Published online Mar 1, 2004. doi: 10.3748/wjg.v10.i5.741
Revised: December 9, 2003
Accepted: December 16, 2003
Published online: March 1, 2004
AIM: Many defense factors of the mother’s colostrum or milk protect infants from intestinal, respiratory and systemic infections. In the present study, we investigated the effect of colostrum and mature human milk on E. histolytica parasites in vitro.
METHODS: Samples of human milk were collected from 5 healthy lactating mothers. The medium with human milk at concentrations of 2%, 5% and 10% was obtained.
RESULTS: The lethal effect of E. histolytica on the medium supplemented with different concentrations of both colostrum and mature human milk was significant during the first 30 min. We also detected that the results of colostrum and mature human milk were similar. No statistically significant differences were found between same concentrations of colostrum and mature human milk at the same times.
CONCLUSION: Colostrum and mature human milk have significant lethal effect on E. histolytica and protect against its infection in breast fed children.
- Citation: Akisu C, Aksoy U, Cetin H, Ustun S, Akisu M. Effect of human milk and colostrum on Entamoeba histolytica. World J Gastroenterol 2004; 10(5): 741-742
- URL: https://www.wjgnet.com/1007-9327/full/v10/i5/741.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i5.741
Entamoeba histolytica has a worldwide distribution with a high prevalence in areas with poor hygiene, overcrowding, and low socioeconomic conditions. When ingested, cyst passes through the stomach and excyst in the distal part of the small intestine and colon. E. histolytica generally lives in the lumen of the large intestine of man. The emerging trophozoites live in the lumen of the bowel close to the mucosa, where they feed by phagocytosis on particulate matter and bacteria and by pinocytosis on liquid nutrients. As such, they may invade the gut wall and produce ulceration and subsequent dysentery. The parasite may be transported by the blood to extraintestinal locations, such as the liver, lungs, and brain[1-3].
The role of breast milk ingestion in passive and active protection of infants is very important. Some reports showed that the incidence of intestinal and systemic E. histolytica infections was decreased in human breast milk fed infants.This protective effect has been attributed to the anti-infective and anti-inflammatory properties of human breast milk[4,5].
The aim of the present study was to investigate the effects of colostrum and mature human milk on E. histolytica. Human colostrum or mature milk, if effective in producing lethal effect on E. histolytica, could be useful in preventing infections caused by infected water and food in breast-fed children.
E. histolytica fresh clinical isolate was obtained from a patient with acute amebiasis. The isolate was cultivated in Robinson medium[6].
Fresh human milk was collected from 5 healthy lactating mothers with no clinical evidence of infection or inflammation, who voluntarily donated up to 10 mL of milk and colostrum samples, with their informed consent, by breast pump (Ameda Egnell SMB Pump, Switzerland), in Ege University Hospital, Izmir, Turkey. None of these mothers received hormones or antibiotics during the postpartum period. Colostrum and mature human milk were obtained during the first 3 d of lactation and the 3rd wk of lactation, respectively. All samples were collected from mothers between 9 and 12 a.m., just before they began to nurse their babies. The colostrum and mature human milk samples were rapidly centrifuged for 15 min at 1000 g. The fresh clear middle layer was collected and immediately frozen (-70 °C) until the study[7].
The trophozoites were adapted by three successive subcultures at 36 ± 0.5 °C. At the end of the period amoebae reached logarithmic growth phase. Trophozoites were chilled for 10 min in an ice water bath, then vigorously shaken to detach amoebae adherent to the walls of the tube. The culture tubes were centrifuged for 2 min (500 r/min) and supernatant was discarded. Once their content was homogenized by repeated inversion. They were counted with a haemocytometer and then 20 × 104 amoebae/mL were transferred to fresh medium. Five tubes including 5 mL medium were prepared for each group. Then, colostrum and mature human milk were added in medium at different concentrations (2%, 5% and 10%). Tubes were incubated at 36 ± 0.5 °C for 30, 60, 120, 180 and 240 min respectively. At the end of incubation, only motile parasites were counted with a haemocytometer[8]. All experiments were done in triplicate and repeated at least twice.
All values were given as mean ± SD. To determine the statistical significance of the difference between study groups, we used nonparametric tests, Kruskal-Wallis analysis of variance and Mann-Whitney test. P value less than 0.05 was considered significant.
In the study, we analyzed the lethal effect of colostrum and mature human milk at three different concentrations on E. histolytica. The number of living parasite was recorded at the different incubation periods. The results of this experiment showed that the best lethal effect (50% killing) was obtained from all samples during the first 30 min exposure. We have also observed that there was no living parasite at the end of 12 h incubation.
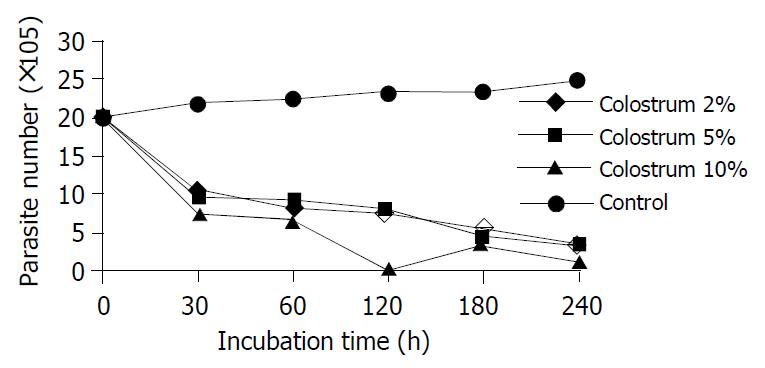
A significant lethal effect of different concentrations of colostrum on E. histolytica was observed during 30 min and 180 min exposure (P < 0.001) (Figure 1). However, no statistically significant differences were shown between them (P > 0.05).
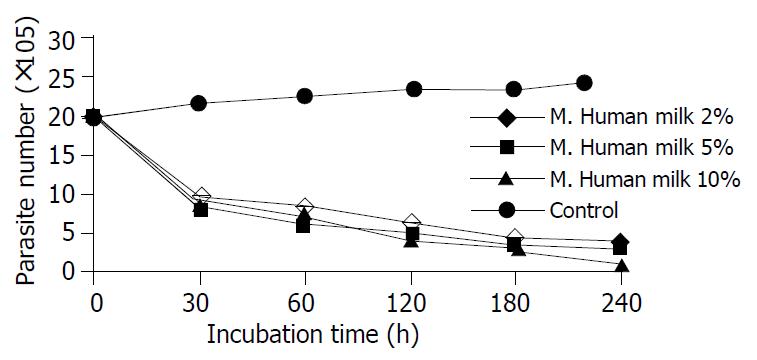
When the lethal effect of mature human milk at different concentrations on E. histolytica was compared with each other, the difference was statistically significant during 30 min and 180 min exposure (P < 0.001) (Figure 2). There was no statistically significant difference between 30 and 180 min exposure (P > 0.005).
The results of colostrum and mature human milk were similar. Colostrum did not prove to be very superior to mature human milk. No statistically significant differences were found between same concentrations of colostrum and mature human milk at 30, 60, 120, 180, and 240 min exposure.
Colostrum and mature human milk may play a protective role in breast-fed children exposed to some parasites (such as G. intestinalis). Both contain considerable amounts of immunglobulins, mainly the secretory immunglobulin A (s IgA) type that may play a role in such protection. It has been shown that such immunglobulins have antibody specificities, which reflect antigenic stimuli in the intestinal tract[9,10].
The demonstration of antibodies in breast milk may have epidemiological significance in population studies. Antibodies of the IgA class were found in serum and colostrum of parturient women in an endemic area of amebiasis[11]. Grundy et al[12] demonstrated sIgA antibodies to E. histolytica in milk (31%) and serum (14%) of mothers living in Kenya. They suggested that serum antibodies indicated past or present invasive amoebasis, milk antibodies were more likely to present intestinal infection in endemic areas. Anti-Eh sIgA antibody titers were significantly increased in colostrum samples of mothers of newborn children with diarrhea[13]. The infants, mostly uninfected, were found to have E. histolytica cysts in small numbers (2/1200 samples), despite the high prevalence of E. histolytica in their mothers[14].
Human milk cells (macrophages, lymphocytes, neutrophils) and antibodies could protect intestinal mucosa, remain active in the neonatal intestine and possibly migrate to other tissues[5]. Colostral macrophages might be cytotoxic to trophozoites of E. histolytica. This has been shown by direct microscopy[7]. It was proposed that colostral macrophages might interrupt colonization and subsequent invasion in infants who were breast-fed. Although there are many epidemiological studies in endemic areas, studies about colostrum and mature human milk in vitro are limited. Gillin et al reported that 90% of E. histolytica were killed in approximally 3 h with 1% milk. But we could not find any publications containing colostrum and mature human milk together in vitro. We established that E. histolytica was killed by exposure to colostrum and mature human milk in vitro. IgA and lactoferrin amounts in colostrum were richer than mature human milk. However, we obtained similar results from both of them. Fifty percent of E. histolytica were killed by all dilutions of both secretions during a 30 min exposure. Thus, the lethal effect did not depend on the amounts of various protectable milk components. Similarly, some studies showed that the lethal effect of sIgA on some parasites did not depend on the amount of sIgA.
In this study, we showed that colostrum and mature human milk had direct lethal effect on E. histolytica in vitro. Therefore, when trophozoites that emerge from cysts in the small intestine are exposed to colostrum and mature human milk, they may be killed. Thus, breast fed children have a lower rate of amebiasis than nonbreast fed children.
Edited by Wang XL Proofread by Zhu LH
| 1. | Ravdin JI. Amebiasis. Clin Infect Dis. 1995;20:1453-164; quiz 1453-164;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Proctor EM. Laboratory diagnosis of amebiasis. Clin Lab Med. 1991;11:829-859. [PubMed] [Cited in This Article: ] |
| 3. | Stanley SL. Amoebiasis. Lancet. 2003;361:1025-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 915] [Cited by in F6Publishing: 769] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Chierici R. Antimicrobial actions of lactoferrin. Adv Nutr Res. 2001;10:247-269. [PubMed] [Cited in This Article: ] |
| 5. | Xanthou M, Bines J, Walker WA. Human milk and intestinal host defense in newborns: an update. Adv Pediatr. 1995;42:171-208. [PubMed] [Cited in This Article: ] |
| 6. | Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Acosta-Altamirano G, Rocha-Ramirez LM, Reyes-Montes R, Cote V, Santos JI. Antiamoebic properties of human colostrum. Adv Exp Med Biol. 1987;216B:1347-1352. [PubMed] [Cited in This Article: ] |
| 8. | Chávez-Dueñas L, Gómez-Dominguez R, López-Revilla R. Effects of Panmede and various horse serum concentrations on the axenic cultivation of Entamoeba histolytica strains in TPS-1 medium. Parasitol Res. 1989;76:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol. 2002;7:275-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Ogra PL, Losonsky GA, Fishaut M. Colostrum-derived immunity and maternal-neonatal interaction. Ann N Y Acad Sci. 1983;409:82-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Berber AC, Escobar A, Zamora M, Acosta G. Identification of Entamoeba histolytica antigens recognized by IgA class human antibodies in sera and colostra of puerperal women using immunoblotting techniques. Arch Invest Med (Mex). 1990;21 Suppl 1:97-101. [PubMed] [Cited in This Article: ] |
| 12. | Grundy MS, Cartwright-Taylor L, Lundin L, Thors C, Huldt G. Antibodies against Entamoeba histolytica in human milk and serum in Kenya. J Clin Microbiol. 1983;17:753-758. [PubMed] [Cited in This Article: ] |
| 13. | Lopez Revilla R, Navarro-Garcia F, Valadez-Sanchez M, Lopez Vidal Y, Calva Mercado J. Dot-enzyme-linked immunosorbent assay (Dot-ELISA) of anti-Entamoeba histolytica antibodies in hu-man serum and colostrum. Arch Invest Med. 1991;22:249-253. [Cited in This Article: ] |
| 14. | Islam A, Stoll BJ, Ljungstrom I, Biswas J, Nazrul H, Huldt G. The prevalence of Entamoeba histolytica in lactating women and in their infants in Bangladesh. Trans R Soc Trop Med Hyg. 1988;82:99-103. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |