Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7292
Revised: August 28, 2006
Accepted: September 16, 2006
Published online: December 7, 2006
AIM: To investigate the effect of transplanted fetal liver epithelial progenitor (FLEP) cells on liver fibrosis in mice.
METHODS: FLEP cells were isolated from embryonal day (ED) 14 BALB/c mice and transplanted into female syngenic BALB/c mice (n = 60). After partial hepatectomy (PH), diethylnitrosamine (DEN) was administered to induce liver fibrosis. Controls received FLEP cells and non-supplemented drinking water, the model group received DEN-spiked water, and the experimental group received FLEP cells and DEN. Mice were killed after 1, 2, and 3 mo, and alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid (HA), and laminin (LN) in serum, and hydroxyproline (Hyp) content in liver were assessed. Alpha-smooth muscle actin (α-SMA) of liver was tested by immunohistochemistry. Transplanted male mice FLEP cells were identified by immunocytochemistry for sry (sex determination region for Y chromosome) protein.
RESULTS: Serum ALT, AST, HA, and LN were markedly reduced by transplanted FLEP cells. Liver Hyp content and α-SMA staining in mice receiving FLEP cells were lower than that of the model group, which was consistent with altered liver pathology. Transplanted cells proliferated and differentiated into hepatocytes and bile duct epithelial cells with 30%-50% repopulation in the liver fibrosis induced by DEN after 3 mo.
CONCLUSION: Transplanted FLEP cells proliferate and differentiate into hepatocytes and bile duct epithelial cells with high repopulation capacity in the fiberized liver induced by DEN, which restores liver function and reduces liver fibrosis.
- Citation: Zheng JF, Liang LJ, Wu CX, Chen JS, Zhang ZS. Transplantation of fetal liver epithelial progenitor cells ameliorates experimental liver fibrosis in mice. World J Gastroenterol 2006; 12(45): 7292-7298
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7292
The incidence of hepatic injury and end-stage liver fibrosis is high in China. Cirrhosis represents a serious health care problem worldwide. The prognosis of patients with the disease is poor, although liver transplantation is a successful treatment for end-stage cirrhosis[1]. Counteracting the success of liver transplantation are problems such as lack of donors, operative damage, rejection, and high costs[2]. As a result, novel effective therapies are needed urgently.
Hepatocyte transplantation has been proposed as an alternative to whole organ transplantation[3]. It not only provides temporary liver function in patients waiting for liver transplantation but has also shown to be curative in certain metabolic conditions[4,5]. However, hepatocyte transplantation has very rarely produced therapeutic effects in human clinical trials, mainly because the number of transplanted hepatocytes is too small to achieve a biological effect[6]. Moreover, hepatocytes are quiescent cells that are difficult to maintain in culture and to cryopreserve.
Progenitor or facultative stem cells reside within or adjacent to the canals of Hering and comprise a quiescent compartment of dormant cells in adult livers. They can be activated to proliferate and differentiate into hepatocytes or bile duct epithelial cells when hepatocytes are impaired persistently[7]. Attempts have been made to identify their counterpart in fetal liver, and it has been suggested that the dormant stem-like cells originate most probably from bipotential fetal liver epithelial progenitor (FLEP) cells[8,9]. It was reported that embryonal day (ED) 14 rat liver contains a subpopulation of bipotent epithelial cells that can differentiate into hepatocytes or bile duct epithelial cells[10-12]. These cells have characteristics of hepatic stem cell, self-renewal potential, and multiple differentiation capability to selectively repopulate the liver. FLEP cell transplantation could offer prospects of novel therapeutic strategies for patients with chronic liver disease or cirrhosis. However, there have been very few reports on the ability of these cells to reduce liver fibrosis after their transplantation.
This study aims to isolate and transplant FLEP cells into the liver of syngenic mice to explore the ability of FLEP cells to proliferate and differentiate into hepatocytes and bile duct epithelial cells and restore impaired liver function and liver fibrosis.
Six to eight week old BALB/c mice were purchased from the Animal Breeding Center of Sun Yat-Sen University (Guangzhou, China). Mice were bred and maintained in an air-conditioned animal house with specific pathogen-free conditions, using an alternate 12 h cycle of daylight and darkness, and unlimited access to chow and water. All animal handling and experimental procedures were all in accordance with the Guidelines of the Animal Care and Use Committee at Sun Yat-Sen University.
FLEP cells were isolated on ED 14 from normal pregnant mice as described previously[8]. The cells were suspended at a concentration of 107 cells/ml and cell viability (> 95%) was measured by trypan blue dye exclusion. The isolated FLEP cells were considered to be composed of 50% male cells and 50% female cells.
Anesthesia was performed with ether and partial hepatectomy (PH) was undertaken using the standard method for two-third resection[11]. Briefly, after ligation of the pedicule and resection of the two largest lobes (median and left), the remaining liver was composed of the caudate and epiploic lobes. Freshly isolated ED 14 FLEP cells were injected into the female liver via the superior mesenteric vein using insulin syringes after hepatectomy[13]. A total of 1 × 106 cells were injected per mouse.
Approximately 2 × 105 FLEP cells were harvested and resuspended in 1 ml phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). The cell suspension was incubated with goat anti-CK-19 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-AFP polyclonal antibody (Santa Cruz Biotechnology), or rabbit anti-albumin polyclonal antibody (Dako; Glostrup, Denmark), for 40 min at 4°C, then washed three times, and fixed in 2% paraformaldehyde. FITC-conjugated secondary antibody or PE-conjugated secondary antibody was used. FACScan was used for flow cytometry analysis (Becton-Dickinson, San Jose, CA) as previously reported[14].
After partial hepatectomy and FLEP cells injection, mice were allowed to recover for one week. Thereafter, DEN (Sigma-Aldrich, St. Louis, MO) was continuously administered in drinking water at a final concentration of 100 μg/L for 12 wk[15]. The weight of the animals was carefully monitored during DEN administration.
Sixty female BALB/c mice were randomly divided into three groups (20 in each group). Mice in the normal control group were given FLEP cells and non-supplemented drinking water. The mice in the model group were continuously administered DEN in drinking water for liver injury induction. The experimental group received FLEP cells and DEN. Mice were killed under anesthesia and the livers were removed 1, 2, and 3 mo after cell transplantation. In most experiments, five animals were used at each time point. Blood was collected for evaluation of liver function and hepatic fibrosis at mo 3. Liver tissue was harvested for examination of hydroxyproline (Hyp) content and for histological evaluation.
Alanine aminotransferase (ALT), aspartate aminotrans-ferase (AST), and total bilirubin in serum were assessed using routine laboratory methods. Hyaluronic acid (HA) and laminin (LN) in serum were also measured with a radioimmunoassay kit (Navy Medical Institute, Shanghai, China).
Hyp determination followed a method designed by Thirunavukkarasu et al[16]. Dried liver tissue after hydrolysis was oxidized by H2O2 and colored by p-dimethylaminobenzoaldehyde and absorbance was determined at 540 nm. The amount of Hyp was expressed in mg/g liver.
To identify hepatocytes in fetal mouse liver sections, we immunostained AFP, albumin, and CK-19 with diaminobenzidine using standard techniques[17]. Liver sections were blocked with 1% BSA and 0.1% normal goat or rabbit sera at room temperature for 1 h, followed by incubation with rabbit anti-AFP polyclonal antibody (dilution 1:100; Santa Cruz Biotechnology), rabbit anti-albumin polyclonal antibody (1:200 dilution, Dako), or goat anti-CK-19 monoclonal antibody (dilution 1:100; Santa Cruz Biotechnology) at 4°C overnight.
HE staining and immunohistochemistry for alpha-smooth muscle actin (α-SMA) were performed to determine the extent of liver inflammation and liver fibrosis development. Liver sections were tested with the antibody against α-SMA (dilution 1:200; NeoMarkers, Fremont, CA, USA) and immunoreactive materials were visualized with diaminobenzidine using a streptavidin-biotin staining kit (UltraSensitive SP kit; Maixin-Bio, Fujian, China).
To identify the origin of cells in the liver, immunohisto-chemistry was performed for sry (sex-determining region for Y chromosome) protein as described previously[18]. It was expected that transplanted cells (originating from fetal liver) would be approximately 50% male and 50% female. For histologic examination, several fields in each slide were randomly selected.
Data were expressed as mean ± SD. Significant differences were determined using ANOVA in SPSS10.0. Results were considered significant when P < 0.05.
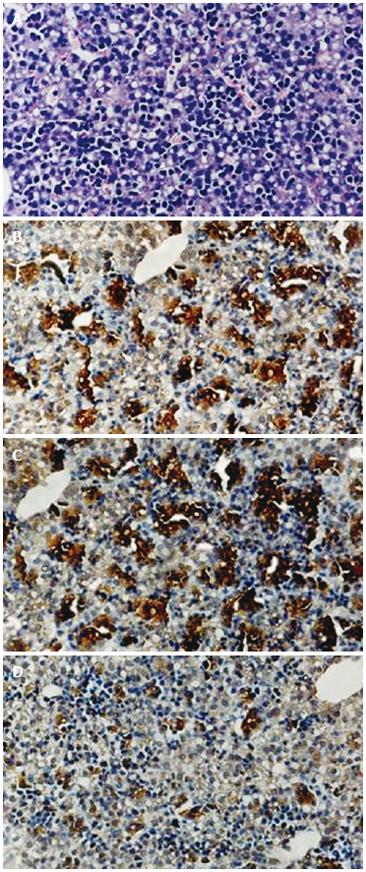
Mouse liver at ED 14 contained immature epithelial cells and hematopoietic cells at different stages of differentiation (Figure 1A). Cells from ED 14 fetal liver were analyzed for expression of AFP, albumin, and CK-19. The percentage of FLEP cells was approximately 20% in the ED 14 fetal liver, determined as the number of cells expressing AFP (Figure 1B), albumin (Figure 1C), or CK19 (Figure 1D).
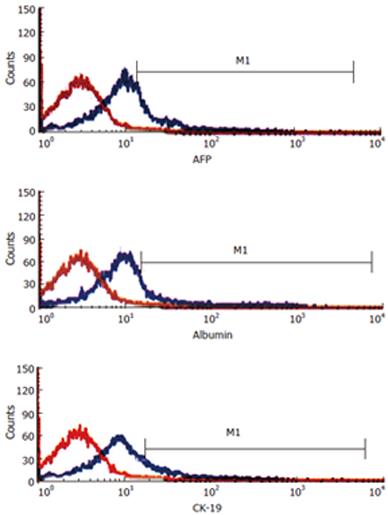
To further characterize the phenotype of immature liver epithelial cells, we tested for expression of AFP, albumin, and CK-19 in isolated ED 14 FLEP cells by flow cytometry. Results demonstrated the existence of at least three distinct subpopulations of epithelial cells in 14-d fetal mouse liver. The percentage of FLEP cells expressing AFP, albumin, and CK-19 was 15.5%, 13.9% and 6.4%, respectively (Figure 2).
The survival rates were not different at the end of the 3-mo experiment in the two groups of DEN-treated mice (P > 0.05), with 90% of DEN-treated mice in the cell transplantation group and 80% of DEN-treated mice in the group without cell transplantation. Table 1 shows the values for the activities of the serum indicator enzymes and the markers of hepatic fibrosis.
DEN administration produced a marked increase in the activities of serum ALT and AST in mice (P < 0.01), compared with those of control group. The DEN plus FLEP cell transplantation group showed a significant decrease in the enzyme levels (P < 0.01), but the levels were still higher than those of control group (P < 0.01). Serum total bilirubin levels were similar among mice in all groups (P > 0.05) (Table 1).
We also examined levels of HA and LN in serum and Hyp contents in liver, which are biochemical markers of liver fibrosis. The serum levels of HA and LN in FLEP cell transplantation group was significantly decreased compared with the model group (P < 0.05), but the levels were still higher than those of control group (P < 0.01). Hyp contents of liver were significantly lower in FLEP cell transplantation group than in the model group (P < 0.01). However, the contents of liver in FLEP cell transplantation group were still higher than those of control group (P < 0.01) (Table 1).
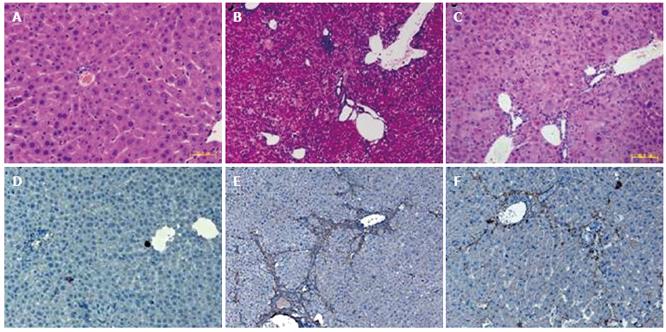
We analyzed liver histology in mice with FLEP cell treatment using HE staining and immunohistochemistry. HE staining for sections of normal control group showed structural integrity without inflammation or fibrosis development (Figure 3A). However, DEN-induced liver injury in the model group showed fibrosis and inflammatory cell infiltration with the loss of structural integrity (Figure 3B). Nevertheless, results from DEN plus FLEP cell transplantation in the experimental group showed that FLEP cells could significantly alleviate DEN-induced fibrosis and inflammation compared with the model group (Figure 3C).
To further verify fibrosis, we analyzed α-SMA, an activating marker of mouse hepatic stellate cells by immunohistochemical staining of liver sections. Only α-SMA expression of vascular smooth muscle cell was observed in normal control group (Figure 3D). An increased amount of α-SMA expression with a dense network by activated HSC was observed in the model group (Figure 3E). However, the expression was reduced significantly in the FLEP cell transplantation group (Figure 3F).
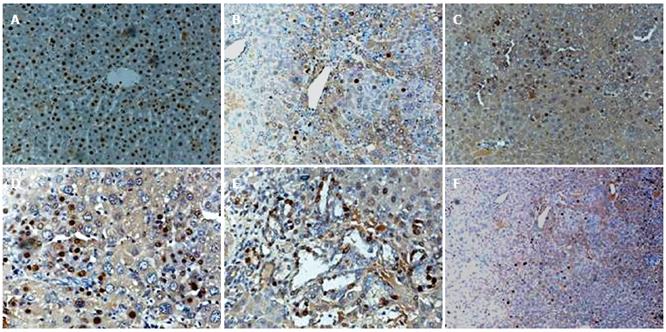
To follow the proliferation and differentiation of FLEP cells, we transplanted FLEP cells into the liver of normal and DEN-treated female mice subject to two-thirds PH. Detection of sry protein served as a marker for proliferation of transplanted FLEP cells. The sry+ cells represented 50% of transplanted cells (originating from fetal liver).
In DEN-treated mice, sry+ cells were distributed in the periportal regions of the liver after cell transplantation. One mo after FLEP cell transplantation into DEN-treated mice, some sry+ cells were observed (Figure 4B). Two months after transplantation, sry+ hepatocytes increased and were already present in the liver parenchyma (Figure 4C). Three months after transplantation, numerous sry+ mature hepatocytes were observed, representing a substantial portion of the liver parenchyma (Figure 4D). We also found sry+ cholangiocytes that formed mature sry positive bile ducts; sry+ bile duct structures became evident as early as 1 mo after ED 14 FLEP cell transplantation, but became more numerous and fully developed in the portal regions at 3 mo (Figure 4E). The number of transplanted cells increased over time and repopulation ranged from 5%-10% at 1 mo, 15%-20% at 2 mo, and 30%-50% at 3 mo.
In the absence of DEN, sry+ cells were still observed 3 mo after FLEP cell transplantation, but were less numerous and contained fewer cells (5%-10% of the total number of cells) than in DEN-treated mice (Figure 4F). In normal mice, PH was required for proliferation of transplanted FLEP cells.
To determine whether ED 14 FLEP cells represent hepatic precursor cells, the characteristics of markers for various liver cell types have been explored, including AFP and albumin for fetal hepatocytes and CK19 for cholangiocytes. Three subpopulations of ED 14 FLEP epithelial progenitor cells have been detected. The first group of cells expressed AFP or albumin but not CK-19, comprising roughly 88% of FLEP cells. The second group (9% of total) expressed CK-19, but not AFP and albumin. The third and smallest group of cells (3% of total) expressed both AFP and albumin and CK-19[19]. Our immunohistochemical and flow cytometric analyses confirmed the existence of three distinct subpopulations of epithelial cells in 14-d fetal mouse liver. Furthermore, the present results demonstrate that immunostaining and flow cytometric analyses for the hepatic epithelial-specific markers AFP, ALB and CK-19 provided a definitive approach to characterize epithelial cell populations in the fetal liver.
We evaluated the anti-fibrosis effects of FLEP cells using a DEN-induced liver fibrosis model. DEN is a hepatotoxin, carcinogen, and mutagen, which can cause liver fibrosis. It provides a suitable experimental fibrosis model to examine human liver fibrosis[20,21]. Our results show that the transplantation of FLEP cells significantly restores the liver function and improves liver fibrosis index in DEN-injured mice. Transplanted FLEP cells can reduce necrosis collagen accumulation of liver and clearly inhibit the fibrosis formation. Moreover, levels of α-SMA positive staining of liver sections decrease significantly after FLEP cells administration in this model. This is exciting because the expression of α-SMA in liver is an indicator of hepatic stellate cell activation, which is recognized as being critical in liver fibrogenesis[22,23]. FLEP cell transplantation thus appears to be effective for the suppression of liver inflammation and fibrosis formation.
We also investigated the underlying mechanisms regarding the repair mechanisms of transplanted FLEP cells to liver recovery after liver injury. We evaluated the stem cell properties and repopulation capacity of ED 14 FLEP cells after their transplantation into normal and chronically damaged livers. The effect of FLEP cell transplantation on reduction of liver fibrosis was studied using immunohistochemistry for sry (sex-determining region for Y chromosome) protein after sex-mismatched transplantation. Immunohistochemical staining for the sry protein offers the advantages of convenience and rapidity to enable the reliable detection and quantification of mixed chimerism in paraffin tissues[18]. We also provided evidence of sry detection in male cells, because the vast majority of cells in all male untreated control livers were positive for sry.
Stem cells are generally considered to exhibit self replication or renewal, differentiation into two or more specific cell phenotypes, and long-term repopulation of the host under appropriate circumstances. Hepatic stem cells include oval cells in canals of Hering of the adult liver and fetal liver hepatoblasts[24,25].
Our results show that FLEP cells selectively proliferate in the normal liver in response to a regenerative stimulus, such as partial hepatectomy. They differentiate into mature hepatocytes and incorporate into the host liver lobule as part of normal hepatocytic cords. Transplanted FLEP cells repopulate to comprise 5%-10% of the liver cells after 3 mo.
In this model, stimulation of the regeneration is amplified by two-thirds hepatectomy in addition to DEN administration. One month after administration of DEN, sry-positive cells were observed around the portal areas. At 2 mo, positive cells were detected in the liver lobes, indicative of FLEP cell migration into the hepatic parenchyma. At 3 mo, there was extensive proliferation and liver repopulation with FLEP cells in DEN-treated mice. The persistent liver damage made by DEN administration is important for the proliferation and differentiation of FLEP cells, and the number of cells migrating into the damaged liver is related to improvement of the liver function and suppression of liver fibrosis. These results suggest that FLEP cells themselves can functionally rescue the recipients by directly substituting the damaged cells.
More interestingly, we demonstrate that transplanted FLEP cells also can differentiate into mature bile duct epithelial cells. Our studies provide direct evidence for differentiation of FLEP cells with dual markers into hepatocytes and bile duct epithelial cells after transplantation into the regenerating liver of DEN-treated mice. This is consistent with other reports that bipotential cells of FLEP cells can proliferate and differentiate into hepatocytes and bile ducts[19,26].
In conclusion, we report that transplanted FLEP cells engraft, proliferate, and differentiate into hepatocytes and bile duct epithelial cells with high repopulation capacity in the liver fibrosis induced by DEN. FLEP cell transplantation restores liver function and reduces liver fibrosis effectively, which rescues the damaged liver by substituting the damaged cells directly. These results suggest that FLEP cell transplantation is an attractive method for chronic liver diseases.
The authors thank Professor Xiu-Qing Xie for her help in histopathology, and the staff of Laboratory of General Surgery, the First Affiliated Hospital, Sun Yat-Sen University.
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004;36:2255-2256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Malhi H, Irani AN, Volenberg I, Schilsky ML, Gupta S. Early cell transplantation in LEC rats modeling Wilson's disease eliminates hepatic copper with reversal of liver disease. Gastroenterology. 2002;122:438-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, Roy-Chowdhury N, Vikram B, Roy-Chowdhury J. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Yin L, Sun M, Ilic Z, Leffert HL, Sell S. Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology. 2002;35:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol. 2000;156:2017-2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679-2688. [PubMed] [Cited in This Article: ] |
| 10. | Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, Weiss MC. Bipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytes. Proc Natl Acad Sci USA. 2004;101:8360-8365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Oertel M, Rosencrantz R, Chen YQ, Thota PN, Sandhu JS, Dabeva MD, Pacchia AL, Adelson ME, Dougherty JP, Shafritz DA. Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology. 2003;37:994-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, Iida H, Ikehara S. Crucial role of donor-derived stromal cells in successful treatment for intractable autoimmune diseases in mrl/lpr mice by bmt via portal vein. Stem Cells. 2001;19:226-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Shi XL, Qiu YD, Wu XY, Xie T, Zhu ZH, Chen LL, Li L, Ding YT. In vitro differentiation of mouse bone marrow mononuclear cells into hepatocyte-like cells. Hepatol Res. 2005;31:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Thirunavukkarasu C, Yang Y, Subbotin VM, Harvey SA, Fung J, Gandhi CR. Endothelin receptor antagonist TAK-044 arrests and reverses the development of carbon tetrachloride induced cirrhosis in rats. Gut. 2004;53:1010-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Vig P, Russo FP, Edwards RJ, Tadrous PJ, Wright NA, Thomas HC, Alison MR, Forbes SJ. The sources of parenchymal regeneration after chronic hepatocellular liver injury in mice. Hepatology. 2006;43:316-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am J Pathol. 2001;159:1323-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Kim MR, Kim HS, Lee MS, Lee MJ, Jang JJ. Cell cycle protein profile of the hepatic stellate cells(HSCs)in dimethylnitrosamine-induced rat hepatic fibrosis. Exp Mol Med. 2005;37:335-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Hu QW, Liu GT. Effects of bicyclol on dimethylnitrosamine-induced liver fibrosis in mice and its mechanism of action. Life Sci. 2006;79:606-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Lau DT, Luxon BA, Xiao SY, Beard MR, Lemon SM. Intrahepatic gene expression profiles and alpha-smooth muscle actin patterns in hepatitis C virus induced fibrosis. Hepatology. 2005;42:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, Theise ND. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology. 2005;41:1252-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci USA. 2006;103:9912-9917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Notenboom RG, van den Bergh Weerman MA, Dingemans KP, Vermeulen JL, van den Eijnde S, Reutelingsperger CP, Hut H, Willemsen R, Offerhaus GJ, Lamers WH. Timing and sequence of differentiation of embryonic rat hepatocytes along the biliary epithelial lineage. Hepatology. 2003;38:683-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |