Published online Dec 16, 2021. doi: 10.12998/wjcc.v9.i35.11016
Peer-review started: May 17, 2021
First decision: June 15, 2021
Revised: June 25, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: December 16, 2021
Surgical therapy of infective endocarditis (IE) involving aortic valves and mitral valves is widespread. However, there are few reports concerning patients with culture-negative endocarditis complicated by the appearance of comorbid valvular perforation and abscess. Therefore, real-time surveillance of changes in cardiac structure and function is critical for timely surgical management, especially in patients who do not respond to medical therapy.
Here, we report an atypical case in a 9-mo-old infant without congenital heart disease but with symptoms of intermittent fever and macular rashes. Physical examination, laboratory tests, and electrocardiograms suggested a diagnosis of IE, although the result of blood cultures was exactly negative. After treatment with antibiotic drugs, the patient got a transient recovery. On the 9th day, we proceeded with continuous echocardiogram due to fever again and the results revealed aortic valve abscess with perforation, regurgitation, vegetation, and pericardial effusion. Intraoperative monitoring revealed aortic valve perforation, presence of apothegmatic cystic spaces below the left coronary cusp of the aortic valve, and severe aortic valve regurgitation. Aortic valve repair was performed by autologous pericardial patch plasty. The patient was discharged after 4 wk of treatment and no complications occurred after surgery.
Our case demonstrated the necessity of serial echocardiography monitoring for possible adverse symptoms of IE in pediatric patients.
Core Tip: We report an atypical case in a 9-mo-old infant without congenital heart disease. Laboratory tests and electrocardiograms suggested a diagnosis of infective endocarditis (IE). After being treated with antibiotic drugs, the patient got a short recovery. Continuous echocardiographic examinations since admission revealed aortic valve abscess with perforation, regurgitation, vegetation, and effusion. Aortic valve repair was performed by using autologous pericardial patch plasty. No postoperative complications occurred and the patient was healthily discharged after 4 wk of treatment. Our case demonstrated the necessity of serial echocardiography monitoring in pediatric patients for possible adverse symptoms of IE.
- Citation: Yang YF, Si FF, Chen TT, Fan LX, Lu YH, Jin M. Early surgical intervention in culture-negative endocarditis of the aortic valve complicated by abscess in an infant: A case report. World J Clin Cases 2021; 9(35): 11016-11023
- URL: https://www.wjgnet.com/2307-8960/full/v9/i35/11016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i35.11016
Infective endocarditis (IE), although uncommon, is a vital disease with an annual incidence ranging between 0.05 and 0.12 cases per 1000 pediatric admissions[1]. The incidence of pediatric IE has significantly increased over the past two decades with changes in risk factors, causative agents, and clinical manifestations deeply impacting its epidemiology[2]. This could be attributed to the increasing use of invasive diagnostic and therapeutic procedures in the management of IE[3]. Further, advances in echocardiography and surgical techniques over the past few years have considerably enhanced the accuracy of diagnosis and treatment for IE, even in patients with a structurally normal heart[4].
IE in children with a normal heart has become a discernible clinical entity[1], which could plausibly be associated with a potential immunosuppressed condition[5]. In an estimated 8%-10% of pediatric cases, IE has been reported to be the consensus of one easily recognizable risk factor with a normally structured heart.
Culture-negative endocarditis is a clinically challenging entity both diagnostically and therapeutically. The spectrum of epidemiology of culture-negative endocarditis has changed over the last five decades. In a recently published series, approximately 8%-36% of patients with clinically diagnosed endocarditis had persistently negative blood cultures[6-8]. The most common causes of culture-negative endocarditis include previous receipt of antimicrobial therapy and infections caused by fastidious organisms also known as the “HACEK” group which includes nutritionally deficient Streptococci, Pasturella spp., Helicobacter spp., Mycobacteria, fungal organisms, infections involving intracellular organisms Bartonella spp., Tropheryma whipplei, Coxiella burnetii (Q fever), and Brucella spp. that are either detectable by serology or polymerase chain reaction of valvular tissue[9]. Moreover, it has been shown that a lower sensitivity of blood cultures for yeast and complete lack of sensitivity for filamentous fungi make the diagnosis of fungal IE limited[1].
Although echocardiogram aids in clinically confirming the diagnosis of endocar
Here, we describe a 9-mo-old infant who was diagnosed with culture-negative endocarditis and complicated with the appearance of valvular perforation and abscess, but did not suffer from congenital heart disease. Further, in view of the above and the scarcity of literature on early surgical therapy in culture-negative endocarditis, we assessed the factors leading to severe valve destruction, the recognition of which is critical and timely for surgical intervention, especially for patients who do not respond to medical therapy.
A male infant aged 9 mo and 8 d, weighing 8 kg, born via spontaneous vaginal delivery, was presented to the emergency department for evaluation of intermittent fevers and red macula.
The patient had intermittent fever (less than 39 °C), red macula, dry and chapped lips, and a red rash around the mouth for 9 d. No other symptoms such as nausea, vomiting, diarrhea, and urinary symptoms were present. He was admitted to the hospital with a presumptive clinical diagnosis of Kawasaki disease on May 12, 2019.
The patient’s past medical history, family medical history, and vaccination status were insignificant.
On physical examination, the patient was conscious and comfortable and responded well. He was febrile with a temperature of 38.7 °C, had tachycardia with a heart rate of 142 beats per minute, but was hemodynamically stable with a normal respiratory rate of 34 breaths/min and normal blood pressure of 80/64 mmHg.
The sound of his breath in both lungs was rough and his neck was supple without lymphadenopathy. Skin examination showed red, needle-point-sized and maculopapular rashes that were non-itchy, faded under pressure, and were distributed on his trunk. Heart examination revealed slightly rough systolic murmur over the third and fourth intercostal space at the left sternal border. Abdominal examination was unremarkable.
Laboratory tests revealed an increased white blood cell count at 32.4 × 109/L (reference range: 10-13 × 109/L) and mild anemia with a hemoglobin level of 78 g/dL (reference range: 100-120 g/dL). The level of brain natriuretic peptide was mildly elevated. The levels of anti-streptolysin O, rheumatoid factors, C-reaction protein (CRP), and myocardial injury markers were normal; urine and stool tests for tetracycline hydrochloride, biochemical tests for antinuclear antibodies, and functional test for the thyroid were negative.
Chest computed tomography suggested the possibility of pneumonia following the admission. B-mode ultrasonography of the neck showed two to three enlarged cervical lymph nodes measuring 1.5 cm × 1.5 cm with good mobility. No abnormalities were observed in the liver and spleen.
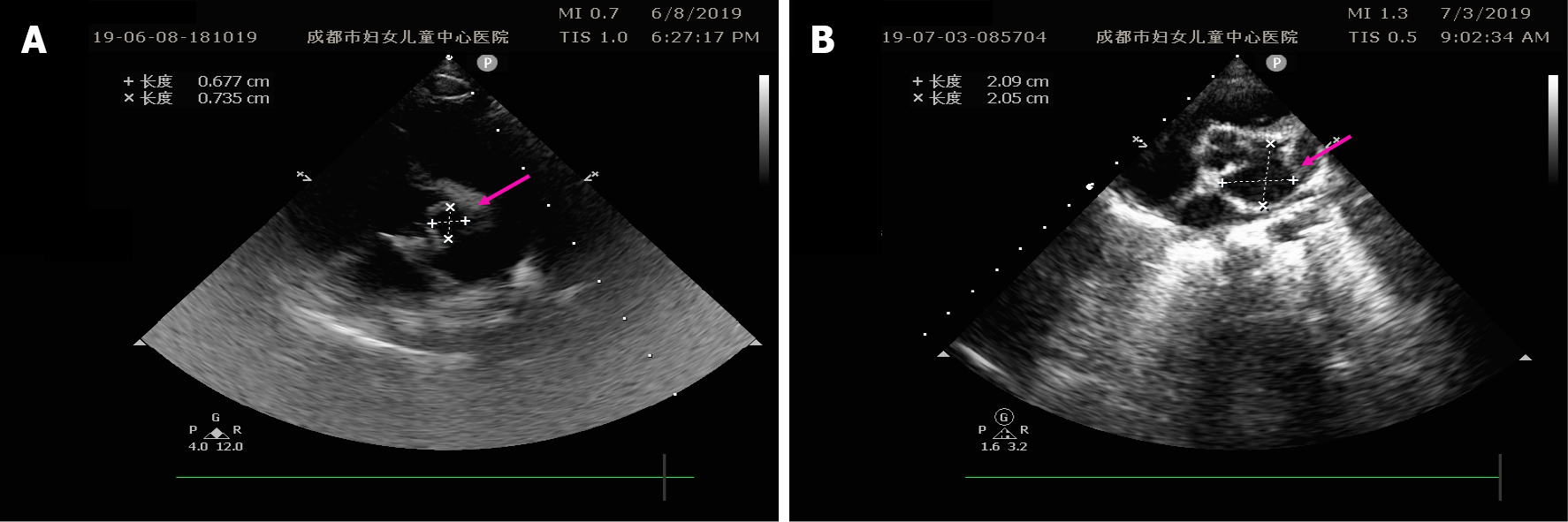
Five consecutive sets of electrocardiograms (ECGs) revealed ST segment depression and a flat T wave. On the 9th day, he developed a high-grade fever (38.4 °C), and color Doppler echocardiography revealed abscess with perforation in addition to the vegetation, aortic regurgitation, and pericardial effusion (Figure 1A). Color Doppler echocardiography was performed thrice on the 2nd (May 13), 6th (May 17), and 9th day (May 20) post-admission (Figure 1).
Four consecutive sets of blood cultures were performed and all of them were negative (May 12/13/17/20, 2019). Although positive blood cultures with Gram-positive cocci were reported from another hospital but paper reports were unavailable.
After admission, the patient did not meet the diagnostic criteria for typical Kawasaki disease and incomplete Kawasaki disease after re-evaluation according to the American Heart Association guidelines in 2017, so infectious disease was considered. He started intravenous piperacillin sulbactam and cefazolin for 8 d. Following the initiation of antibiotics, his clinical symptoms improved significantly. His sensorium and body temperature were normal, respiratory status improved, and heart sound was louder and audible with an even heart rhythm besides rashes on his trunk and limbs disappeared.
However, on the 9th day, he developed a high-grade fever (38.4 °C). A definite diagnosis was attained considering the clinical features as well as the results of laboratory tests and UCGs.
The final diagnosis of the presented case was culture-negative endocarditis.
On the 10th day, aortic valve repair was planned for assistance in management. During surgery, no significant enlargements of the heart and aorta/pulmonary artery (1:1) were seen; aortic valve perforation, severe aortic regurgitation, vegetation, and apothegmatic cystic spaces on the left coronary cusp of the aortic valve were identified. Multiple vegetations were surgically excised from the left coronary cusp of the aortic valve. The abscess of the inferior aortic valve was drained. The left coronary valve was repaired by using autologous pericardial patch plasty, and the perforation of the left ventricle was closed with direct sutures.
The follow-up evaluations included complete medical history, clinical examination, and color Doppler echocardiography. Following surgery, the culture of vegetation obtained during surgery was negative. Two sets of blood cultures were documented to be negative. Postoperative reexamination of echocardiogram at weeks 2, 3, and 4 showed mild aortic regurgitation, normal cystic echo of the left coronary valve, and normal left ventricular systolic function (Figure 2). After 4 wk of treatment with intravenous piperacillin sulbactam (245 mg/kg/d, q8h), the patient was healthily discharged.
We describe the case of a 9-mo-old male infant who presented with intermittent fever and macular rashes that were persistent for 9 d after the admission. On our evaluation, the patient was febrile with a temperature of 38.7 °C, had tachycardia with a heart rate of 142 beats per minute, and had a slightly rough systolic murmur over the third and fourth intercostal space at the border of the left sternum. Persistent and “apparently” negative blood cultures together with ECGs and color Doppler echocardiogram confirmed the clinical diagnosis of culture-negative IE. According to the revised Duke criteria, the diagnosis and classification of IE mainly depend on blood culture.
However, the sensitivity of these criteria for diagnosing culture-negative endocar
It is thought that culture-negative endocarditis in patients with prior antibiotic therapy is caused by Gram-positive cocci, such as Staphylococci, Streptococci, and Enterococci—the bacteria usually associated with culture-positive endocarditis[16].
The possibility of “culture-negative” endocarditis after antibiotic use arises in our case as blood culture was carried out prior to the initiation of empirical antimicrobial therapy. Thereby, echocardiography is a crucial tool in the diagnosis and management of culture-negative endocarditis in the absence of positive blood cultures. In this case, we had performed serial color Doppler echocardiography for monitoring the minor changes of cardiac structure and coronary condition to ensure timely interventions before any possible clinical deterioration. We detected the vegetation and abscess in time, and then arranged surgery immediately under the condition that antibiotic treatments were not well responded to. Similarly, earlier studies have shown that in adult patients with culture-negative endocarditis and large vegetation, monitoring vegetation size by means of serial transesophageal echocardiography might prove to be useful to determine the efficacy of treatment[17].
Given that the patient had a structurally normal heart and did not show any risk factors for congenital heart disease, the clinical situation resembles atypical culture-negative IE. It is thought that many factors predispose pediatric patients with IE to potentially life-threatening complications that call for an early surgery[1]. Although a latent heart disease is the main predisposing factor for pediatric IE, many cases of IE without a preexisting heart disease have been reported[18]. A study by Zamorano et al[19] showed that patients with culture-negative IE have a higher rate of complications, such as valve rupture and perforation requiring immediate surgical attention, compared to those with positive blood culture. There is a paucity of data about pediatric IE with a normally structured heart and without predisposing factors. Of note, a review by Russell et al[20] related to the surgical outcome of IE identified that, of 35 cases of endocarditis requiring surgical intervention, 14 (40%) presented with no potential congenital heart defect. Other possible latent factors such as immunodeficiency, chronic parenteral nutrition, and those with central venous catheters near the heart or tunneled central venous catheters could be considered as predisposing conditions for IE[1]. A study by Carceller et al[21] showed that approximately 26% of pediatric patients with IE had a serious systemic underlying disease without congenital heart defect, and about 7% were completely healthy. However, these potential predisposing factors were not identified in this case.
Interestingly, the lesion was located on the left side in this case, similar to that reported by Pachirat et al[22] who showed that about 92% of patients had lesions located on the left side in contrast to only 8% on the right side.
Furthermore, Shamszad et al[23] demonstrated that the left-sided lesions were the most that needed surgical intervention. The complex nature of this disease necessitates surgical treatment in about one half of patients with IE[13]. According to the guidelines (2016) published by the American Association for Thoracic Surgery, surgical indications for IE include severely-compromised valve function resulting in symptoms of heart failure, left-sided IE caused by Staphylococcus aureus, fungi, or other highly resistant microorganisms, IE complicated by a heart block, annular or aortic abscess or penetrating lesions, and persistent infection for 5-7 d despite an appropriate antibiotic course[24]. Irrespective of whether the nature of IE is culture-positive or culture-negative, the major indications for surgical treatment to prevent embolization are the presence of left-sided lesion(s) with severe stenosis or regurgitation or intractable heart failure or very large vegetation (> 30 mm)[5]. In this case, antibiotic therapy was performed at first and clinical symptoms were relieved. However, the situation deteriorated rapidly on the 9th day as the fever came back and the vegetation and abscess were detected. It reminds clinicians that even if there are no indications for surgery for the time being, it is necessary to keep an eye out for changes of cardiac construction and function.
An already complex etiology of IE is further complicated by the appearance of comorbid abscesses and valvular perforations. In the present case, intraoperative monitoring revealed aortic valve perforation, presence of apothegmatic cystic spaces below the left coronary cusp of the aortic valve, and severe aortic valve regurgitation.
Surgical treatment involving valve repair and valve replacement, could get excellent outcomes for native valve endocarditis including lesions of either the aortic or the mitral valves[25-28]. However, there is little information concerning aortic valve repair in patients with culture-negative endocarditis. For this case, aortic valve repair was performed by using autologous pericardial patch plasty. A previous study demonstrated that the augmentation or partial replacement of defective aortic cusps with autologous pericardium is a safe and feasible surgical alternative, and further advocated that aortic regurgitation can be treated effectively by aortic valve repair using pericardial patch plasty[29]. Nevertheless, the reason for the enlarged echo range of the aortic valve lateral flap in the postoperative children is not clear, and whether the infection still exists or the cystic cavity is normal after the operation remains to be followed.
Collectively, our case report suggests tailored management of pediatric IE in children without predisposing factors. Further, for cases with persistent fever and abnormal elevation of inflammatory factors (white blood cells and/or CRP), repeated blood cultures and color Doppler ultrasonography need to be performed to determine the most appropriate treatment option. Last but not least, we advocate that timely surgical intervention guided by serial echocardiography monitoring is crucial to prevent any further complications and enhance quick recovery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim J, Ph.D. JLM S-Editor: Chang KL L-Editor: Wang TQ P-Editor: Chang KL
| 1. | Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, Pahl E, Schutze GE, Shulman ST, Willoughby R Jr; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young and the Council on Cardiovascular and Stroke Nursing. Infective Endocarditis in Childhood: 2015 Update: A Scientific Statement From the American Heart Association. Circulation. 2015;132:1487-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B; AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 430] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 3. | Cabell CH, Jollis JG, Peterson GE, Corey GR, Anderson DJ, Sexton DJ, Woods CW, Reller LB, Ryan T, Fowler VG Jr. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162:90-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 313] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: challenges and perspectives. Lancet. 2012;379:965-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 5. | Nasser BA, Al Qwaee A, Almesned AR, Akhfash A, Mohamad T, Chaikhouni F, Alhabshan F, Kabbani MS. Infective endocarditis in children with normal heart: Indication for surgical intervention. J Saudi Heart Assoc. 2019;31:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Wei HH, Wu KG, Sy LB, Chen CJ, Tang RB. Infectious endocarditis in pediatric patients: analysis of 19 cases presenting at a medical center. J Microbiol Immunol Infect. 2010;43:430-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Weber R, Berger C, Balmer C, Kretschmar O, Bauersfeld U, Pretre R, Nadal D, Knirsch W. Interventions using foreign material to treat congenital heart disease in children increase the risk for infective endocarditis. Pediatr Infect Dis J. 2008;27:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Alshammary A, Hervas-Malo M, Robinson JL. Pediatric infective endocarditis: Has Staphylococcus aureus overtaken viridans group streptococci as the predominant etiological agent? Can J Infect Dis Med Microbiol. 2008;19:63-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Tattevin P, Watt G, Revest M, Arvieux C, Fournier PE. Update on blood culture-negative endocarditis. Med Mal Infect. 2015;45:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Siciliano RF, Mansur AJ, Castelli JB, Arias V, Grinberg M, Levison ME, Strabelli TM. Community-acquired culture-negative endocarditis: clinical characteristics and risk factors for mortality. Int J Infect Dis. 2014;25:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Al Abri SS, Zahedi FI, Kurup PJ, Al-Jardani AK, Beeching NJ. The epidemiology and outcomes of infective endocarditis in a tertiary care hospital in Oman. J Infect Public Health. 2014;7:400-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Piciche M, Ranocchi F, Fiorani B, Bergonzini M, Feccia M, Montalto A, Alessandro CD, Cottini M, Gherli R, Mariani B. Surgical Treatment of Valvular Infective Endocarditis Complicated by An Abscess: A Single Center's Experience. Interv Cardiol J. 2017;26:1. [DOI] [Cited in This Article: ] |
| 13. | Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL; ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2661] [Cited by in F6Publishing: 3046] [Article Influence: 338.4] [Reference Citation Analysis (0)] |
| 14. | Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart. 2003;89:258-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Siddiqui BK, Tariq M, Jadoon A, Alam M, Murtaza G, Abid B, Sethi MJ, Atiq M, Abrar S, Smego RA Jr. Impact of prior antibiotic use in culture-negative endocarditis: review of 86 cases from southern Pakistan. Int J Infect Dis. 2009;13:606-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Barnes PD, Crook DW. Culture negative endocarditis. J Infect. 1997;35:209-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Rohmann S, Erhel R, Darius H, Makowski T, Meyer J. Effect of antibiotic treatment on vegetation size and complication rate in infective endocarditis. Clin Cardiol. 1997;20:132-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Lin YT, Hsieh KS, Chen YS, Huang IF, Cheng MF. Infective endocarditis in children without underlying heart disease. J Microbiol Immunol Infect. 2013;46:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Zamorano J, Sanz J, Moreno R, Almería C, Rodrigo JL, Samedi M, Herrera D, Aubele A, Mataix L, Serra V, Sánchez-Harguindey L. Comparison of outcome in patients with culture-negative vs culture-positive active infective endocarditis. Am J Cardiol. 2001;87:1423-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Russell HM, Johnson SL, Wurlitzer KC, Backer CL. Outcomes of surgical therapy for infective endocarditis in a pediatric population: a 21-year review. Ann Thorac Surg. 2013;96 171-4:discussion 174-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Carceller A, Lebel MH, Larose G, Boutin C. [New trends in pediatric endocarditis]. An Pediatr (Barc). 2005;63:396-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Pachirat O, Chetchotisakd P, Klungboonkrong V, Taweesangsuksakul P, Tantisirin C, Loapiboon M. Infective endocarditis: prevalence, characteristics and mortality in Khon Kaen, 1990-1999. J Med Assoc Thai. 2002;85:1-10. [PubMed] [Cited in This Article: ] |
| 23. | Shamszad P, Khan MS, Rossano JW, Fraser CD Jr. Early surgical therapy of infective endocarditis in children: a 15-year experience. J Thorac Cardiovasc Surg. 2013;146:506-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs, Pettersson GB, Coselli JS; Writing Committee, Pettersson GB, Coselli JS, Hussain ST, Griffin B, Blackstone EH, Gordon SM, LeMaire SA, Woc-Colburn LE. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg. 2017;153:1241-1258.e29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 25. | Wakasa S, Matsui Y. [Early Surgery for Active Infective Endocarditis]. Kyobu Geka. 2015;68:586-590. [PubMed] [Cited in This Article: ] |
| 26. | Musci M, Hübler M, Amiri A, Stein J, Kosky S, Meyer R, Weng Y, Hetzer R. Surgical treatment for active infective prosthetic valve endocarditis: 22-year single-centre experience. Eur J Cardiothorac Surg. 2010;38:528-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Larbalestier RI, Kinchla NM, Aranki SF, Couper GS, Collins JJ Jr, Cohn LH. Acute bacterial endocarditis. Optimizing surgical results. Circulation. 1992;86:II68-II74. [PubMed] [Cited in This Article: ] |
| 28. | Muehrcke DD, Cosgrove DM 3rd, Lytle BW, Taylor PC, Burgar AM, Durnwald CP, Loop FD. Is there an advantage to repairing infected mitral valves? Ann Thorac Surg. 1997;63:1718-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Lausberg HF, Aicher D, Langer F, Schäfers HJ. Aortic valve repair with autologous pericardial patch. Eur J Cardiothorac Surg. 2006;30:244-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |