Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10075
Peer-review started: July 19, 2021
First decision: August 19, 2021
Revised: September 1, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 26, 2021
Pegylated liposomal doxorubicin (PLD) uses the hydrophilic layer of liposomes to reach the sweat on the skin surface or accumulate in the sweat glands, producing toxic free radicals and oxidative damage, resulting in hand-foot syndrome (HFS). Regional cooling can induce vasoconstriction to reduce the release of drugs in the limbs and reduce the accumulation of drugs in sweat glands; thus, decreasing the incidence and severity of HFS.
To study the efficacy of cooling patches to prevent HFS caused by PLD in the short-term.
This is a retrospective cohort study. Female breast cancer patients (n = 101) who were treated with PLD in two breast wards at our department from February 2020 to February 2021 were enrolled in the study and were randomly divided into the cooling group (51 patients) and the control group (50 patients). Patients in the control group only received routine care, while the patients in the cooling group applied cooling patches, based on routine care, to the palm and back of the hands 15 min before chemotherapy infusion for 10 h. All patients took a corresponding dose of dexamethasone orally one day before chemotherapy, on the day of chemotherapy, and one day after chemotherapy. SPSS23.0 version was used to analyze the data in this study. The occurrence and severity of HFS was analyzed by the Mann-Whitney U test, and scores were analyzed by the Student’s t test or Wilcoxon rank-sum test. A P value < 0.05 was regarded as statistically significant.
In this study, neither group of patients developed Grade 3 HFS. In the control group, the incidence of Grade 1 HFS and Grade 2 HFS was 38% and 2%, respectively. However, in the cooling group, only one person developed Grade 1 HFS (2%), and none of the patients developed Grade 2 HFS. These findings showed that cooling patches can effectively reduce the frequency and severity of HFS (P < 0.0001) in the short-term. Before the fourth chemotherapy cycle, although general self-efficacy scale scores in the cooling group were low, they were still significantly higher than those in the control group (17.22 ± 5.16 vs 19.63 ± 6.42, P = 0.041). Compared with the control group, the mean Hand-Foot Skin Reaction and Quality of Life Questionnaire score in the cooling group was significantly lower (18.08 ± 7.01 vs 14.20 ± 7.39, P = 0.008).
Cooling patches can effectively reduce the frequency and severity of HFS caused by PLD in the short-term. In addition, it may help delay the decline in patients’ self-efficacy.
Core Tip: The significance of cooling patches to prevent hand-foot syndrome (HFS) caused by pegylated liposomal doxorubicin (PLD) in breast cancer patients was evaluated. We retrospectively analyzed 101 breast cancer patients treated with PLD. Fifty-one patients applied cooling patches to their hands (the cooling group), and fifty patients did not apply cooling patches (the control group). We observed and recorded the occurrence of HFS. In the short-term, patients in the cooling group had a lower incidence of HFS than those in the control group (40% vs 2%), and patients' self-efficacy in the cooling group decreased more slowly than that in the control group, and the difference was statistically significant.
- Citation: Zheng YF, Fu X, Wang XX, Sun XJ, He XD. Utility of cooling patches to prevent hand-foot syndrome caused by pegylated liposomal doxorubicin in breast cancer patients. World J Clin Cases 2021; 9(33): 10075-10087
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10075.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10075
Breast cancer has been a major factor that threatens the lives and health of women worldwide[1]. Pegylated liposomal doxorubicin (PLD) is an effective chemotherapeutic drug commonly used in the treatment of breast cancer, ovarian cancer, lymphoma, and other malignant tumors. Hand-Foot Syndrome (HFS) and mucositis are the most common side effects of PLD treatment[2]. PLD is often administered in combination with other drugs, and patients may develop HFS after the treatment for 1-21 d or a few months, depending on the dose used[3].
HFS, also known as palmar–plantar erythrodysesthesia[4], and its symptoms vary due to different drugs used. For example, the characteristic symptoms of HFS caused by doxorubicin are mainly erythema and swelling[5]. Patients experience paresthesias initially, such as numbness, tingling, burning, and erythema. The affected area includes hands, feet, buttocks, groin, sagging breasts, armpits, etc. (especially hands and feet)[6]. As the disease progresses, HFS will lead to reduced quality of life (QOL)[7]. When patients develop HFS, they may not only risk dose adjustment or withdrawal due to physical pain but also limitation of their social activities as a result of psychological disorders[8].
The mechanism of PLD-induced HFS is not yet clear. Doxorubicin can penetrate the capillary wall and interact with metallic Cu (II) ions in the skin tissue to generate reactive oxygen species that can promote the release of chemokines and inflammatory cytokines and induce specific apoptosis of keratinocytes, triggering skin symptoms[9]. PLD may use the hydrophilic coating of liposomes to reach the skin surface via sweat, and the circulation time of PLD in the body is also long, which makes the above situation worse[10]. Local cooling plays a vasoconstricting role in the blood vessels alleviating pain, and acts as an antiperspirant, without adverse reactions[11]. Although cold therapy has prevented HFS to some extent in previous studies, there is still room for improvement[12-14]. The purpose of this study was to evaluate the efficacy of a local cooling patch on the prevention of HFS caused by PLD chemotherapy in breast cancer patients.
