Manuscript accepted on :21-01-2020
Published online on: 28-01-2020
Plagiarism Check: Yes
Reviewed by: Virendra S. Ligade
Second Review by: Chunyan Ren
Final Approval by: Dr. Mohamed Abdel-Daim
Sunita S Patil1* and Vaishali S Patil2
and Vaishali S Patil2
1Department of Pharmacology, D Y Patil Medical college and University, Kaolhapur, India, 416006
2Department of Biochemistry, D Y Patil Medical college and University ,Kaolhapur, India, 416006
Corresponding Author E-mail : drpsunita@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/1866
Abstract
The people living with HIV/AIDs have various types of hematological disorders of various degree of varying severity. Aim is to study different hematological profile before and after HAART and its correlation with CD4 count. Study conducted in western Maharashtra. Socio-demographic characteristics were recorded & Hematological profile and CD4 count before and 6 months after HAART in AIDs patients, collected from the data book of laboratory after permission from physician. Correlation of these blood profile and CD4 count before and after HAART which will help for prognosis and clinical evaluation of AIDs patients during treatment. In 100 patients 56% were female and 44% were male.Most of the patients female were in age group of 31-40(35.7%) and males were in age group of 21-30(34%).Significant improvement was seen in hemoglobin level, total leucocyte count & CD4 count after HAART. Significant macrocytosis observed after ART treatment. Thus Anaemia is a very important and common presentation. HAART produces a definitive improvement of hematological parameters and CD4 cell count. There is significant association between increase in hemoglobin level and CD4 cell count, which can be used for prognosis and clinical evaluation.
Keywords
Anaemia; AIDS; CD4; HIV; HAART; Hematological Parameters
Download this article as:| Copy the following to cite this article: Patil S. S, Patil V. S. Correlation of Blood Profile and CD4 Count in AIDS Patients Before and After HAART, Study in Western Maharashtra. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Patil S. S, Patil V. S. Correlation of Blood Profile and CD4 Count in AIDS Patients Before and After HAART, Study in Western Maharashtra. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2RAErs2 |
Introduction
HIV(Human immunodeficiency virus) infection is global pandemic with cases reported from virtually every country. As per the HIV estimation 2015 report, adult (15-49 yrs) HIV prevalence in India was estimated at 0.26%(0.22%-0.32%). Infection with HIV commonly associate with hematopoietic system. Hematological complications have been documented to be second most common cause of morbidity and mortality in HIV patients. [1]
Disorder of hematological system including anemia, leucopenia,and thrombocytopenia are common throughout the course of HIV infection and may be direct result of HIV manifestations, secondary infections or side effect of therapy. Among all anemia is the most common hematologicalabnormality and a significant predictor of progression to AIDs(Acquired immune deficiency syndrome) or death. [2’3]
Now Highly active antiretroviral therapy (HAART) is recommended as a standard medication for the management of HIV infection.The HAART,includes nucleoside and non-nucleoside analogues with potential to inhibit HIV reverse transcriptase and protease inhibitors.Combination of three or four drugs which create multiple obstacles to HIV replication to keep the number low and reduce the possibility of a superior mutation arising.[4] This however, results in improved quality of life of people living with HIV/AIDS.[5]Use of antiretroviral drugs could positively or negatively affect the hematological parameters depending on choice of combination used. It is observed that the HIV drugs show some adverse effects like nausea, diarrhea, anemia, neutropenia etc. [6]
There are studies describing hematological parameters of HIV infected individuals after receiving HAART, however very few published reports seen in western Maharashtra, hence the aim of the present study is to assess hematological profile in HIV infected adults after initiation of HAART and to compare the mean difference of hematological profile and CD4 count between baseline and 6 months after initiation of HAART.
Materials and Methods
This study includes 100 diagnosed patients attending ART(antiretroviral treatment) Centre. Investigations(hematological profile and CD4) were done of each patient in the laboratory. We have taken the investigation data of HIV patients from the data book of laboratory and explored to find the correlation of the hematological profile and CD4 count in patients before and after HAART (highly active antiretroviral retroviral treatment).Permission to take data was taken from physician.All procedure done and method used in this study were in accordance with the ethical standards of institutional research committee.
Data collection
The sociodemographic characteristics, clinical information and hematological parameters of the study subjects were recorded from ART log book. All parameters for each patient were collected at 0 and 6 months following HAART initiation. Data includes Hemoglobin level, WBC(white blood cell) count, differtial WBC, MCV(mean cell volume), Platelets by automated blood analyzer and CD4 count by flow-cytometry were collected.
Hematological Abnormalities
Certain abnormalities were determined in the subject on HAART. Anemia defined as hemoglobin less than 12 gm/dl. Mild anaemia as hemoglobin value of 10-11 gm/dl. Moderate anaemia as hemoglobin value of 8-9gm/dl and severe anaemia as hemoglobin value less than 8gm/dl, Leucopenia as total WBC count less than 4000 cells/µl. Neutropenia as absolute neutrophils/granulocyte count less than 1000 cells/µl and lyphocytopenia considered at lymphocyte count of less than 800 cells/µl. Thrombocytopenia considered as platelet count less than 150x 103 /µl. Macrocytosis as(MCV) mean cell volume more than 100 fl.
Statistical Analysis
All data analysis was done using SPSS version 17 statistical software package. Data was presented as mean±standard deviation(SD)and calculation done by using student t test. Difference were considered to be of statistical significant at an error probability of less than 0.05(p< 0.05)
Results
In present study total 100 patients data was studied.
Table 1: Hematological parameters of HIV patients
| Parameters | Before starting ARV drugs(0 month)
mean ± SD |
After starting ARV drugs (6 month)
mean ± SD |
P- value |
| Hemoglobin(g/dl) | 10.10 ±1.13 | 11.50 ±1.30 | 0.001(S) |
| WBC (103 /µl) | 3.10 ±1.33 | 4.68 ±1.30 | 0.002(S) |
| Neutrophil(103 /µl)) | 2.30±1.05 | 1.10 ±0.99 | 0.001(S) |
| Lymphocyte(103/µl) | 2.16±0.87 | 2. 14±0.78 | 0.870(NS) |
| MCV(fl) | 82.44±1,62 | 103.2±1.78 | 0.001(S) |
| Platelets(103/µl) | 235.52± 40.20 | 230.60±45.60 | 0.414(NS) |
| CD4 cell/ µl) | 263.20± 160.10 | 360.40±140.90 | 0.002(S) |
(S)-statistical significant. (NS)-statistical not significant.
From table 1 -Following parameters, hemoglobin level, WBC count, neutrophil count, lymphocyte count, MCV, Platelets and CD4 count were estimated and shows the mean hemoglobin at baseline (before HAART) is 10.10 ±1.13 which after 6 months of HAART is 11.50 ±1.30(p=0.001) Thus significant increase in hemoglobin level was seen after 6 month of HAART treatment. MCV shows significant difference (p=0,001) macrocytosis after 6 month of HAART treatment. CD4 count shows significant increase (p=0.002) after HAART treatment. Neutrophil level show significant reduction (p=0.001) after 6months of HAART treatment.
Table 2: Cytopenia seen in number of patients
| Hematological abnormalities | Before treatment no of patients. (n=100) | 6-month after treatment no of patients. (n=100) | |||
| Number | % | Number | % | ||
| Leukopenia (<4000cell/ µl) | 12 | 12 | 3 | 3 | |
| Neutropenia (<1000cell/ µl) | 10 | 10 | 4 | 4 | |
| Lymphopenia (<800cell/ µl) | 18 | 18 | 6 | 6 | |
| Thrombocytopenia(<150X103cell/ µl) | 10 | 10 | 7 | 7 | |
| Anaemia
Mild -(Hb 10-11gm/dl) Moderate(Hb 8-9gm/dl) Severe(Hb< 8 gm/dl)
|
25
23 6 |
25
23 6 |
6
10 4 |
6
10 4 |
|
| CD4 (<200cell/ µl) | 48 | 48 | 20 |
20 |
|
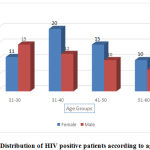 |
Graph 1: Distribution of HIV positive patients according to age and sex |
From Graph 1, shows 56% were female and 44% were male. Patients were grouped in four groups according to age between21-30(26%), 31-40(32%), 41-50(25%),51-60(17%).Most of the patients female were in age group of 31-40(35.7%) and males were in age group of 21-30(34%).
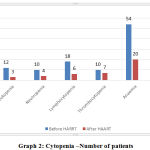 |
Graph 2: Cytopenia – Number of patients |
From Graph 2, out of 100 cases leucopenia was seen in 12%(n=12) cases while rest had TLC within normal limits .After 6 months of HAART 75%(n=9) of these cases showed improved. Neutropenia was seen in 10%(n=10) of total cases which after 6 months of HAART regimen 60%(n=6) of these cases showed improvement. Lymphopenia was present in 18% of total cases and after HAART regimen 66.6%(n=12) of these cases showed improvement. Thrombocytopenia was seen in 10% of total cases , after HAART only 30%(n= 3) showed improvement.Anaemia was seen in 54% of total cases after HAART 63%(n=34) of these cases showed improvement. CD4 level less than 200 cell/ µl were seen in 48 of total cases among these 58.3%(n=28) showed improvement after HAART.
Discussion
The result of the data analysis obtained shows a predominance of female among 100 patients that is female constituted 56% in present study. Same findings also reported by Manisha SP et.al.[7]The patients of age in the present study with 35.7% of female patients in the age group 31 to 40 years, which is sexually active part of life similar finding mentioned by J.K,Mitra et.al[8]and 34% of male patients in age group 21-30.
Anaemia, leucopenia especially neutropenia and thrombocytopenia were common finding in present study with anaemia in 54%, leucopenia in 12% and thrombocytopenia in 10%.These findings was also documented in different studies.[9,10]Anemia was the most common hematological abnormality detected in our study about 54%.HIV infection may lead to anaemia in many ways like changes in cytokine productions, decreased erythropoietin concentrations, opportunistic infectious agents. Similar more prevalence of anaemia seen in studies, 65.5% by Dikshitet.al[11] and 61% by Kasthuri et.al.[12] This may be due to difference in study population,socio-demographic characteristics of study subjects.In present study prevalence of anaemia was significantly reduced to 37% after receiving antiretroviral therapy(ART) . This finding is in agreement with other studies[13,14] showing use of ART results in decrease in prevalence of anaemia.
In present study highly significant increase in MCV level (macrocytosis) was seen after 6 month of ART. Similar findings were seen in various studies and this increase in MCV used as surrogate marker for adherence to HAART.[15]Out of 100 patients 48%(n=48) cases with CD4-T cell count< 200 cell/cumm, indicating stage of immunosuppression. Similarly other study conducted in India showed 89.2% cases at baseline were with CD4-T cell count < 200 cell/cumm.[16]In our study we found haemoglobin level significantly increased with high CD4 cell count.This is consistent with other studies that have shown an association between leucopenia and low CD4 counts with anaemia.[17,18]Our further extension of project is to study correlation of haematological profile, CD4 count with oxidative stress parameters in HIV patients before and after HAART.
Conclusion
On the basis of above observations, the hematological disorders are very common in HIV patients. Anaemia is a very important and common presentation.HAART produces a definitive improvement of haematilogical parameters and CD4 cell count. There is significant association between increase in hemoglobin level and CD4 cell count,which can be used for clinical evaluation.
Acknowledgement
I acknowledge the ART center for collection of data.
Conflict of interest
There is no conflict of interest.
Ethical Approval
Institutional ethical approval was taken.
References
- Cosby CD. Hematologic disorders associated with human immunodeficiency virus and AIDS. J InfusNurs. 2007; 30(1):22–32
CrossRef - Volberding P. The impact of anemia on quality of life in human immunodeficiency virus-infected patients. J Infect Dis. 2002; 185(Suppl 2):S110–S114.
CrossRef - Odunukwe N, Idigbe O, Kanki P, Adewole T, Onwujekwe D, Audu R, Onyewuche J. Haematological and biochemical response to treatment of HIV-1 infection with a combination of nevirapine + stavudine + lamivudine in Lagos, Nigeria. Turkish J Haem. 2005;22:125–131
CrossRef - Amegor O, Bigila D, Oyesola O, Oyesola T and Buseni S. Hematological changes in HIV patients placed on anti retroviral therapy in markurdi, Benue state of Nigeria. Asian J of Epidemiol 2009;2(4) : 97-103.
CrossRef - Study confirms effectiveness of antiretroviral drugs for HIV patients. Lancet 2003;362:1267-1274.
CrossRef - Farizo KM, Buchler JW, Ckhamberland ME. Spectrum of Spectrum of disease in person with human immunodeficiency virus infection in the United States. IDMA. 1992;267:1798-1805.
CrossRef - ManishaSP,Anil SG, JayatR,Abhaynkar,Madhavi CA. Haematological profile of HIV positive patients. Indian J PatholMicrobiol 2002;45(2):147-150.
- K.Mitra,Sandeep.M.Horo.Analysis of Haematological profile in HIV Positive patients before and after Antiretroviral Therapy. Inte Journal of Health Sciences and Research 2015 vol,5;11:18-24.
- Muluneh A, Fessahaye A. Hematologic abnormalities among children on HAART in Jimma University Specialized Hospital, Southwestern Ethiopia. Ethiop J Health Sci. 2009;14(2):83–89.
- Akinbami A, Oshinaike O, Adeyemo T. Hematologic abnormalities in treatment-naïve HIV patients. Lagos, Nigeria. Infect Dis: Res Treat. 2010;14:45–49.
CrossRef - Dikshit B, Wanchu A, Sachdeva RK, Sharma A, Das R. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disorders. 2009;9:5.
CrossRef - Kasthuri AS, Sharma S, Kar PK. A study of hematological manifestations of HIV Infection. Indian J Sex Transm Dis. 2006;27:1-9
- Mathews S, Srivastava D, Yadav RB, Sharma A. Association of haematological profile of human immunodeficiency virus-positive patients with clinicoimmunologic stages of the disease. J Lab Physicians. 2013;5:34-7
CrossRef - Enawgaw et al. Determination of hematological and immunological parameters among HIV positive patients taking highly active antiretroviral treatment and treatment naive in the antiretroviral therapy clinic of Gondar University Hospital, Gondar, Northwest Ethiopia: a comparative cross-sectional study. BMC Hematology. 2014 14:8
CrossRef - Steele, R., et al., Mean cell volume (MCV) changes in HIV-positive patients taking nucleoside reverse transcriptase inhibitors (NRTIs): a surrogate marker for adherence. International Journal of STD & AIDS, 2002. 13(11): p. 748-54.
CrossRef - Gautam H, Bhalla P, Dewan R. Correlation between baseline CD4+ T lymphocyte count and viral load in AIDS patients and their early clinical and immunological response to HAART: a preliminary study. Indian J of Med Microbiology 2008; 26(3):256-258.
CrossRef - Semba, R.D., N. Shah, and D. Vlahov, Improvement of Anemia Among HIV-Infected Injection Drug Users Receiving Highly Active Antiretroviral Therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes, 2001. 26(4): p. 315-319.
CrossRef - Levine, A.M., et al., Prevalence and Correlates of Anemia in a Large Cohort of HIV-Infected Women: Women’s Interagency HIV Study. JAIDS Journal of Acquired Immune Deficiency Syndromes, 2001. 26(1): p. 28-35.
CrossRef







