Manuscript accepted on :23-12-2022
Published online on: 12-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Daya Shankar Gautam
Second Review by: Dr. Prakash Balu
Final Approval by: Dr. Jihan Seid Hussain
Sneha Ghildiyal1 , Manjari Baluni1
, Manjari Baluni1 , D. Himanshu Reddy3 and Alok Kumar2*
, D. Himanshu Reddy3 and Alok Kumar2*
1Department of Microbiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareily Road, Lucknow, 226014, Uttar Pradesh, India.
2*Department of Molecular Medicine and Biotechnology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareily Road, Lucknow, 226014, Uttar Pradesh, India.
3Department of Medicine, King George's Medical University, Lucknow, Uttar Pradesh, 226003, India.
Corresponding Author E-mail: dralokkumar03@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2662
Abstract
Identifying potential biomarkers, which can be used for diagnostic and therapeutic purposes, is urgently needed for successful Japanese encephalitis (J.E.) viral infection disease management. In the present study, we identified key CSF protein biomarkers of J.E. patients. We compared them to those from non-JE acute encephalitis syndrome and other neurological non-infectious patients to determine their discriminatory potential to detect JEV infection. Demographic and clinical information including fever, headache, vomiting, altered sensorium, behavioral abnormalities, neck stiffness, and GCS score were recorded for all patients. CSF protein biomarkers were analyzed using 2D gel electrophoresis and liquid chromatography-tandem mass spectrometry (LC-MS/MS). Total 22 CSF based protein biomarkers were identified and a out of them three protein spots were further processed for biomarkers identification on the basis of size and density. Functional enrichment analyses of Gene Ontology (G.O.) were performed using Cytoscape software to explore the biological functions and relevant pathways. G.O. enrichment analysis showed that the G.O. terms were mainly enriched in immune responses, inflammatory and apoptotic cell death pathways, autophagy regulation, cellular organization, cellular protein modification, lipid transportation, fatty acid metabolism and iron regulation specifically associated with JEV disease. Taken together, it showed that a combination of multiple CSF protein biomarkers constitutes a founding set for the discrimination of JEV infection individuals, which can be used for diagnosis and therapeutic targets; however, it demands further extensive independent cohorts study.
Keywords
Cerebrospinal fluid; 2D-gel electrophoresis; Japanese encephalitis disease; liquid chromatography-tandem mass spectrometry; Proteomics; virus
Download this article as:| Copy the following to cite this article: Ghildiyal S, Baluni M, Reddy D. H, Kumar A. Comprehensive Assessment of Human Cerebrospinal Fluid for Protein Bio-Marker Identification Following Japanese Encephalitis Viral Infection. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Ghildiyal S, Baluni M, Reddy D. H, Kumar A. Comprehensive Assessment of Human Cerebrospinal Fluid for Protein Bio-Marker Identification Following Japanese Encephalitis Viral Infection. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3Bkevqz |
Introduction
Acute encephalitis syndrome (AES) is characterized by an acute onset of fever and clinical neurological manifestation that includes mental confusion, disorientation, delirium, or coma. It is triggered by many infectious viruses for eg; Dengue, West Nile Virus, Epstein-Barr virus, including the Japanese encephalitis virus (JEV) or other infectious agents such as fungus, bacterial and even non-infectious when the immune system responds to a previous infection or, it mistakenly attacks brain tissue.1, 2 Japanese encephalitis (J.E.) virus infection emerged as Asia’s most common viral encephalitis. Globally, JEV is responsible for ~68,000 clinical cases each year. The mortality associated with J.E. is as high as 25-30% in India, and even after treatment, 50% of survivors, especially children; suffer from lifelong disabilities due to late intervention and treatment.3, 4 This emphasizes the need for detection of JEV infection with a comprehensive understanding of the biology of the disease so that the pathogenesis could be controlled in a better way.
J.E. virus is a single-stranded, positive-sense RNA virus that belongs to the family Flaviviridae.5 The virion is composed of three structural proteins– nucleocapsid or core protein (C), non-glycosylated membrane protein (M) and glycosylated envelope protein (E), andseven non-structural (N.S.) proteins – NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5.6 J.E. viral infection has a complex molecular strategy to evade the host immune cell response. NS Proteins play a crucial role in generating neurovirulence in host neuronal cells by assisting the virus in the neuronal invasion, eliciting host immune response, and protecting immunity via generation of host antibodies.7, 8 Furthermore, it is evident that immune cells (e.g., microglia) act as a plausible reservoir of the virus particles in JEV infection, thus mediating the neuroinflammatory response by producing chemokines and cytokines, which further leads to neuronal cell death in bystander fashion.9, 10
During JE viral infection, systemic protein analysis of host and viral proteins can provide dynamic insights on virus-host interaction. These include immunity components, viral-host protein interaction products, and proteins targeted for degradation by viruses. Cerebrospinal fluid (CSF) proteomic analysis is a well-accepted powerful technique to examine protein changes in the brain.11 This analysis will allow us to see how host cells change after being infected with the J.E. virus and help identify critical targets for JEV disease. In the present study, we compared the CSF protein profiles of JE-AES patients with non-JE AES and other neurological disorders using 2-dimensional gel electrophoresis (2-DGE) and LC/MS-MS.
Materials and Methods
Human samples
Patients with acute encephalitis syndrome admitted to the Department of Medicine and Department of Pediatrics, King George Medical University, Lucknow, India, were investigated for JEV infection and divided into J.E. positive and non-JE AES patients. Other non-infectious neurological disease patients were included as a control in the study. This study has been approved by the Institutional ethics committee (Ref: 92nd ECM II A/P3). Demographic and clinical data of patients were recorded at the time of enrolment with the help of a questionnaire, as provided in table 1. The groups of this study as follows:
A: JE patients (n=25)
B: Non-JE AES patients (n=25).
Inclusion criteria for JEV and non-JEV AES patients were acute onset of fever with change in mental status (including symptoms such as confusion, disorientation, coma, or inability to talk) and new onset of seizures (excluding simple febrile seizures)1.
C: Non infectious other neurological diseases (n=20). Note: this group consists non-infectious other neurological diseases like seizures patients (n=7), stroke patients (n=11) and glioblastoma (n=2).
CSF sample collection
CSF samples were collected from all individuals by lumbar puncture followed by centrifugation for 15 min at 2,000 rpm. The supernatant was transferred into new vials and protease inhibitor cocktails were added in each sample and were stored at -80˚C for further analysis.
Detection of J.E.Virus
Enzyme-Linked Immunosorbent Assay (ELISA) was performed for all CSF samples using JE virus IgM ELISA kit (National Institute of Virology, Pune, India). JEV-IgM positive samples were further tested for IgM antibodies against Dengue by using Dengue IgM ELISA kit (National Institute of Virology, Pune, India) to avoid the possibility of cross-reaction with Dengue virus infection.
CSF protein sample preparation
40μl CSF samples from each patient per group were pooled and precipitated using acetone procedure. Briefly, CSF and acetone were mixed uniformly at a ratio of 1:6 and kept overnight at -20˚C and were centrifuged at 10,000 × g for 30 min at 4˚C. The supernatant was discarded, and the protein sample pellets were air-dried for further proteomic analysis.12
Protein quantification
The protein concentration of all CSF samples was determined by using Bradford’s method according to the manufacturer’s instructions (Bradford Protein Assay Kit, Bio-Rad).
2D Gel Electrophoresis and mass spectrometry analysis
2D Gel Electrophoresis
The first part of the 2 D gel electrophoresis was performed using the SCI-PLAS Isoelectric focusing System (IEF-SYS), Germany. Total 200 μg protein samples were dissolved in 125 μl of strip rehydration solution (8M urea, 2%CHAPS, 0.2M DTT, 0.2% bromophenol blue, and 0.4% resolytes). The prepared samples were loaded onto IPG Stip (3-10 pH, 7 cm, Biorad ReadyStrip™ IPG Strips) by in-gel sample rehydration overnight at room temperature. IEF strips were run in IEF unit at 500 V for 30 min, followed by 1,000 V for 30 min, 3,000 V for 6 hr, and 300V for 2 hr. The IPG strips were equilibrated in reducing buffer (6 M Urea, 20% glycerol, 2% SDS, 375 mM Tris-HCl pH 8.8 and 2% DTT) for 10 min, and in alkylating buffer containing 6 M Urea, 20% glycerol, 2% SDS, 375 mM Tris-HCl pH 8.8 and 2.5% iodoacetamide for additional 10 min. The IPG strips and molecular weight standard were placed on top of 13% SDS-PAGE gel and were sealed with 0.5% agarose. The second dimension electrophoresis protein separation was carried out under 60V for 10 min and then under 90V until the front of dye reached the bottom of the gel. After electrophoresis, gels were stained by silver nitrate.
Sample Preparation for mass spectrometry
Selected protein spots were excised from the stained gel and were transferred into microcentrifuge tubes for in-gel digestion. Destaining solution [40 mM ammonium bicarbonate (NH4CO3) and 40% acetonitrile (ACN)] was added to cover the gel pieces and was agitated until the gel pieces completely destained. 100% ACN was added to each tube and was incubated for 10-15 min followed by addition of sufficient reduction solution (5 mM DTT in 40 mM NH4CO3). The mixture was incubated at 60°C for 30 min. After cooling down to room temperature, sufficient alkylation solution (20 mM iodoacetamide (IAA) in 40 mM NH4CO3) was added. The mixture was kept in the dark at room temperature for 10 min. The solution was removed, and gel pieces were dehydrated with 100% ACN. Trypsin solution was prepared with a concentration of 10ng/µl in 40 mM NH4CO3 and enough trypsin solution was added to each microcentrifuge tube. The tubes were placed on ice for 45 minutes until the gel pieces were completely rehydrated. Subsequently, 40 mM NH4HCO3 was added to each tube. The tubes were incubated at 37°C overnight. The next day, 100 µl of 5% formic acid was added to each tube and incubated for 10 min at 37°C for peptide extraction, and the supernatant was collected into a fresh microcentrifuge tube. After that, 100 µl extraction buffer (5% Formic acid; 40% ACN) was added to each tube and was incubated for 10 min. For final extraction, 100% ACN was further added and was incubated for 10 min, and was dried down by vacuum drying procedure.
Mass spectrometry analysis
Digested peptides samples were analyzed using 120 minutes runtime on Orbitrap QE+ mass spectrometer interfaced with Vanquish3000 LC system (Thermo Scientific, Bremen, Germany). Each fraction was reconstituted in 0.1% formic acid and then loaded onto a 2 cm long pre-column (50 µ x 2 cm, 3 µ particle, and 100 Å pore size). The peptides were resolved on an analytical column (50 µ x 20 cm, 3 µ particle, and 100 Å pore size) using a linear gradient of 5% to 30% of solvent B (0.1% formic acid in 95% Acetonitrile) over 100 minutes and flow rate of 300 nl/min. Both MS and MS/MS were acquired using Orbitrap mass analyzer. MS scans were acquired in 350-1800 m/z range with an ion injection time of 60 ms, AGC target of 500,000, and resolution of 120,000 at 400 m/z. Most abundant peptides with 2-5 charges were acquired in data-dependent mode, and exclusion duration was set to 30 seconds. Higher-energy collisional dissociation (HCD) was used for fragmentation and set at 37%. Fragment ions were detected in Orbitrap with a mass resolution of 30,000 at 400 m/z and AGC target value was set to 50,000. MS3 scans were acquired on Orbitrap for accurate relative quantitation. Synchronous precursor selection was enabled, and HCD collision energy of 65% was used with AGC target set to 2,000 and maximum ion injection time of 200 ms. Internal calibration was carried out using the lock mass option (m/z 445.1200025) from ambient air.
Biological pathways analysis
The identified proteins were then used to fetch Gene Ontology terms using Cluego plugging.13-15 Only p-Value significant Gene Ontology terms associated processes and pathways were considered.
Results
Demography and clinical characteristics
The demographic and clinical characteristics of patients are detailed in table 1.
Table 1: Demographic and clinical characteristics of all group patients
| S.NO. | Characteristics | Non JE AES patients (N=25) | JE patients (N=25) | Other neurological
(N=20) |
| Demographic characteristic | ||||
| 1 | Mean age (±SD) | 14.80 ± 2.572 | 20.42 ± 3.000 | 21.58 ± 4.118 |
| 2
3 |
Male
Female |
17
08 |
14
11
|
13
07 |
| Clinical features | ||||
| 1 | Fever | 21 | 24 | 0 |
| 2 | Headache | 23 | 24 | 03 |
| 3 | Vomiting | 17 | 16 | 07 |
| 4 | Altered sensorium | 16 | 21 | 0 |
| 5 | Behavioral abnormalities | 17 | 18 | 02 |
| 6 | Neck Stiffness | 05 | 03 | 0 |
| 7 | GCS≤8 | 09 | 11 | 11 |
| 8 | GCS˃8 | 16 | 14 | 09 |
2-DGE and mass spectrometry analysis
The final protein concentration of CSF samples was found 1223.81μg/ml, 1392.21μg/ml, and 1561.91μg/ml of JEV (group A), non-JEV AES (group B), and non-infectious other neurological diseases (group C), respectively. We observed 22 protein spots in JE CSF, 32 protein spots in non-JE AES patients, and 49 protein spots in non-infectious other neurological diseases patients after silver staining (Figure 1). Inter gel comparison of these three groups was analyzed using Same Spots v 5.1.012 online software. Total 16 protein spots were differentially expressed in JE CSF. Out of these sixteen differentially expressed protein spots, three spots based on size and density were further processed for biomarker identification using mass spectrometry LC-MS/MS. The proteins identified in these three spots are illustrated in table 2.
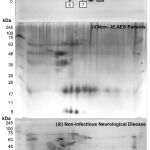 |
Figure 1 |
Table 2: Protein Identified by 2D-GE LC-MS/MS
| SPOT No. | Protein ID | Accession No. | Peptides | Unique
Peptides |
| 1 | Serum albumin
Keratin, type II cytoskeletal 1 Tumor-associated calcium signal transducer 2 Immunoglobulin lambda-like polypeptide 5 isoform 1 Cystatin-C Immunoglobulin heavy variable 4-38-2-like Ubiquitin-60S ribosomal protein L40 isoform X1 Metalloproteinase inhibitor 1 Chitinase-3-like protein 1 Tumor necrosis factor receptor superfamily member 6B
|
gi|4502027
gi119395750 gi|166795236 gi|295986608 gi|4503107 gi|768041374 gi|1034609204 gi|4507509 gi|144226251 gi|4507585
|
11
6 2 2 1 1 1 1 1 1 |
11
5 2 2 1 1 1 1 1 1 |
| 2.
3.
|
Serum albumin
Immunoglobulin lambda-like polypeptide 5 isoform 1 Keratin, type II cytoskeletal 1 Hemoglobin subunit beta Immunoglobulin J chain Fatty acid-binding protein, heart isoform 1 Immunoglobulin heavy variable 4-38-2-like Apolyprotein E Isoform a Cystatin C
Serum albumin Serotransferrin isoform 1 Hemoglobin subunit beta Apolipoprotein D Alpha-2-macroglobulin isoform X1 Eukaryotic translation initiation factor 5A-1 isoform X1 Alpha-crystallin A chain isoform 1 Dermcidin isoform 2 preproprotein Beta-2-microglobulin isoform X1 Compliment C3
|
gi|4502027
gi|295986608 gi|119395750 gi|4504349 gi|1004426878 gi|1004426878 gi|21489959 gi|705044002 gi|4503107
gi|4502027 gi|4557871 gi|4504349 gi|4502163 gi|578822814 gi|1370470360 gi|4503055 gi|665505971 gi|530406197 gi|115298678
|
21
2 4 1 1 1 1 5 5
10 3 2 2 1 1 1 1 1 1 |
21
2 4 1 1 1 1 5 5
10 3 2 2 1 1 1 1 1 1 |
Proteins biological function
Biological functions of JE CSF proteins were analyzed by using Cytoscape system for direct and indirect relationships. P-value of proteins for eg,serum albumin, keratin, type II cytoskeletal 1, immunoglobulin lambda-like polypeptide 5 isoform 1, immunoglobulin heavy variable 4-38-2-like, metalloproteinase inhibitor 1, hemoglobin subunit beta, immunoglobulin J chain, serotransferrin, alpha 2 microglobulin found significant in Gene Ontology terms associated biological pathways. Serotransferin and immunoglobulin J chain were found related to antimicrobial humoral response while immunoglobulin lambda five, A2M were found related to complement activation and apoptotic pathway. Keratin 1 protein was found involved in the establishment of skin barriers as a cell organization process. Tissue metalloproteinase inhibitor (TIMP-1) was found associated with endopeptidase inhibitor activity and apoptotic processes. Serum albumin and hemoglobin subunit beta were found associated with blood plasma lipoprotein particle remodeling and platelet aggregation. Similarly, other CSF proteins for eg; tumor-associated calcium signal transducer 2, Ubiquitin-60S ribosomal protein L40 isoform X1, Cystatin-C, Chitinase-3-like protein 1, Tumor necrosis factor receptor superfamily member 6B, Fatty acid-binding protein, heart isoform 1, Fatty acid-binding protein, heart isoform 1, Apolipoprotein D, Eukaryotic translation initiation factor 5A-1 isoform X1, Alpha-crystallin A chain isoform 1, Dermcidin isoform 2 preproprotein, Beta-2-microglobulin isoform X1, Compliment C3 and their biological functions are summarized in table 3A and B.
Table 3: Proteins biological functions
| A. p-value significant Gene Ontology terms associated biological pathways of proteins | ||||||
| S.No. | Protein | Biological functions | ||||
| 1 | Serum albumin | Blood coagulation | ||||
| 2 | Keratin, type II cytoskeletal 1 | Cell organization | ||||
| 3 | Immunoglobulin lambda-like polypeptide 5 isoform 1 | Immune responses | ||||
| 4 | Immunoglobulin heavy variable 4-38-2-like | Immune response | ||||
| 5 | Metalloproteinase inhibitor 1 | Apoptotic pathway | ||||
| 6 | Hemoglobin subunit beta | Blood coagulation | ||||
| 7 | Immunoglobulin J chain | Immune responses | ||||
| 8 | Serotransferrin | Immune response and iron transport | ||||
| 9 | Alpha 2 microglobulin | Immune response & apoptotic pathway | ||||
| B. Other proteins biological pathways | ||||||
| 1 | Tumor-associated calcium signal transducer 2 | Sensory transduction | ||||
| 2 | Ubiquitin-60S ribosomal protein L40 isoform X1 | Cellular protein modification, | ||||
| 3 | Cystatin-C | Inflammation | ||||
| 4 | Chitinase-3-like protein 1 | Immune responses (Inflammation) | ||||
| 5 | Tumor necrosis factor receptor superfamily member 6B | Immune responses(Inflammation, apoptosis) | ||||
| 6 | Fatty acid-binding protein, heart isoform 1 | Fatty acid metabolism | ||||
| 7 | Apolyprotein E Isoform a | Lipid Transport | ||||
| 8 | Apolipoprotein D | Lipid Transport, Immune response | ||||
| 9 | Eukaryotic translation initiation factor 5A-1 isoform X1 | Cellular response to virus, Protein transport | ||||
| 10 | Alpha-crystallin A chain isoform 1 | Apoptotic process | ||||
| 11 | Dermcidin isoform 2 preproprotein | Antimicrobial humoral response | ||||
| 12 | Beta-2-microglobulin isoform X1 | Immune response | ||||
| 13 | Compliment C3 | Immune response | ||||
 |
Figure 2 |
Discussion
In our study, we found that all three differential protein spots from CSF of JEV patients contained multiple immunological proteins such as α2M (Alpha 2 microglobulin), beta-2microglobulins (β2M), immunoglobulin lambda-like polypeptide 5, immunoglobulin heavy variable 4-38-2-like, immunoglobulin J chain, indicating host immune cell response against JEV infection and blood-brain barrier (BBB) impairment. α2M is a member of alpha macroglobulin, which acts as a marker for BBB impairment and has protein transport function and anti-protease activity.16-18 Conversely, α2M has also been shown to bind and internalize viral proteins, regulate immune responses, and increase virus infectivity.16-19 Similarly, we also observed another key protein, beta 2 microglobulins (β2M) which is a consistent marker in different inflammatory diseases, neuroinfectious disorders such as HIV and other neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease.20-26 Previous study investigated that Beta-2-microglobulin (B2M) protein upregulated after JE viral infection.27
Sero-transferrin (Tf) was found in the CSF of J.E. patients. Tf is an important beta-globin that helps in iron transportation, energy balance, and metabolism. The presence of Tf in CSF represents a case of BBB damage and disease pathology following J.E. viral infection.28 Along with this, we found tissue inhibitor metalloproteinases (TIMP1) in J.E. patients, which is consistent with our previous findings, that highlights that matrix metalloproteinase (MMPs) and TIMP play key roles in the pathogenesis of J.E. viral infection by modulating BBB integrity and infiltration of peripheral immune cells in CNS29 and found significantly higher TIMP-1 concentration in CSF of JEV infected children compared to control group.30
Complement protein C3 that complement system activation may play a role in JEV infection pathogenesis. Privious study shows that in flavivirus infections, the complement system plays an antagonistic role, either by restricting viral replication and protecting the host, or by exacerbating the inflammatory response, increasing illness severity.31 Jongen PJH et al. (2000) demonstrated that the CSF level of C3 protein represents intrathecal C3 production and significantly affects immunopathogenesis or effector mechanism in immune-mediated inflammatory neurodegeneration disorders. In continuation of these findings, it has also been observed that the CSF level of complement protein (C1q/C3) correlates with patients’ neuronal injury level.32
Along with this, we also noted ubiquitin-60S ribosomal protein L40 isoform X1 and cystatin C protein in CSF of J.E. patients, which are found to be an essential component of cellular pathways for viral processing, protein transport, protein degradation, and cell-matrix interactions.33
In addition, we found Chitinase-3-like protein 1 (YKL-40), which belongs to the conservative Chitinase family in mammals. This protein has been investigated previously in the role of the inflammatory process like multiple sclerosis, inflammatory bowel diseases, rheumatoid arthritis, inflammatory lung diseases, osteoarthritis, cardiovascular disease, psoriasis, and viral hepatitis.34-36 In previous observations of other viral diseases,the level of YKL-40 is observed to be increased in CSF and found to be correlated with the increased level of axonal injury in HIV.37, 38 Our results agree with the hypothesis that JEV infection in the brain results in activation of glial cells, for instance microglia and astrocytes and leads to neuronal injury, suggesting the importance of using YKL-40 as a prognostic marker in neuroinflammation mediated neurodegenerative diseases.
Tumor necrosis factor receptor (TNFR) superfamily member 6b (TNFRSF6B) is known as Decoy receptor 3 (DcR3) and expressed after inflammatory stimuli by T cells, monocytes, and epithelial cells.39 DcR3 was shown to block the signaling of three TNF ligand members, FasL, LIGHT, TL1A, and disease pathogenesis.40-42 In a previous study, detecting DcR3 in cerebrospinal fluid is helpful as a diagnostic and prognostic marker of different neurological diseases and other infectious disorders such as bacterial meningitis acute respiratory diseases syndrome (ARDS).43, 44 However, the presence of DcR3 in CSF of J.E. patients needs further investigation.
Interestingly, Apolipoprotein E (ApoE) and Apolipoprotein D (ApoD) are found in CSF of J.E. patients. In one study, apoE level is found increased in CSF from patients with Alzheimer’s disease (A.D.) and other neurological and psychiatric disorders, which indicates that CSF apoE tests can generally represent neuronal damage and inflammatory brain reactions.45 However, their role in viral infectious diseases is controversial as it is involved in both pro and antiviral activity of HIV.46-48 Similarly, we also noted ApoD in CSF of JEV patients; likeApoE, ApoD is also expressed in glial cells in the brain and associated with the development and repair of the nervous system.49, 50 In another study Apolipoprotein D (ApoD) expression was found to be elevated in the central nervous system (CNS) of mice after Japanese encephalitis virus (JEV) infection.51
Along with this, the biological functions of proteins were identified by Cytoscape in gene ontology (G.O.) term and found many proteins related to immune responses, inflammatory and apoptotic cell death pathways, and other key pathways e.g., autophagy regulation, cellular organization, cellular protein modification, lipid transportation, fatty acid metabolism, iron regulation, are explicitly involved in JEV pathologies.
Conclusion
In summary, we mainly found protein biomarkers, which related to autophagy, inflammation and neurodegenration (Figure 2) and may serve as a reference list for future CSF proteome studies in JE disease. Extensive prospective studies would be required to evaluate the efficacy of these biomarkers in JE-specific biomarkers for global application.
Acknowledgment
The authors, duly acknowledges, late Dr T. N. Dhole and Gourav Day for providing technical as well as methodology assistance during the work.
Conflict of Interest
There is no conflict of interest
Funding Sources
This study was approved by the Institutional ethics committee (Ref: 92nd ECM II A/P3) and was supported by the Ramalingaswami fellowship (BT/RLF/Re-entry/13/2014) from the Department of Biotechnology, Ministry of Science and Technology, Govt. of India to A.K.
References
- Baluni, M., Ghildiyal, S., Singh, D., Reddy, D. H., Kumar, R., Dhole, T. N. Increased serum microRNA-29b expression and bad recovery in Japanese encephalitis virus infected patients; A new component to improve the disease recovery. Journal of Neuroimmunology, 2018; 323, 56-61.
CrossRef - Misra, U.K. and J. Kalita, Changing spectrum of acute encephalitis syndrome in India and a syndromic approach. Annals of Indian Academy of Neurology. 2022; 25(3): p. 354.
CrossRef - Baluni M, Fatima T, Zia A, Reddy DH, Dhole TN. Association of ICAM-1 (K469E) and MCP-1-2518 A> G polymorphism with risk of Japanese encephalitis in North Indian population. Cytokine. 2018; 111: p. 420-427.
CrossRef - Kulkarni R, Sapkal GN, Kaushal H, Mourya DT. Suppl-2, M8: Japanese Encephalitis: A Brief Review on Indian Perspectives. The open virology journal. 2018; 12: p. 121.
CrossRef - Thounaojam MC, Kundu K, Kaushik DK, Swaroop S, Mahadevan A, Shankar SK, Basu A. MicroRNA 155 regulates Japanese encephalitis virus-induced inflammatory response by targeting Src homology 2-containing inositol phosphatase 1. Journal of virology. 2014; 88(9): p. 4798-4810.
CrossRef - Tiwari S, Chitti SV, Mathur A, Saxena SK. Japanese encephalitis virus: an emerging pathogen. American Journal of Virology. 2012; 1(1):1-8.
CrossRef - Lin YL, Chen LK, Liao CL, Yeh CT, Ma SH, Chen JL, Huang YL, Chen SS, Chiang HY. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. Journal of virology. 1998: 1; 72(1):191-200.
CrossRef - Yang Y, Ye J, Yang X, Jiang R, Chen H, Cao S. Japanese encephalitis virus infection induces changes of mRNA profile of mouse spleen and brain. Virology journal. 2011; 8(1):1-1.
CrossRef - Yang Y, Ye J, Yang X, Jiang R, Chen H, Cao S. Japanese encephalitis virus infection induces changes of mRNA profile of mouse spleen and brain. Virology journal. 2011; 8(1):1-1.
CrossRef - Thongtan T, Cheepsunthorn P, Chaiworakul V, Rattanarungsan C, Wikan N, Smith DR. Highly permissive infection of microglial cells by Japanese encephalitis virus: a possible role as a viral reservoir. Microbes and infection. 2010; 1;12(1):37-45.
CrossRef - Tumani H, Teunissen C, Süssmuth S, Otto M, Ludolph AC, Brettschneider J. Cerebrospinal fluid biomarkers of neurodegeneration in chronic neurological diseases. Expert review of molecular diagnostics. 2008; 1;8(4):479-94.
CrossRef - Chen RL, Sage EA, Dunn MJ, Wait R, Preston JE. Optimising ovine cerebrospinal fluid preparation for two‐dimensional gel electrophoresis. Proteomics. 2006: 6(10):3170-5.
CrossRef - Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research. 2003; 1;13(11):2498-504.
CrossRef - Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. 2014; 15(12):1-21.
CrossRef - Bindea G, Galon J, Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics. 2013; 1;29(5):661-3.
CrossRef - Alonso M, Dimitrijevic A, Recuero M, Serrano E, Valdivieso F, López-Guerrero JA. Interaction of α-2-macroglobulin and HSV-1 during infection of neuronal cells. Journal of neurovirology. 2001; 7(6):556-63.
CrossRef - Cucullo L, Marchi N, Marroni M, Fazio V, Namura S, Janigro D. Blood-brain barrier damage induces release of α2-macroglobulin. Molecular & Cellular Proteomics. 2003; 1;2(4):234-41.
CrossRef - Gupta, A.K., Pokhriyal, R., Khan, M.I., Kumar, D.R., Gupta, R., Chadda, R.K.,et al.. Cerebrospinal fluid proteomics for identification of α2-macroglobulin as a potential biomarker to monitor pharmacological therapeutic efficacy in dopamine dictated disease states of Parkinson’s disease and schizophrenia. Neuropsychiatric Disease and Treatment. 2019; 15, p.2853.
CrossRef - Krause K, Azouz F, Nakano E, Nerurkar VR, Kumar M. Deletion of pregnancy zone protein and murinoglobulin-1 restricts the pathogenesis of west nile virus infection in mice. Frontiers in Microbiology. 2019; 13; 10:259.
CrossRef - Hansson SF, Puchades M, Blennow K, Sjögren M, Davidsson P. Validation of a prefractionation method followed by two-dimensional electrophoresis–Applied to cerebrospinal fluid proteins from frontotemporal dementia patients. Proteome science. 2004; 2(1):1-1.
CrossRef - Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 2003; 53(S3):S49-60.
CrossRef - Mattsson N, Insel P, Nosheny R, Zetterberg H, Trojanowski JQ, Shaw LM, Tosun D, Weiner M. CSF protein biomarkers predicting longitudinal reduction of CSF β-amyloid42 in cognitively healthy elders. Translational psychiatry. 2013; 3(8):e293.
CrossRef - Batista Muñoz A, Hadley S, Iriondo Sanz M, Agut Quijano T, Camprubí Camprubí M. Role of beta-2-microglobulin as a biomarker in very preterm and extremely preterm infants with CNS inflammation. Plos one. 2019; 7; 14(5):e0216498.
CrossRef - Peterslund NA, Black FT, Geil JP, Mogensen CE. Beta‐2‐microglobulin in the cerebrospinal fluid of patients with infections of the central nervous system. Acta neurologica scandinavica. 1989; 80(6):579-83.
CrossRef - Svatoňová J, Bořecká K, Adam P, Lánská V. Beta2-microglobulin as a diagnostic marker in cerebrospinal fluid: a follow-up study. Disease markers. 2014; 8;2014.
CrossRef - Zhang, J., D.R. Goodlett., T.J. Montine. Proteomic biomarker discovery in cerebrospinal fluid for neurodegenerative diseases. Journal of Alzheimer’s Disease, 2005; 8(4): p. 377-386.
CrossRef - Zhang LK, Chai F, Li HY, Xiao G, Guo L. Identification of host proteins involved in Japanese encephalitis virus infection by quantitative proteomics analysis. Journal of proteome research. 2013; 12(6):2666-78.
CrossRef - Przyjałkowski W, Lipowski D, Kolasa T, Issa E, Olejnik Z. Blood-cerebrospinal fluid barrier in purulent cerebrospinal meningitis. Neurologia i Neurochirurgia Polska. 1996; 30(1):39-48.
- Shukla V, Shakya AK, Dhole TN, Misra UK. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of children with Japanese encephalitis virus infection. Archives of virology. 2013; 158(12):2561-75.
CrossRef - Shukla V, Shakya AK, Dhole TN, Misra UK. Matrix metalloproteinases and their tissue inhibitors in serum and cerebrospinal fluid of children with Japanese encephalitis virus infection. Archives of virology. 2013; 158(12):2561-75.
CrossRef - Conde JN, Silva EM, Barbosa AS, Mohana-Borges R. The complement system in flavivirus infections. Frontiers in microbiology. 2017: 14; 8:213.
CrossRef - McGuire JL, Gill AJ, Douglas SD, Kolson DL. The complement system, neuronal injury, and cognitive function in horizontally-acquired HIV-infected youth. Journal of neurovirology. 2016; 22(6):823-30.
CrossRef - Nycander M, Estrada S, Mort JS, Abrahamson M, Björk I. Two‐step mechanism of inhibition of cathepsin B by cystatin C due to displacement of the proteinase occluding loop. FEBS letters. 1998; 23; 422(1):61-4.
CrossRef - Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World journal of gastroenterology: WJG. 2009; 11;15(42):5249.
CrossRef - Salomon J, Matusiak Ł, Nowicka-Suszko D, Szepietowski JC. Chitinase-3-like protein 1 (YKL-40) is a new biomarker of inflammation in psoriasis. Mediators of inflammation. 2017; 28;2017.
CrossRef - Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annual review of physiology. 2011; 17; 73:479-501.
CrossRef - Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, et al. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. The American journal of pathology. 2008; 1;173(1):130-43.
CrossRef - Hermansson L, Yilmaz A, Axelsson M, Blennow K, Fuchs D, Hagberg L,et al. Cerebrospinal fluid levels of glial marker YKL-40 strongly associated with axonal injury in HIV infection. Journal of neuroinflammation. 2019; 16(1):1-9.
CrossRef - Hsieh SL, Lin WW. Decoy receptor 3: an endogenous immunomodulator in cancer growth and inflammatory reactions. Journal of biomedical science. 2017; 24(1):1-9.
CrossRef - Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002; 1;16(3):479-92.
CrossRef - Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998; 396(6712):699-703.
CrossRef - Yu KY, Kwon B, Ni J, Zhai Y, Ebner R, Kwon BS. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. Journal of Biological Chemistry. 1999; 14;274(20):13733-6.
CrossRef - Liu YJ, Shao LH, Wang Q, Zhang J, Ma RP, Liu HH, et al. Predictive value of decoy receptor 3 in postoperative nosocomial bacterial meningitis. International Journal of Molecular Sciences. 2014; 3;15(11):19962-70.
CrossRef - Mueller AM, Pedré X, Killian S, David M, Steinbrecher A. The Decoy Receptor 3 (DcR3, TNFRSF6B) suppresses Th17 immune responses and is abundant in human cerebrospinal fluid. Journal of neuroimmunology. 2009; 30;209(1-2):57-64.
CrossRef - Lindh M, Blomberg M, Jensen M, Basun H, Lannfelt L, Engvall B, et al. Cerebrospinal fluid apolipoprotein E (apoE) levels in Alzheimer’s disease patients are increased at follow up and show a correlation with levels of tau protein. Neuroscience letters. 1997; 27;229(2):85-8.
CrossRef - Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE ε4/ε4 genotype accelerates HIV disease progression. Proceedings of the National Academy of Sciences. 2008; 24;105(25):8718-23.
CrossRef - Dobson CB, Sales SD, Hoggard P, Wozniak MA, Crutcher KA. The receptor-binding region of human apolipoprotein E has direct anti-infective activity. The Journal of infectious diseases. 2006; 1;193(3):442-50.
CrossRef - Kelly BA, Neil SJ, McKnight Á, Santos JM, Sinnis P, Jack ER, et al. Apolipoprotein E‐derived antimicrobial peptide analogues with altered membrane affinity and increased potency and breadth of activity. The FEBS journal. 2007; 274(17):4511-25.
CrossRef - Lyoumi S, Tamion F, Petit J, Dechelotte P, Dauguet C, Scotte M, et al. Induction and modulation of acute-phase response by protein malnutrition in rats: comparative effect of systemic and localized inflammation on interleukin-6 and acute-phase protein synthesis. The Journal of nutrition. 1998; 1; 128(2):166-74.
CrossRef - Münch V, Trentin L, Herzig J, Demir S, Seyfried F, Kraus JM, et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood, The Journal of the American Society of Hematology. 2017; 3; 130(5):643-54.
CrossRef - Saha S, Rangarajan PN. Common host genes are activated in mouse brain by Japanese encephalitis and rabies viruses. Journal of General Virology. 2003; 1; 84(7):1729-35.
CrossRef