We aimed to evaluate the short-term preventive effect of local cooling using cooling patches on HFS caused by chemotherapy with PLD. Female breast cancer patients (n = 101) were selected who underwent PLD in two breast wards at our department from February 2020 to February 2021. The patients were randomly divided into the cooling group (51 patients) and the control group (50 patients).
Patients enrolled in the experiment met the following eligibility criteria: (1) Patients who had been diagnosed with breast cancer by pathology or cytology; (2) Patients aged between 20 and 75 years, and who could cooperate with investigators and complete the scale by themselves; (3) At least 4 cycles of PLD were administered; and (4) Patients agreed to sign the informed consent. Patients with the following conditions were excluded: (1) Breast cancer patients suffering from other serious heart, brain, kidney, or serious metabolic diseases; (2) Those with local bleeding, ulcers, and infection tendency; (3) Those with impaired consciousness, unable to communicate normally; (4) Patients who do not understand the meaning of the terms of the scale; and (5) Patients who refused to participate in the study. Two chemotherapy regimens were administered in this study, including chemotherapy plan 1 (PLD + 0.6 mg/m2 cyclophosphamide) and chemotherapy plan 2 (PLD + 0.6 mg/m2 cyclophosphamide and sequential 80-100 mg/m2 docetaxel treatment). Most patients received 30-35 mg/m2 PLD. Briefly, chemotherapy plan 1 included 4 cycles, chemotherapy plan 2 also included 4 cycles of sequential docetaxel treatment based on chemotherapy plan 1. We only discussed the effect of the use of cooling patches on HFS during the first 4 cycles of chemotherapy.
In the control group, patients received routine care, including: (1) Wearing loose shoes, socks, and gloves to avoid frequent friction and excessive pressure on the hands and feet, and they avoided heavy physical labor and intense exercise; (2) Sun protection was advised to avoid direct sunlight on the skin; (3) Sitting or lying on a soft surface with legs as high as possible; and (4) For patients with abnormal skin sensations, contact with too cold, hot, sharp, and irritating objects was avoided. In the cooling group, patients applied cooling patches based on routine care. In addition, all patients received the corresponding dose of dexamethasone orally one day before, on the day of chemotherapy, and one day after chemotherapy, according to the doctor's advice. Supportive therapy was given when necessary.
We explained the purpose and method of the investigation to the patients. The survey method was face-to-face data collection. The patients voluntarily participated in the research and completed the questionnaire according to the actual situation. We only explained unclear points but did not interfere with the patient's selection. The questionnaire was handed out before each chemotherapy cycle. A higher score of the Hand-Foot Skin Reaction and Quality of Life Questionnaire (HF-QOL) indicates poorer QOL or worsening symptoms[15]. Self-efficacy plays a positive role in the psychological resilience of breast cancer patients after surgery[16], which positively correlated with QOL[17]. To understand QOL and self-efficacy of the patients, we also conducted scale assessments, including HF-QOL and the general self-efficacy scale (GSES).
The main components of the cooling patch (50 mm × 110 mm) were pure water (77%), hydrophilic polymer gel, and mint extract (Japan DIA Pharmaceutical Co., Ltd). The cooling patch absorbs the heat from the skin through the polymer hydrogel layer and uses vaporization to dissipate heat so that the local skin is continuously cooled. Cooling patches were applied to the palm and back (avoiding the needle hole for intravenous infusion) of the hands 15 min before infusion of chemotherapy, and the cooling effect was maintained for 10 h. Routine care was applied to the feet without cooling treatment. Each patient used four cooling patches per chemotherapy cycle. If skin ulcers or symptoms were too severe to apply the cooling patches during treatment, local cold therapy was terminated.
When symptoms first appeared on the hands, we evaluated the symptoms and classified HFS before each chemotherapy cycle (starting from the second chemotherapy cycle). Patients with corresponding symptoms in one or both hands were classified as HFS. Evaluation of the occurrence of HFS continued until 2 or 3 cycles after the 4th cycle due to the medicinal properties of PLD.
SPSS version 23.0 was used for statistical analysis of the data in this study. The occurrence and severity of HFS were analyzed by the Mann-Whitney U test, and the Student's t test was used to analyze the scores of the scales. A P value < 0.05 was regarded as statistically significant.
One hundred and one female patients who met the criteria were enrolled, and all of them were treated with intravenous PLD in our department. None of these patients had received previous chemotherapy, and all followed the research protocol.
Moreover, there were no significant differences between the two groups of patients in terms of some baseline. However, there were significant differences between the two groups in terms of character traits, such as facing major events in life, and attitudes to suffering from breast cancer (Table 1). However, multivariate analysis of variance indicated that these three items were not independent influencing factors on the occurrence of HFS, and their main effects and interaction effects did not affect the HF-QOL and GSES scores (P > 0.05) (Table 2).
| Control group | Cooling group | F | Z | P value | |
| Age | 51.18 ± 9.27 yr | 51.24 ± 10.16 yr | 0.863 | 0.977 | |
| Weight | 64.20 ± 10.29 kg | 65.37 ± 9.74 kg | 0.404 | 0.558 | |
| BMI | 24.50 ± 3.60 kg/m2 | 24.98 ± 3.66 kg/m2 | 0.042 | 0.503 | |
| Height | 162 (158-165) cm | 162 (160-165) cm | -0.044 | 0.965 | |
| Body surface area | 1.6975 (1.6005-1.799) m2 | 1.723 (1.646-1.802) m2 | -0.802 | 0.423 | |
| PLD dose | 60 (54.25-60) mg | 60 (60-60) mg | -1.53 | 0.126 | |
| Total PLD dose | 240 (220-240) mg | 240 (240-240) mg | -1.04 | 0.299 | |
| Chemotherapy cycle | 4 cycles, 21 d/cycle | ||||
| Leukocytes | 5.66 (4.48-7.0875) | 6.02 (4.92-7.47) | -0.971 | 0.331 | |
| Platelets | 249.5 (225.5-301.25) | 233 (203-270) | -1.96 | 0.05 | |
| Albumin | 47.59 (43.375-49.025) | 46.5 (44.8-48.424) | -0.194 | 0.846 | |
| Alanine aminotransferase | 21 (13.5-33.525) | 17 (12.47-24) | -1.502 | 0.133 | |
| Aspartate aminotransferase | 21.9 (17-27.5) | 20 (17-22.03) | -1.251 | 0.211 | |
| Total bilirubin | 12.715 (7.9225-14.9) | 10.58 (8.25-13.78) | -1.274 | 0.203 | |
| Creatinine | 45.6 (41.465-48.8) | 45.4 (42.8-49.5) | -0.234 | 0.815 | |
| Urea Nitrogen | 4.69 (4.3-5.1218) | 5.1 (4.12-5.9) | -1.485 | 0.138 | |
| Race | -1.400 | 0.161 | |||
| Marital status | -0.323 | 0.747 | |||
| Profession | -0.999 | 0.318 | |||
| Education | -0.467 | 0.640 | |||
| Household income level (in recent year) | -0.050 | 0.960 | |||
| Resident population (in recent year) | -1.272 | 0.203 | |||
| Medical procedures | -0.475 | 0.635 | |||
| Whether the patient has hypertension | -0.499 | 0.618 | |||
| Whether the patient has diabetes | -0.988 | 0.323 | |||
| Whether the patient ever had heart disease | -0.020 | 0.984 | |||
| Whether the patient has other tumors | -0.566 | 0.571 | |||
| Whether the patient has a long-term medication history | -0.823 | 0.410 | |||
| Whether the patient has a smoking history | -1.010 | 0.313 | |||
| Whether the patient has a drinking history | 0.000 | 1.000 | |||
| Whether the patient has the habit of taking health products | -1.036 | 0.300 | |||
| Attitude | -1.293 | 0.196 | |||
| Character traits | -2.479 | 0.013 | |||
| Facing major events in life | -3.258 | 0.001 | |||
| Attitude to this matter (suffering from breast cancer) | -2.654 | 0.008 | |||
| Whether the patient has undergone surgery | -1.364 | 0.172 | |||
| Surgical approach | -0.543 | 0.587 | |||
| Chemotherapy administration | -1.344 | 0.179 | |||
| Chemotherapy regimen | -1.143 | 0.253 | |||
The incidence of HFS was 40% in the control group and 2% in the cooling group, with a statistically significant difference (P < 0.0001) (Table 3). In the control group, the incidence of Grade 1 and Grade 2 HFS was 38% and 2%, respectively. However, up to the end of the 5th course of treatment, only one patient in the cooling group developed Grade 1 HFS. None of the patients developed Grade 3 HFS in either group. Most patients developed HFS after the 3rd or 4th course of treatment, and two patients developed HFS after the 5th course of treatment.
| HFS occurrence | |||||
| No HFS | Grade 1 | Grade 2 | Z | P value | |
| Control group | 30 (60.0) | 19 (38.0) | 1 (2.0) | 4.686 | 0.000003 |
| Cooling group | 50 (98.0) | 1 (2.0) | 0 (0.0) | ||
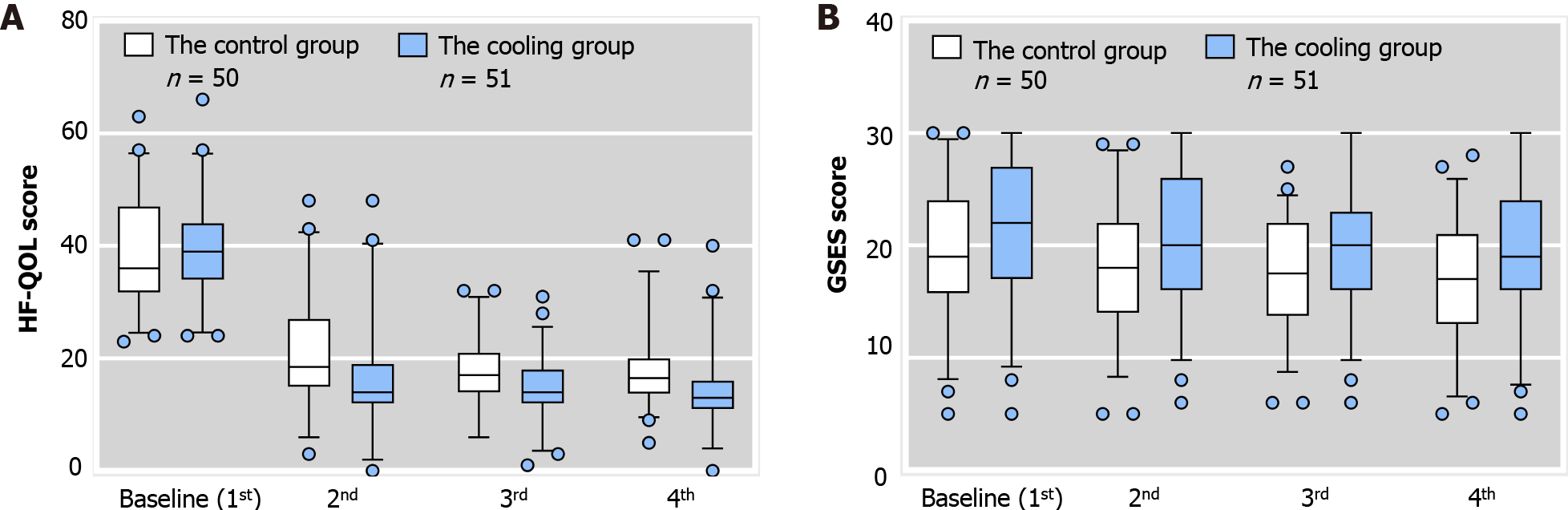
The GSES and HF-QOL scores are shown in Figure 1A and B, respectively. We also performed intra-group comparisons (Table 4). In the third assessment in the control group, the GSES score was significantly different to the baseline score (the first evaluation). Several subsequent scores were also significantly different from the baseline. In the cooling group, the GSES score was significantly different from the baseline score in the second evaluation, and the difference persisted until the fourth evaluation. Compared with the baseline scores, the GSES scores showed a difference in the fourth evaluation in both the control group (19.48 ± 5.88 vs 17.22 ± 5.16, P = 0.012) and the cooling group (21.61 ± 6.13 vs 19.63 ± 6.42, P = 0.008), and the mean value of both groups was low, indicating that most patients had a lower sense of self-efficacy (the total score was 40 points, and below 24 points was regarded as low self-efficacy). A comparison between the two groups was then performed (Table 5). No significant difference in GSES scores between the two groups in the first assessment (19.48 ± 5.88 vs 21.61 ± 6.13, P = 0.078) was observed. And 78% of patients had low self-efficacy in the control group, and 64.7% of patients had low self-efficacy in the cooling group. In the third evaluation, there were no significant differences in GSES scores between the two groups. In the fourth assessment, although mean GSES scores were low in the cooling group, they were still significantly higher than those in the control group (17.22 ± 5.16 vs 19.63 ± 6.42, P = 0.041). Low self-efficacy was observed in 94% of patients in the control group, and in 78.4% of patients in the cooling group.
| GSES score | 2nd and 1st | 3rd and 1st | 4th and 1st | |
| Control group | t | -1.982 | -2.373 | -2.604 |
| P value | 0.053 | 0.022 | 0.012 | |
| Cooling group | t | -2.047 | -2.385 | -2.773 |
| P value | 0.046 | 0.021 | 0.008 |
| GSES score | 1st | 2nd | 3rd | 4th |
| Control group | 19.48 ± 5.88 | 18.40 ± 5.55 | 17.52 ± 4.82 | 17.22 ± 5.16 |
| Cooling group | 21.61 ± 6.13 | 20.67 ± 6.08 | 19.94 ± 5.81 | 19.63 ± 6.42 |
| F | 0.021 | 0.479 | 0.552 | 1.647 |
| P value | 0.078 | 0.053 | 0.025 | 0.041 |
At baseline, there were no significant differences in HF-QOL scores between the two groups (39.14 ± 10.29 vs 39.06 ± 8.56, P = 0.966) (Table 6). From the second evaluation, the HF-QOL scores in the two groups were significantly different, and this difference persisted until the fourth evaluation. In the fourth evaluation, the HF-QOL scores showed very significant differences (18.08 ± 7.01 vs 14.20 ± 7.39, P = 0.008) between the two groups, and compared with the control group, the median HF-QOL score in the cooling group was significantly lower. In the intra-group comparisons (Table 7), in both groups in the second evaluation, the HF-QOL score was significantly different from baseline. Several subsequent scores were also significantly different from baseline. There were significant differences in HF-QOL scores before the 1st chemotherapy and the 4th chemotherapy in both the cooling group (39.06 ± 8.56 vs 14.20 ± 7.39, P < 0.05) and the control group (39.14 ± 10.29 vs 18.08 ± 7.01, P < 0.05).
| HF-QOL score | 1st | 2nd | 3rd | 4th |
| Control group | 39.14 ± 10.29 | 21.80 ± 10.10 | 18.02 ± 5.96 | 18.08 ± 7.01 |
| Cooling group | 39.06 ± 8.56 | 16.65 ± 10.46 | 14.22 ± 6.01 | 14.20 ± 7.39 |
| F | 4.559 | 0.061 | 0.192 | 0.025 |
| P value | 0.966 | 0.013 | 0.002 | 0.008 |
| HF-QOL score | 2nd and 1st | 3rd and 1st | 4th and 1st | |
| The control group | t | -10.224 | ||
| F | 20.226 | 13.952 | ||
| P value | 0.000 | 0.000 | 0.000 | |
| The cooling group | t | -14.500 | ||
| F | 4.781 | 1.570 | ||
| P value | 0.000 | 0.000 | 0.000 |
In order to identify the factors that may have caused HFS, univariate analysis of the factors that may affect the occurrence of HFS was performed (Table 4). The results showed that occupation, albumin level, and whether the patient had a long-term medication history were the factors that may have caused HFS. However, after binary logistic regression analysis, none of these factors were found to be associated with the occurrence of HFS. Moreover, character traits, such as facing major events in life, attitudes toward cancer, body mass index (BMI), age, PLD dose, and body surface area were not significantly associated with the incidence of HFS (Table 8).
| Possible risk factors | F | Z | P value |
| Age | 3.354 | 0.387 | |
| BMI | 0.088 | 0.581 | |
| Body surface area | -0.649 | 0.517 | |
| Race | -0.2 | 0.842 | |
| Profession | -3.023 | 0.003 | |
| Education | -0.597 | 0.55 | |
| Whether the patient has hypertension | -0.887 | 0.375 | |
| Whether the patient has diabetes | -1.008 | 0.313 | |
| Whether the patient ever had heart disease | -1.04 | 0.298 | |
| Whether the patient has other tumors | -0.896 | 0.37 | |
| Whether the patient has a long-term medication history | -2.083 | 0.037 | |
| Whether the patient has a smoking history | -0.512 | 0.608 | |
| Whether the patient has a drinking history | 0 | 1 | |
| Whether the patient has the habit of taking health products | -1.04 | 0.298 | |
| Attitude | -0.363 | 0.717 | |
| Character traits | -1.459 | 0.145 | |
| Facing major events in life | -1.229 | 0.219 | |
| Attitude to this matter (suffering from breast cancer) | -1.26 | 0.208 | |
| Whether the patient has undergone surgery | -0.127 | 0.899 | |
| Surgical approach | -1.137 | 0.255 | |
| Chemotherapy administration | -0.182 | 0.856 | |
| Chemotherapy regimen | -1.398 | 0.162 | |
| Total PDL dose | -0.289 | 0.773 | |
| Leukocytes | -0.816 | 0.415 | |
| Platelets | -1.712 | 0.087 | |
| Albumin | -2.428 | 0.015 | |
| Alanine aminotransferase | -1.177 | 0.239 | |
| Aspartate aminotransferase | -1.517 | 0.129 | |
| Total bilirubin | -0.833 | 0.405 | |
| Creatinine | -0.448 | 0.654 | |
| Urea Nitrogen | -0.088 | 0.93 |
PLD is the most common cause of HFS. Approximately 83.7% of female cancer patients who received PLD developed HFS and the incidence of Grade 3 HFS was 52.9%[18]. The management of HFS involves preventive measures, health education, symptom management, and dose adjustment[19]. Here we only discuss preventive measures for HFS caused by chemotherapy.
Plasma filtration can safely and effectively remove circulating PLD and decrease the incidence of HFS and mucositis[20]. However, the success rate depends on the technical equipment and experience of the operator in the therapeutic plasma filtration[2]. Topical antiperspirants (containing aluminum chlorohydrate) seemed to reduce the incidence of HFS, but the effect of preventing Grade 2 or Grade 3 HFS was insignificant (58%)[21]. Jung et al[22] used high concentrations of topical antioxidants to neutralize free radicals in the skin and found that they were more effective in preventing Grade 3 HFS. Pyridoxine has been used to prevent HFS caused by chemotherapy, but the evidence for its related efficacy is still controversial[23].
It has been recognized that as a non-drug therapy, local cooling is an effective means of preventing HFS caused by chemotherapy[24]. However, local cooling has disadvantages such as high shedding rate and cumbersome cold therapy process (Table 9). The cooling patch can make up for the insufficiency of ice packs, ice gloves, and ice socks. For example, it can be cut into suitable shapes according to affected areas (some uneven parts, such as underarms and breasts[6]) and can reduce the risk of skin friction. The cooling patch can result in a 0.5-1 degree temperature decrease within 10 min and has the advantages of low price, safety, comfort, good adhesion, simple operation, and the cooling effect can last for 10h. It also has a moisturizing effect. Although some of the water in the cooling patch will evaporate, the higher water content can keep the skin moist and prevent sweating, reducing the possibility of PLD accumulating in sweat glands[10]. We studied the preventive effect of cooling patches on the hands that are mostly affected by HFS. In our study, the incidence of HFS was much lower than in the previous studies on local cold therapy, and the dropout value was 0. Moreover, compared with the control group, the HF-QOL score in the cooling group was significantly lower. Our study also showed that the use of cooling patches may help delay the decline in patients' self-efficacy, which may be due to psychological factors.
| n | Tumor | Chemotherapy regimen | Dosage of PLD | Cold therapy equipment | Cooling parts of the body | Cooling time | Incidence of HFS | Ref. |
| 20 | Ovarian cancer | Monotherapy | 30-50 mg/m2 | Ice packs | Around the wrists and ankles | After chemotherapy for 24 h | 1/17 (5.9%) | Molpus et al[12] |
| 53 | Ovarian cancer | Combination or monotherapy | 30-50 mg/m2 | Ice packs | Around the wrists and ankles | During chemotherapy infusion | 2/28 (7.1%) | Mangili et al[13] |
| 55 | Ovarian cancer | Combination | 30 mg/m2 | FGS | Whole hands and feet | From 15 min before infusion to 15 min after infusion | ≥ Grade 2 (31.70%) | Bun et al[14] |
| 41 | Monotherapy | 50 mg/m2 | ≥ Grade 2 (5.5%) | |||||
| 51 | Breast cancer | Combination | 30-35 mg/m2 | The cooling patches | Palm and back of hands | Start 15 min before infusion and lasted for 10 h | 1/51 (2.0%) | Our study |
Liang et al[25] found that BMI is an independent risk factor for moderate to severe HFS, and the two are directly proportional. Patients with high BMI seemed more likely to develop HFS in our study (Table 10), although this was statistically non-significant. Yamada et al[26] thought that the severity of HFS and mucositis caused by PLD may be a predictor of its efficacy. Jandu et al[27] believed that HFS was a biological marker of improved survival rate in cancer patients. Our data may provide another possible explanation for HFS being associated with a good prognosis. It was shown that HFS was more likely to occur in women who were healthier (fewer diseases) and who were optimistic in personality (Table 10), although this was statistically non-significant.
| Characteristics | Percentage (%) |
| Age (yr) | |
| ≤ 17 | 0 |
| 18-45 | 38.1 |
| 46-69 | 61.9 |
| > 70 | 0 |
| BMI (kg/m2) | |
| Underweight (< 18.5) | 0 |
| Normal (18.5-24.9) | 42.9 |
| Overweight (25.0-29.9) | 52.4 |
| Obese class I (30.0-34.9) | 4.8 |
| Obese class II (35.0-39.9) | 0 |
| Obese class III (≥40.0) | 0 |
| Whether the patient ever had hypertension | |
| Yes | 9.5 |
| No | 90.5 |
| Whether the patient has diabetes | |
| Yes | 4.8 |
| No | 95.2 |
| Whether the patient ever had heart disease | |
| Yes | 0 |
| No | 100 |
| Whether the patient has other tumors | |
| Yes | 0 |
| No | 100 |
| Whether the patient has a long-term medication history | |
| Yes | 9.5 |
| No | 90.5 |
| Whether the patient has a smoking history | |
| Yes | 0 |
| No | 100 |
| Whether the patient has a drinking history | |
| Yes | 0 |
| No | 100 |
| Whether the patient has the habit of taking health products | |
| Yes | 0 |
| No | 100 |
| Attitude | |
| Impatient | 61.9 |
| Slowcoach | 4.8 |
| Somewhere in between | 33.3 |
| Character traits | |
| Extroverted | 38.1 |
| Introverted | 28.6 |
| Somewhere in between | 33.3 |
| Facing major events in life | |
| Accept frankly | 90.5 |
| Accept after enlightenment | 9.5 |
| Difficult to accept after enlightenment and rethinking | 0 |
| Attitude to this matter (suffering from breast cancer) | |
| Optimistic | 95.2 |
| Slightly optimistic | 4.8 |
| Confused | 0 |
| Worried | 0 |
Our study also had limitations. The observation period was short, only the hands received the cooling treatment, and it is impossible to directly evaluate the difference in the cooling effect between this method and other methods. In the future, more randomized studies should be conducted to determine the optimal duration, optimal temperature, and effectiveness of local cooling.
The cooling patch can effectively reduce the frequency and severity of HFS caused by PLD in the short term. In addition, it may help to improve patients' quality of life and delay the decline of their self-efficacy.
Hand-foot syndrome (HFS) is one of the most common skin toxicities of pegylated liposomal doxorubicin (PLD). When patients develop HFS, they may be at risk for dose adjustment (or withdrawal) and limitation of activities of daily living due to physical pain, even lead to limited social activities due to psychological disorders. Although cold therapy as a non-drug therapy to prevent and treat HFS has been effective in previous studies, there is room for improvement. If local cold therapy has the advantages of good effect, easy operation, and low price, it will be a huge benefit for patients with HFS.
Current methods of preventing chemotherapy-induced HFS are mostly pharmacological prevention, and local cooling as a non-drug therapy is effective in previous studies, but has disadvantages such as cumbersome implementation steps, low patient tolerance, and high shedding rate.
The main goal is to study the efficacy of the cooling patch in preventing HFS caused by PLD in the short term (for the prevention of hand symptoms). Improve the current situation that patients face the risks of restricted activities of daily living due to physical pain caused by HFS and limited social activities due to psychological disorders. We apply a cooling patch originally used to reduce fever in infants and children to prevent HFS caused by PLD in breast cancer patients to make up for some of the shortcomings of ice packs, ice gloves, and ice socks.
This study was a retrospective cohort study in which using purposive sampling to select the research objects. The research objects answered the questions in the scale regularly, and we distributed and collected the scale.
The cooling patch can effectively reduce the frequency and severity of HFS in the short-term. Before the fourth chemotherapy cycle, although general self-efficacy scale scores in the cooling group were low, they were still significantly higher than those in the control group. Compared with the control group, the mean Hand-Foot Skin Reaction and Quality of Life Questionnaire score in the cooling group was signi
The cooling patch can effectively reduce the frequency and severity of HFS caused by PLD in the short term. In addition, it may help to improve patients' quality of life and delay the decline of their self-efficacy. The cooling patch is often applied to treat fever in infants and young children. We use it to prevent HFS caused by PLD chemotherapy in breast cancer patients.
In the future, we will study whether the cooling patch also has a good effect on the feet, underarms, and other parts that may be affected and conduct more rigorous randomized controlled studies to clarify the optimal duration, temperature, and effectiveness of local cooling.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liehr T S-Editor: Wang JL L-Editor: A P-Editor: Ma YJ
| 1. | Yao J, Pan S, Fan X, Jiang X, Yang Y, Jin J, Liu Y. Pegylated liposomal doxorubicin as neoadjuvant therapy for stage II-III locally advanced breast cancer. J Chemother. 2020;32:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Filip S, Kubeček O, Špaček J, Lánská M, Bláha M. Therapeutic Apheresis, Circulating PLD, and Mucocutaneous Toxicity: Our Clinical Experience through Four Years. Pharmaceutics. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Salzmann M, Marmé F, Hassel JC. Prophylaxis and Management of Skin Toxicities. Breast Care (Basel). 2019;14:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Hartung B, Thiel W, Ritz-Timme S, Häussinger D, Erhardt A. Hand-foot syndrome induced changes of the palmar epidermal ridge configurations during and after treatment with capecitabine. Leg Med (Tokyo). 2020;45:101710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Saif MW. Capecitabine and hand-foot syndrome. Expert Opin Drug Saf. 2011;10:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia ('hand-foot' syndrome). Ann Oncol. 2007;18:1159-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Urakawa R, Tarutani M, Kubota K, Uejima E. Hand Foot Syndrome Has the Strongest Impact on QOL in Skin Toxicities of Chemotherapy. J Cancer. 2019;10:4846-4851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Komatsu H, Yagasaki K, Hirata K, Hamamoto Y. Unmet needs of cancer patients with chemotherapy-related hand-foot syndrome and targeted therapy-related hand-foot skin reaction: A qualitative study. Eur J Oncol Nurs. 2019;38:65-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Yokomichi N, Nagasawa T, Coler-Reilly A, Suzuki H, Kubota Y, Yoshioka R, Tozawa A, Suzuki N, Yamaguchi Y. Pathogenesis of Hand-Foot Syndrome induced by PEG-modified liposomal Doxorubicin. Hum Cell. 2013;26:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Jacobi U, Waibler E, Schulze P, Sehouli J, Oskay-Ozcelik G, Schmook T, Sterry W, Lademann J. Release of doxorubicin in sweat: first step to induce the palmar-plantar erythrodysesthesia syndrome? Ann Oncol. 2005;16:1210-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kepinska-Szyszkowska M, Misiorek A, Kapinska-Mrowiecka M, Tabak J, Malina K. Assessment of the Influence Systemic Cryotherapy Exerts on Chosen Skin Scores of Patients with Atopic Dermatitis: Pilot Study. Biomed Res Int. 2020;2020:5279642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Molpus KL, Anderson LB, Craig CL, Puleo JG. The effect of regional cooling on toxicity associated with intravenous infusion of pegylated liposomal doxorubicin in recurrent ovarian carcinoma. Gynecol Oncol. 2004;93:513-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Mangili G, Petrone M, Gentile C, De Marzi P, Viganò R, Rabaiotti E. Prevention strategies in palmar-plantar erythrodysesthesia onset: the role of regional cooling. Gynecol Oncol. 2008;108:332-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Bun S, Yunokawa M, Tamaki Y, Shimomura A, Shimoi T, Kodaira M, Shimizu C, Yonemori K, Fujiwara Y, Makino Y, Terakado H, Tamura K. Symptom management: the utility of regional cooling for hand-foot syndrome induced by pegylated liposomal doxorubicin in ovarian cancer. Support Care Cancer. 2018;26:2161-2166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Anderson RT, Keating KN, Doll HA, Camacho F. The Hand-Foot Skin Reaction and Quality of Life Questionnaire: An Assessment Tool for Oncology. Oncologist. 2015;20:831-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Huang Y, Huang Y, Bao M, Zheng S, Du T, Wu K. Psychological resilience of women after breast cancer surgery: a cross-sectional study of associated influencing factors. Psychol Health Med. 2019;24:866-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Zhou Y, Cui Y, Yang J. The effectiveness of a rehabilitation programme for Chinese cancer survivors: A pilot study. Int J Nurs Pract. 2016;22:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Hackbarth M, Haas N, Fotopoulou C, Lichtenegger W, Sehouli J. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women's cancers. Results of a prospective study. Support Care Cancer. 2008;16:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Kwakman JJM, Elshot YS, Punt CJA, Koopman M. Management of cytotoxic chemotherapy-induced hand-foot syndrome. Oncol Rev. 2020;14:442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Kubeček O, Martínková J, Chládek J, Bláha M, Maláková J, Hodek M, Špaček J, Filip S. Plasmafiltration as an effective method in the removal of circulating pegylated liposomal doxorubicin (PLD) and the reduction of mucocutaneous toxicity during the treatment of advanced platinum-resistant ovarian cancer. Cancer Chemother Pharmacol. 2020;85:353-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Templeton AJ, Ribi K, Surber C, Sun H, Hsu Schmitz SF, Beyeler M, Dietrich D, Borner M, Winkler A, Müller A, von Rohr L, Winterhalder RC, Rochlitz C, von Moos R, Zaman K, Thürlimann BJ, Ruhstaller T; Swiss Group for Clinical Cancer Research (SAKK) Coordinating Center. Prevention of palmar-plantar erythrodysesthesia with an antiperspirant in breast cancer patients treated with pegylated liposomal doxorubicin (SAKK 92/08). Breast. 2014;23:244-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Jung S, Sehouli J, Chekerov R, Kluschke F, Patzelt A, Fuss H, Knorr F, Lademann J. Prevention of palmoplantar erythrodysesthesia in patients treated with pegylated liposomal doxorubicin (Caelyx®). Support Care Cancer. 2017;25:3545-3549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Charalambous A, Tsitsi T, Astras G, Paikousis L, Filippou E. A pilot randomized double-blind, placebo-controlled study on the effects of the topical application of pyridoxine on palmar-plantar erythrodysesthesia (PPE) induced by capecitabine or pegylated liposomal doxorubicin (PLD). Eur J Oncol Nurs. 2021;50:101866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Breast Cancer Group; Chinese Association for Clinical Oncologist (CACO). [Chinese expert consensus on management of adverse events of pegylated liposomal doxorubicin (2020 edition)]. Zhonghua Zhong Liu Za Zhi. 2020;42:617-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Liang G, Ma W, Zhao Y, Liu E, Shan X, Tang D, Li L, Niu X, Zhao W, Zhang Q. Risk factors for pegylated liposomal doxorubicin-induced moderate to severe hand-foot syndrome in breast cancer patients: assessment of baseline clinical parameters. BMC Cancer. 2021;21:362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Yamada Y, Kawaguchi R, Ito F, Iwai K, Niiro E, Shigetomi H, Tanase Y, Kobayashi H. Skin-mucous membrane disorder and therapeutic effect of pegylated liposomal doxorubicin in recurrent ovarian cancer. J Obstet Gynaecol Res. 2017;43:1194-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Jandu H, Church D, Paula J, Tomlinson I, Ivesons T, Kerr R, Kerr D, Panes C. Hand-foot syndrome is a biomarker of improved survival following treatment with capecitabine. Ann Oncol. 2019;30:iv117-iv118. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |