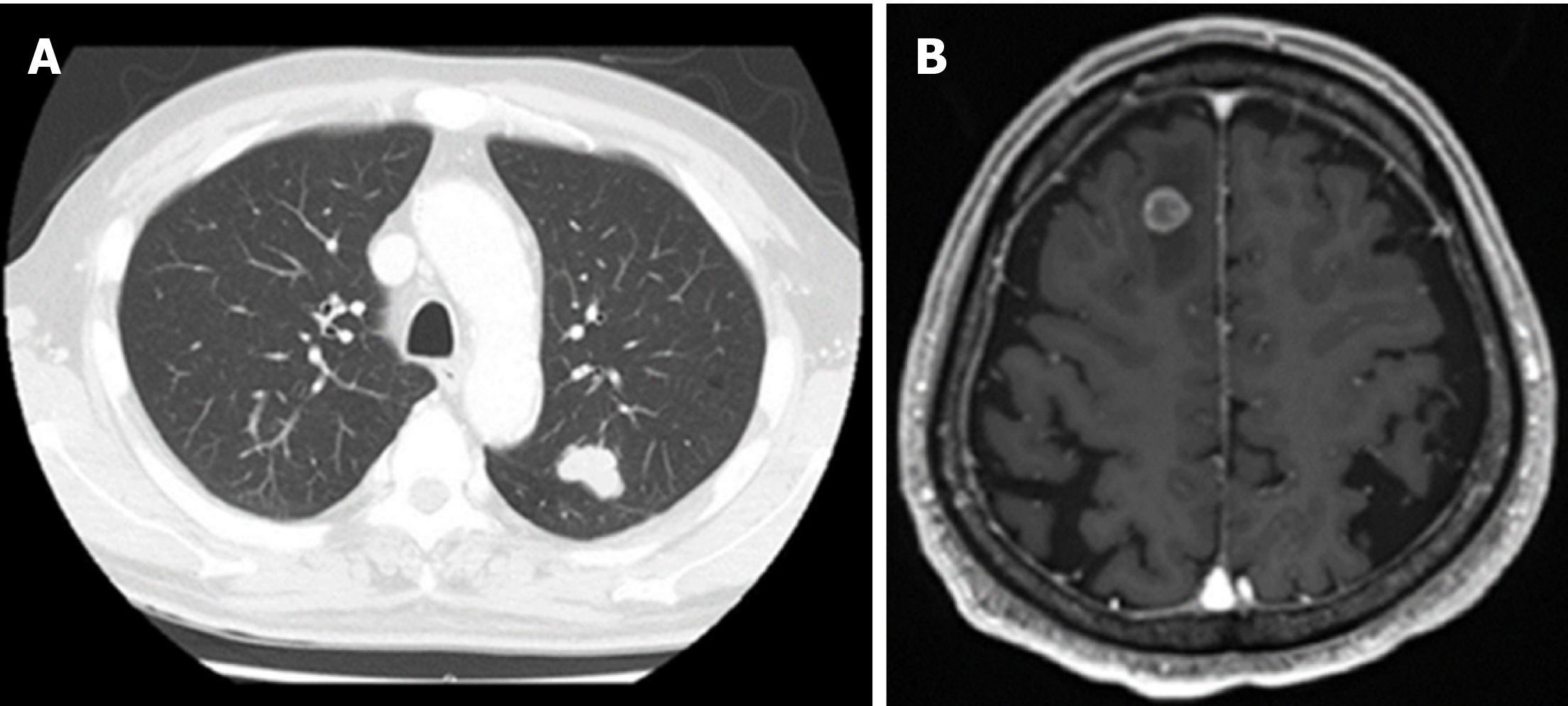
The postoperative course showed a high-grade fever caused by acute cholangitis, which was successfully treated by the intravenous administration of teicoplanin and piperacillin/tazobactam for 6 d and was classified as Clavien-Dindo complication grade II. Glycemia and blood pressure were effectively controlled during the perioperative time by insulin therapy and antihypertensive drugs. The patient was discharged at 31 d after surgery. The patient did not receive further supportive treatment and was monitored at regular intervals through clinical examination, biochemical investigations and imaging diagnosis. Unfortunately, at 32 mo after the operation, a 26 mm tumor in the left upper lobe lung field was detected following a suspicious elevation of CA 19-9 (Figure 5), and a suspected metastasis 12 mm brain tumor in the right frontal lobe was determined (Figure 6). The initial diagnosis was confidently proposed as primary lung cancer with brain metastasis (cT1bN0M1b). Lung tumor markers, such as squamous cell carcinoma antigen, neuron-specific enolase, sialyl stage-specific embryonic antigen-I and cytokeratin 19 fragment (CYFRA 21-1), were within the normal range.
Video-assisted thoracoscopic surgery of the left upper lobectomy and stereotactic radiotherapy for brain tumor given as dose of 44.2 Gy in 13 fractions are indicated. The postoperative course was uneventful, and he was discharged on postoperative day 10. The gross findings of the lung tumor revealed well-circumscribed yellowish-white nodules located in the peripheral lung (Figure 7). Histopathological examination showed a well-demarcated nodule with marked necrosis in the center of the tumor (Figure 8). At the high power field, the tumor exhibited a cribriform pattern consisting of eosinophilic, tall, columnar cells with nuclear pseudostratification. Immunohistological staining showed that the tumor cells were positive for CK7, CK20 and CDX2 and negative for TTF-1, which was the same profile as the primary bile duct lesion. The lung tumor was diagnosed as metastatic adenocarcinoma of a bile duct origin. Subsequently, a genomic profiling analysis using whole exome sequencing (WES) with paired tissue and blood samples was performed (Riken Genesis Co., LTD., Tokyo, Japan) to investigate common variants between two specimens. We obtained informed consent from the patient for undergoing WES tests. A comparison between primary and lung metastatic tumors revealed 100 somatic single nucleotide variants (SNVs) and 168 insertion/deletions (InDels) that were common in the two tumors (Supplementary table 1). We annotated the variants detected by WES using SnpEff (http://snpeff.sourceforge.net/SnpEff.html)[5]. This result confirmed lung metastasis originating from bile duct cancer. The patient currently remains in a stable condition without recurrence and continues to receive treatment with S-1.
Distant metastasis after an antecedent diagnosis of high grade intraepithelial neoplasm is extraordinarily illogical and infrequent IPNB is a rare tumor characterized by a lesion protruding into the biliary lumen, which might cause cholestasis. According to the degree of dysplasia and depth of invasion, IPNB is classified into four stages: (stage 1) IPNB with low-to-intermediate grade dysplasia, (stage 2) IPNB with high-grade dysplasia, (stage 3) intraductal growth-type cholangiocarcinoma, American Joint Committee on Cancer stage T1 and (stage 4) intraductal growth-type cholangiocarcinoma, American Joint Committee on Cancer stage T2 or higher[1]. As without an invasive component and evidence of metastasis, our present case was defined as an early stage of IPNB, which has a good prognostic value through radical resection. Regrettably, after more than two years of careful follow-up, distant metastasis was revealed without primary site recurrence and accompanied by an incomprehensible malignancy mechanism. Thus, two potential hypotheses were proposed: (1) Pulmonary enteric adenocarcinoma, a very rare type of primary lung cancer that shares some identical components with colorectal carcinoma and is difficult to discriminate from the metastasis of intestinal adenocarcinoma, even with immunohistochemical staining; and (2) Escape of the invasive components from the observation of the pathologist due to intervals between the adjacent slices. In the second supposition, even if it occurs, the dimension of the lesion should be restricted and smaller than the intervals between the adjacent slices (usually 3-4 mm). Another situation that could have happened is that a skip lesion escaping from our observation metastasized to the lungs and brain. However, this scenario also appears unlikely as the skip lesion should have developed unobstrusively in small dimension enough to escape from our observation and should have rarely metastasized.
This study is a very rare report of IPNB without locoregional lymph node metastasis and extension to distant organs. In retrieving the current literature, a unique case report by Yoshida et al[6] delineated an advanced stage of IPNB with metastases to the ovary and lymph nodes. Basically, IPNB was considered less malignant than conventional bile duct cholangi-ocarcinoma[1]. Far fewer lymph node metastases were identified in patients with IPNB than in those with cholangiocarcinoma[7]. Strikingly, a single center retrospective study over 16 years by Wu et al[8] revealed only three metastatic lymph nodes in one patient among 88 harvested lymph nodes in a series of 28 patients with IPNB. Fundamentally, the detention of neoplastic cells by the entire basal lamina layer prevents the risk of metastasis. Despite a favorable prognosis, such as R0 resection achievement, an intraepithelial neoplasia pattern without an invasive component, no evidence of extended metastasis, negative metastatic lymph node and ascites cytology, our patient suffered from an unpredictable metastasis to the lungs and brain. This phenomenon seems to imply a complex mechanism that defies a simple explanation. Lymphatic permeation, blood circulation, perineural invasion and intestinal subtype may somehow suggest an answer. Using WES, the detection of shared 100 somatic SNVs and 168 InDels confirmed the diagnosis of metastatic lung tumor from IPNB. Thus, all physicians are forewarned to engage in extensive research toward cancer metastasis.
Another important clinical implication of this study was the association between variations in the CA 19-9 level and metastasis. At the time of lung and brain metastasis detection, the CA 19-9 level was four-fold higher than the baseline value. Subsequently, in performing VATS lobectomy and SRT, CA 19-9 level returned to the normal range. Luvira V et al[9] found that the serum CA 19-9 level was a significant prognostic factor for malignant IPNB. In addition, Yeh et al[10] observed an enhanced serum CA 19-9 level in 35% of benign lesions, whereas that in malignant lesions was 61%. Furthermore, Lee et al[11] demonstrated a higher mean CA 19-9 level in IPNB with mucin hypersecretion. Thus, CA 19-9 screening during follow-up should be valuable for the detection of extended lesions.
In conclusion, we report a distant and simultaneous metastasis (brain and lungs) of an extrahepatic IPNB case, which was diagnosed as high grade intraepithelial neoplasia (T1N0M0) and was rigorously treated with curative resection (R0 resection without malignant lymph node). Regardless of the rarity of this lesion, our case report raises the following question: What is the actual mechanism of distant metastasis in the early stage of IPNB without an invasive component? Further studies should be performed to elucidate this unusual phenomenon.
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Viswanath Y S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 108] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press 2010; 417. [Cited in This Article: ] |
| 3. | Choi SC, Lee JK, Jung JH, Lee JS, Lee KH, Lee KT, Rhee JC, Jang KT, Choi SH, Heo JS, Choi DW, Lim JH. The clinicopathological features of biliary intraductal papillary neoplasms according to the location of tumors. J Gastroenterol Hepatol. 2010;25:725-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Gordon-Weeks AN, Jones K, Harriss E, Smith A, Silva M. Systematic Review and Meta-analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates. Ann Surg. 2016;263:656-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6:80-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6032] [Cited by in F6Publishing: 6679] [Article Influence: 556.6] [Reference Citation Analysis (0)] |
| 6. | Yoshida Y, Ajiki T, Ueno K, Ohtsubo I, Murakami S, Shinozaki K. Advanced Intraductal Papillary Neoplasm of Bile Duct (IPNB) in a young female with metastases to lymph nodes and ovary. Tando. 2012;231-236. [DOI] [Cited in This Article: ] |
| 7. | Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Wu X, Li B, Zheng C, Chang X, Zhang T, He X, Zhao Y. Intraductal papillary neoplasm of the bile duct: a single-center retrospective study. J Int Med Res. 2018;46:4258-4268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Luvira V, Pugkhem A, Bhudhisawasdi V, Pairojkul C, Sathitkarnmanee E, Luvira V, Kamsa-Ard S. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J Gastroenterol Hepatol. 2017;32:527-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Yeh TS, Tseng JH, Chen TC, Liu NJ, Chiu CT, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma of the intraductal growth-type and its precursor lesions. Hepatology. 2005;42:657-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E, Lee SG, Min YI. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |