Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2136
Revised: March 16, 2004
Accepted: April 5, 2004
Published online: April 14, 2005
AIM: To provide hepatic protection through administration of doxorubicin before stop-flow chemotherapy (SFC) and to investigate the expression of heat shock protein 72 (HSP72) and role of nuclear factor kappa B (NF-κB) in this effect.
METHODS: The hepatic preconditioning of doxorubicin was established in a porcine model by injection of doxorubicin (1 mg/kg) before SFC. The experimental animals were randomized into two groups: groups receiving doxorubicin (DOX) and normal saline (NS). Serial serum and tissue samples were taken from both groups to evaluate the protection of doxorubicin. Western blot and immuno-precipitation were applied to detect the expression of HSP72, NF-κB p65 protein, inhibitor κB-α (IκB-α) and phosphorylated IκB-α as well. The expression of tumor necrosis factor α (TNF-α) was estimated by semiquantitative RT-PCR. And the extent of the hepatic injury was estimated with the level of serum aminotransferases.
RESULTS: An abundance production of HSP72 in porcine liver was observed after 24 h of intravenous administration of doxorubicin, but without any change in the expression of NF-κB p65 subunit in cytoplasm. NF-κB p65 subunit accumulated in nuclei at the end of SFC and reached its highest level at 30 min after the restoration of the abdominal circulation and decreased gradually during the 6 h after SFC in NS group, while there was little change in DOX group. There was also a slight decrease of IκB-α at 30 min after the restoration of the abdominal circulation in NS group accompanying with the appearance of phosphorylated IκB-α. The expression of TNF-α was significantly higher in NS group than that in DOX group (average 1.40±0.17 vs 0.62±0.22, P<0.01) at serial time points after SFC. Serum ALT and AST levels of NS group were higher after 24 h than those of DOX group (93.2±7.8 IU/L vs 53.3±13.9 IU/L, 217.0±29.4 IU/L vs 155.0±15.6 IU/L for ALT and AST respectively, P<0.05) and after 48 h than those of DOX group (66.6±18.1 IU/L vs 43.3±16.7 IU/L, 174.4±21.3 IU/L vs 125.7±10.5 IU/L for ALT and AST respectively, P<0.05).
CONCLUSION: Doxorubicin renders the liver to be tolerant to the hepatic influence in SFC in a porcine model through the NF-κB/IκB-α pathway with the expression of HSP72.
- Citation: Lu H, Zhu ZG, Yao XX, Zhao R, Yan C, Zhang Y, Liu BY, Yin HR, Lin YZ. Hepatic preconditioning of doxorubicin in stop-flow chemotherapy: NF-κB/IκB-α pathway and expression of HSP72. World J Gastroenterol 2005; 11(14): 2136-2141
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2136
Although the surgical excision is currently the most ideal treatment for abdominal cancer, its application in the management of malignant disorders in the advanced stage is limited. The severe untoward effects, however, preclude the administration of high-dose chemotherapeutic drugs systematically. So it is rationale to perform regional chemotherapies to increase the local tumor cells’ exposure to the chemotherapeutic agents and obviate the toxic effect on other organs and tissues. As one of the newly developed means of regional chemotherapies, the stop-flow chemotherapy (SFC) procedure blocks the blood supply of tumor bearing tissues with inflated balloons and provides higher local concentration of chemotherapeutic medicines than intra-arterial and intravenous drugs injection[1-4].
Taking the entire abdominal cavity as target territory, the clinical trial of SFC in the treatment of upper digestive tract cancer is rather optimistic; however, some modifications are indicated, especially the protection of some vital organs, such as liver, kidney and pancreas, which are subjected to hypoxia and chemotherapeutic agents during the process. The liver, which is highly sensitive to short period of hypoxia, may suffer from another injury upon re-oxygenation. It has been suggested that the ischemic/reperfusion injury is initialized by over production of active oxygen species upon re-exposure to the oxygen. The oxygen stress will impair the balances among members of NF-κB family and increase the expression of various inflammatory factors, such as TNF-α and IL-1β[5-10], which propagate the inflammatory response and cause sequestration of polymorphonuclear neutrophils in liver. And there are abundant evidences supporting that ischemia/reperfusion injury can be alleviated by interfering the activation of NF-κB in various organs including liver[11-13].
Thus we propose that the hepatic influence caused by SFC should be related to the activation of NF-κB and inhibition of this activation through induction of HSP72 via intravenous administration of doxorubicin can mitigate this influence.
Stop-flow balloon catheters and Gambro roller were purchased from PfM (Germany). TRIzol reagent was purchased from Invitrogen Life Technologies (USA). Reverse transcription system was from Promega (USA). BCA Protein Assay Reagents and Seize X Immunoprecipitation Kit were from Pierce (USA). All antibodies were purchased from Santa Cruz (USA).
Healthy female hybrid prepubertal pigs weighing 25.0±4.4 kg were employed in accordance with the guideline for animal research and were approved by the Ethical and Research Committee of the hospital. Twelve pigs were randomized into two groups, six animals in each. Animals in preconditioning and SFC group (DOX group) were anesthetized with an intramuscular injection of ketamine hydrochloride and doxorubicin (DOX) 1 mg/kg was then injected through peripheral veins. After recovery for 24 h, animals were subjected to SFC as described below. Meanwhile, animals in SFC group (normal saline (NS) group) were the same as in DOX group, but were injected with NS instead of DOX.
After overnight fast, all animals were subject to intravenous anesthesia with orotracheal intubation and controlled mechanic ventilation. A midline incision was performed. The abdominal aorta and inferior vena cava were exposed and separated about 2-3 cm. After systemic heparinization with 0.75 mg/kg of heparin, the abdominal aorta and inferior vena cava were clamped by Satinsky clamps just above the aortic and venous bifurcation. Then two small incisions were made above the clamp sites of the abdominal aorta and inferior vena cava, respectively. The isolation of abdomen was achieved by insertion of two 3-lumen stop-flow balloon catheters (PfM, Cologne, Germany) into the abdominal aorta and inferior vena cava respectively. The balloons were positioned above the celiac axis and hepatic venous under fluoroscopic control and filled with NS. As soon as the inflation of balloons, mitomycin C was perfused through an extra-corporeal circuit connected to both catheters at the dosage of 0.2 mg/kg, the Gambro roller (PfM, Cologne, Germany) was then started and the flow rate was adjusted at about 100 mL/min. No oxygen was added to this extra-corporeal circuit. After a total of 20-min hypoxic perfusion, the circulation to abdomen was restored by deflation of the balloons in aorta and inferior vena cava. The catheters were extracted after a brief stabilization period and the incisions were sutured. The circulation of hind legs was recovered by unclamping the Satinsky clamps. After the biopsies of liver tissue and blood samples were taken, the abdominal cavity was lavaged and the incision was sutured.
The hepatic biopsies were collected before, at the end of SFC and at 30 min, 3 and 6 h following the restoration of the abdominal circulation. Hepatic biopsy’s sites were rotated randomly, but were in all cases as remote from each other as possible. The liver tissues were frozen immediately in liquid nitrogen and stored at -80 °C until analyzed. Peripheral blood samples were taken before, at the end of SFC and at 30 min, 3, 6, 24, 48 h and 7 d following the restoration of the abdominal circulation. Blood samples were centrifuged at 4000 r/min immediately after taken from peripheral veins. Serum was separated and stored in aliquots at -80 °C until analyzed. All animals were killed at d 7 after SFC.
Total RNA was extracted using TRIzol reagent (Invitrogen, USA) from stored liver tissues at each given time point and 2 μg of it was reversely transcribed to complementary DNA (cDNA) using a reverse transcription system according to the manufacturer’s guide (Promega, USA). An aliquot of 1 μL of cDNA products was amplified using specific primers for TNF-α and β-actin as described below. And housekeeping gene (β-actin) was amplified as internal control to verify the equal loading of RNA and cDNA in the reverse transcription and PCR creations.
PCR primers were as follows: TNF-α sense, 5’-atcggc-ccccagaaggaagag-3’ and TNF-α antisense, 5’-gatggcagagag-gaggttgac-3’ to give a 351-bp product; β-actin sense, 5’-ggacttcgagcaggagatgg-3’ and antisense, 5’-gcaccgtgttgg-cgtagagg-3’ to give a 233-bp product. All primers spanned at least one intron of genomic DNA. Eight microliters of each PCR products were electrophoresed in a 2% agarose gel and stained with ethidium bromide. The intensities of the specific bands were analyzed to determine whether the differences were statistically significant.
Frozen liver tissues were homogenized in 1 mL of homogenization buffer A [10 nmol/L HEPES (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L DTT, 0.2 mmol/L PMSF, 0.1 mmol/L EDTA] on ice. The homogenates were incubated for 15 min on ice and centrifuged at 14000 g for 15 min. The supernatants were stored at -80 °C as cytoplasmic extracts. The pellet was further suspended in ice-cold buffer B [20 mmol/L HEPES (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.42 mol/L NaCl, 25% glycerol, 0.5 mmol/L DTT, 0.2 mmol/L PMSF, 0.2 mmol/L EDTA] and incubated for 30 min on ice with frequent vortex. After the suspensions were centrifuged at 14000 g for 15 min, the supernatants were collected as nuclear extracts in aliquots and stored at -80 °C.
Western blot was applied to determine the expression of HSP72, NF-κB p65 subunit and I-κBα in liver tissues. In brief, the protein contents of cytoplasmic and nuclear extracts were measured by a BCA protein assay (Pierce, USA), and 100 μg per lane was separated on a denaturing 10% SDS-PAGE gel (Laemmli). The proteins were then transferred to a nitrocellulose membrane (HybondTM-C, Amersham, UK) using a semi-dry transfer system (BioRad, USA). The membrane was stained by Ponceau S to check for the efficiency of transfer and subsequently blocked by 2% BSA for 1 h at room temperature. Membrane was then washed thrice for 5 min in TBS and then incubated for 2 h at room temperature with polyclonal goat anti-HSP72 antibody, polyclonal rabbit anti-p65 antibody, and polyclonal rabbit anti-IκB-α antibody in TBS. The membrane was washed thrice for 10 min in TBS and incubated with an appropriate secondary antibody (anti-goat or anti-rabbit) coupled to alkaline phosphatase in TBS. After the membrane was washed thrice for 10 min, it was developed with NBT/BICP staining kit (Sino-American, China) according to the manufacturer’s protocol.
The immunoprecipitation was performed using a commercial immunoprecipitation kit (Pierce, USA) according to the manufacturer’s guide. After immunoprecipitation, the products were analyzed by Western blot as described above. An antibody, polyclonal rabbit anti-p-IκB-α antibody, special for phosphorylated inhibitor κB-α (IκB-α) was applied to detect the phosphorylated IκB-α.
ALT and AST were determined according to the manufacturer’s protocols. The serum levels of ALT and AST in serum were analyzed to determine whether the differences were statistically significant.
Experimental results were expressed as mean±SD. SPSS statistical package was applied. Statistical comparisons were made using a general linear model (SNK). P value less than 0.05 was considered statistically significant.
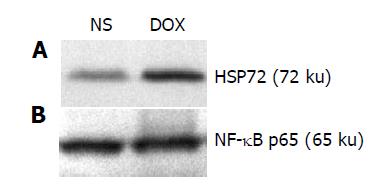
After 24 h intravenous administration of doxorubicin (1 mg/kg), we observed a substantial production of HSP72 in liver tissue (Figure 1A, lane 2), whereas there was a minor expression in liver in NS group (Figure 1A, lane 1). But there was no observed change in the expression of cytoplasmic NF-κB p65 subunit between NS group and DOX group (Figure 1B), suggesting that administration of doxorubicin could induce the expression of HSP72 in liver and did not alter that of NF-κB p65 subunit in porcine liver.
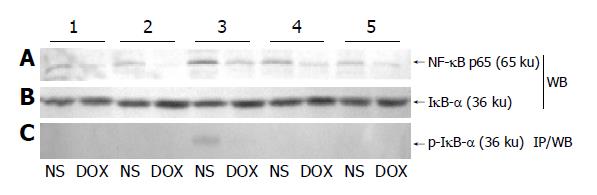
The dynamic change of NF-κB p65 subunit presenting in hepatic tissue nuclear extracts is shown in Figure 2A. The p65 subunit of NF-κB was absent in the nuclei in both NS and DOX groups. But it began to accumulate in nuclei at the end of SFC in NS group, reached its highest level in 30 min after the restoration of abdominal circulation and then decreased gradually. Compared with NS group, there was little change of p65 subunit in DOX group at corresponding time points. Thus we investigated the IκB-α level in cytoplasm at these time points and tried to figure out the relationship between NF-κB p65 and its inhibitor. A slight decrease of IκB-α in cytoplasm was noticed at 30 min after the restoration of the abdominal circulation and then recovered at following time points in NS group. In contrast to this, IκB-α level in DOX group remained constant during SFC (Figure 2B). Then we performed immunoprecipitation to evaluate the phosphorylation course of IκB-α during and after SFC procedure. The phosphorylated IκB-α solely appeared at 30 min after the restoration of the abdominal circulation in cytoplasm in NS group, which was consistent with the slight decrease of IκB-α (Figure 2C).
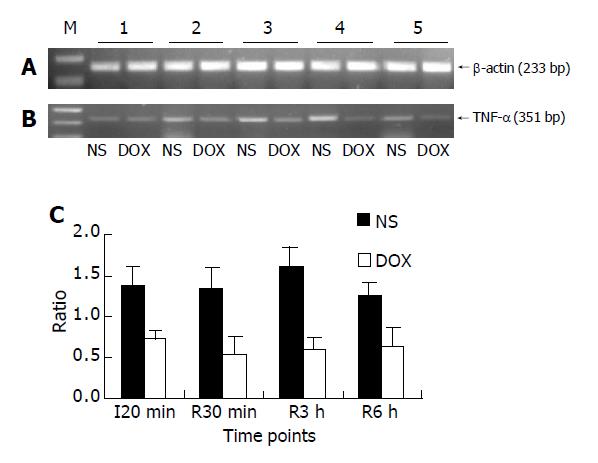
The transcriptional activity of NF-κB downstream gene, TNF-α, was evaluated by semiquantitative RT-PCR (Figure 3).
According to the difference of activated NF-κB between NS and DOX groups, the transcriptional activity of TNF-α mRNA in DOX group was significantly lower than that in NS group at different time points (average 1.40 vs 0.62 for NS vs DOX respectively, P<0.01).
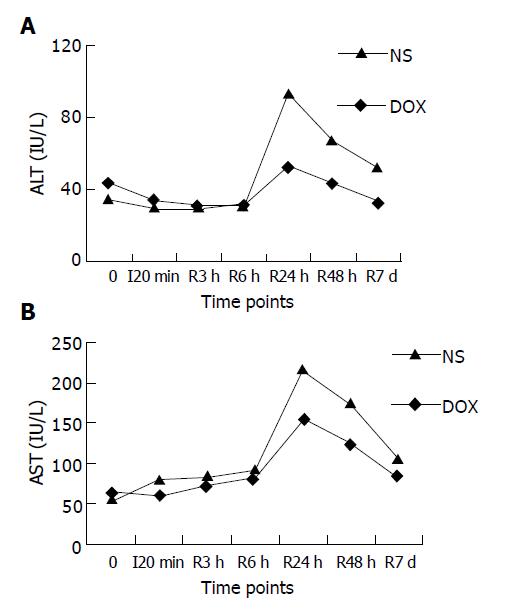
The serum level of ALT and AST of NS group was significantly higher than that of DOX group at 24 and 48 h after SFC (P<0.05, Figure 4).
Although a lot of work has been done in hepatic ischemia/reperfusion injury, little is known at such a particular situation that liver was under hypoxia and low-flow but not no-flow in SFC procedure. We have previously shown a mild and reversible hepatic influence[4], and there was a mild elevation in serum aminotransferases within 48 h after SFC procedure. The purpose of this study was to investigate the role of NF-κB pathway during SFC and tried to alleviate this influence through pharmacologically hepatic preconditioning by administration of doxorubicin before SFC procedure and further investigate the potential mechanism of this effect.
With regard to the activation of nuclear factor kappa B (NF-κB) p65 subunit, the presence of p65 protein was investigated in both hepatic cytoplasmic and nuclear extracts. We first examined the expression of cytoplasmic p65 subunit obtained from liver before SFC procedure and there was no difference between two groups (Figure 1B). It means that the expression of p65 subunit was not affected by the administration of doxorubicin before SFC procedure. However, there was a significant change of p65 subunit in nuclei during and after SFC procedure between NS and DOX groups. It was that in NS group, the p65 subunit in hepatic nuclear extract accumulated gradually from the end of SFC and reached its highest level in 30 min after the restoration of the abdominal circulation and then decreased in the following 6 h after SFC procedure, while there was only a little change in DOX group during the same time period. Meanwhile, we also observed a slight decrease of IκB-α and the appearance of phosphorylated IκB-α in cytoplasm at 30 min after the restoration of the abdominal circulation in NS group when compared with DOX group at that time point. NF-κB was first thought to be primarily a lymphocyte and macrophage transcription factor, but it now has been demonstrated in various tissues and organs including liver, in which it responds to hypoxia and re-oxygenation and is involved in immune and inflammatory responses. Cells must retain their NF-κB proteins in cytoplasm from activating gene transcription wantonly by means of NF-κB inhibitors such as IκB family. Once cells receive an appropriate stimulus such as pro-inflammatory cytokines, metal ions, and reactive oxygen species as well, the IκB kinase (IKK) complexes become active and phosphorylate the IκB inhibitors. The most common active dimer of NF-κB is composed of p50 and p65, in which p65 is a transcription-activating subunit and p50 contributes to the binding of specific DNA sequence[10]. The activated NF-κB promotes transcriptional activity of pro-inflammatory genes like TNF-α. We then investigated the mRNA level of TNF-α in liver tissues at the same time points and observed an elevated transcriptional activity of TNF-α after the restoration of the abdominal circulation in NS group, but there was a little change of it in DOX group. This difference is compatible with the activation pattern of NF-κB in NS and DOX groups respectively. TNF-α is one of the key pro-inflammatory cytokines that play a pivotal role in augmenting the inflammatory response in hepatic ischemia/reperfusion injury. The extent of hepatic ischemia/reperfusion injury was limited via applying anti-TNF-α mono-antibody or ischemic preconditioning to restrain the production or activity of TNF-α[14,15]. Teoh[16] proved that the hepatic ischemia/reperfusion injury in TNF-α knock-out mice was suppressed during both the early (2 h) and late (24 h) phases of ischemia/reperfusion injury. In our model, the inhibition of NF-κB was also followed by the inhibition of transcriptional activity of TNF-α. Furthermore, we also observed a significant change in the serum level of ALT and AST between NS and DOX groups. The serum level of aminotransferases in NS group significantly increased within 48 h and the peak value of ALT in NS group almost doubled at 24 h after SFC when compared with that of DOX group. These results indicate that NF-κB/IκB-α pathway acts quickly to the hypoxia/re-oxygenation in liver caused by SFC procedure and cause the following inflammatory response. Administration of doxorubicin preceding SFC procedure protects the liver from this insult by the inhibition of the activity of NF-κB through stabilization of IκB-α.
The remaining paradoxical phenomenon is how doxorubicin, a potential deleterious agent to liver, provides protection for liver in SFC procedure. Interestedly, we observed obvious expression of HSP72 protein in porcine liver after 24 h of the administration of doxorubicin via peripheral venous when compared with injection of NS, which is consistent with the work of Kume[17]. If administrated intravenously, doxorubicin accumulates in liver and is reductively metabolized to semiquinone radical intermediate participating in the formation of reactive oxygen species[18]. Because of the enterohepatic circulation of doxorubicin and its metabolites, there might be appreciable reactive oxygen species triggering oxidant stress and might induce the production of another stress reaction protein that is heat shock protein (HSP) family. HSP72 is the inducible member of HSP70 family, the other is constitutively expressed, HSP73. As a molecular chaperon, HSP72 serves as preventing the aggregation and misfolding of proteins. In addition, it also assists in the folding of newly synthesized proteins, translocating proteins to appropriate organs. Induction of HSP72 pharmacologically or through heat shock preconditioning renders liver to be tolerant to ischemia/reperfusion injury[13,17,19]. It has been speculated that binding of HSP72 and NF-κB/IκB-α may contribute to block the activation of NF-κB, and Shimizu et al[20], has reported the formation of complex of HSP72-IκB-α in the model of myocardial infarct. Another hypothesis is that it is the activity of IKK that is inhibited after the induction of HSP72[21-23].
Combination NF-κB/IκB-α with the expression of HSP72 in our animal model of SFC, administration of doxorubicin before SFC did not down-regulate the expression of p65 subunit in liver, and the inhibitory effect of NF-κB is blunted at the phosphorylation and degradation of IκB-α. Our results are in agreement with those studies showing that NF-κB/IκB-α pathway can be blocked by various stimuli[13,21,22,24]. Further work in the interaction between HSP72 and NF-κB/IκB-α is needed to elucidate the exact role of HSP72 in pharmacological preconditioning of doxorubicin in SFC.
In conclusion, we have revealed the role of NF-κB/IκB-α pathway in the model of abdominal SFC. Our study also shows that doxorubicin is not only beneficial to patients with advanced abdominal cancer, but also can interfere with the activation of NF-κB through stabilizing the IκB-α probably by inducing HSP72 in liver. The understanding of doxorubicin in pharmacological hepatic preconditioning sheds light on a new approach to prevent the hepatic influence in SFC and makes this new chemotherapy feasible in clinical practice.
| 1. | Averbach AM, Stuart OA, Sugarbaker TA, Stephens AD, Fernandez-Trigo V, Shamsa F, Sugarbaker PH. Intraaortic stop-flow infusion: pharmacokinetic feasibility study of regional chemotherapy for unresectable gastrointestinal cancers. Ann Surg Oncol. 1995;2:325-331. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Turk PS, Belliveau JF, Darnowski JW, Weinberg MC, Leenen L, Wanebo HJ. Isolated pelvic perfusion for unresectable cancer using a balloon occlusion technique. Arch Surg. 1993;128:533-538; discussion 538-539. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Guadagni S, Fiorentini G, Palumbo G, Valenti M, Russo F, Cantore M, Deraco M, Vaglini M, Amicucci G. Hypoxic pelvic perfusion with mitomycin C using a simplified balloon-occlusion technique in the treatment of patients with unresectable locally recurrent rectal cancer. Arch Surg. 2001;136:105-112. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Yao XX, Zhu ZG, Zhao R, Su J, Zhou S, Yu BW, Yin HR, Lin YZ. The influence of Stop-flow chemotherapy on the abdominal visceral function of swine. J Surg Concepts Pract. 2003;8:223-225. [Cited in This Article: ] |
| 5. | Bronk SF, Gores GJ. Efflux of protons from acidic vesicles contributes to cytosolic acidification of hepatocytes during ATP depletion. Hepatology. 1991;14:626-633. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Gasbarrini A, Borle AB, Farghali H, Bender C, Francavilla A, Van Thiel D. Effect of anoxia on intracellular ATP, Na+i, Ca2+i, Mg2+i, and cytotoxicity in rat hepatocytes. J Biol Chem. 1992;267:6654-6663. [PubMed] [Cited in This Article: ] |
| 7. | Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169-173. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] [Cited in This Article: ] |
| 9. | Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35-47. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-179. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Vos IH, Govers R, Gröne HJ, Kleij L, Schurink M, De Weger RA, Goldschmeding R, Rabelink TJ. NFkappaB decoy oligodeoxynucleotides reduce monocyte infiltration in renal allografts. FASEB J. 2000;14:815-822. [PubMed] [Cited in This Article: ] |
| 12. | Kis A, Yellon DM, Baxter GF. Role of nuclear factor-kappa B activation in acute ischaemia-reperfusion injury in myocardium. Br J Pharmacol. 2003;138:894-900. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Uchinami H, Yamamoto Y, Kume M, Yonezawa K, Ishikawa Y, Taura K, Nakajima A, Hata K, Yamaoka Y. Effect of heat shock preconditioning on NF-kappaB/I-kappaB pathway during I/R injury of the rat liver. Am J Physiol Gastrointest Liver Physiol. 2002;282:G962-G971. [PubMed] [Cited in This Article: ] |
| 14. | Iwasaki Y, Tagaya N, Hattori Y, Yamaguchi K, Kubota K. Protective effect of ischemic preconditioning against intermittent warm-ischemia-induced liver injury. J Surg Res. 2002;107:82-92. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Ben-Ari Z, Hochhauser E, Burstein I, Papo O, Kaganovsky E, Krasnov T, Vamichkim A, Vidne BA. Role of anti-tumor necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation. 2002;73:1875-1880. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412-421. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Kume M, Yamamoto Y, Yamagami K, Ishikawa Y, Uchinami H, Yamaoka Y. Pharmacological hepatic preconditioning: involvement of 70-kDa heat shock proteins (HSP72 and HSP73) in ischaemic tolerance after intravenous administration of doxorubicin. Br J Surg. 2000;87:1168-1175. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727-741. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Fudaba Y, Ohdan H, Tashiro H, Ito H, Fukuda Y, Dohi K, Asahara T. Geranylgeranylacetone, a heat shock protein inducer, prevents primary graft nonfunction in rat liver transplantation. Transplantation. 2001;72:184-189. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Shimizu M, Tamamori-Adachi M, Arai H, Tabuchi N, Tanaka H, Sunamori M. Lipopolysaccharide pretreatment attenuates myocardial infarct size: A possible mechanism involving heat shock protein 70-inhibitory kappaBalpha complex and attenuation of nuclear factor kappaB. J Thorac Cardiovasc Surg. 2002;124:933-941. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Roussel RR, Barchowsky A. Arsenic inhibits NF-kappaB-mediated gene transcription by blocking IkappaB kinase activity and IkappaBalpha phosphorylation and degradation. Arch Biochem Biophys. 2000;377:204-212. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416-5423. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Kohn G, Wong HR, Bshesh K, Zhao B, Vasi N, Denenberg A, Morris C, Stark J, Shanley TP. Heat shock inhibits tnf-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock. 2002;17:91-97. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Kiemer AK, Gerbes AL, Bilzer M, Vollmar AM. The atrial natriuretic peptide and cGMP: novel activators of the heat shock response in rat livers. Hepatology. 2002;35:88-94. [PubMed] [DOI] [Cited in This Article: ] |