Published online Feb 26, 2019. doi: 10.12998/wjcc.v7.i4.500
Peer-review started: November 13, 2018
First decision: November 27, 2018
Revised: December 21, 2018
Accepted: December 29, 2018
Article in press: December 30, 2018
Published online: February 26, 2019
Severe hyperthyroidism is a life-threatening exacerbation of thyrotoxicosis, characterized by high fever and multiorgan failure. The most common medical treatments are administration of antithyroid drugs and radioactive iodine, and thyroidectomy. In some patients, antithyroid therapy is limited due to serious adverse effects or failure to control disease progression. In some extreme cases, such as thyroid storm, conventional therapy alone does not yield effective and rapid improvement before the development of multiorgan failure.
This report describes a Chinese patient with severe hyperthyroidism accompanied by multiorgan failure, who was transferred to the medical intensive care unit of our hospital. The patient presented with palpitations, vomiting, diarrhea, and shortness of breath for a week. Laboratory tests showed elevation of thyroid hormones. Hepatic failure occurred with high aminotransferase levels and jaundice. Given her abnormal liver function and medication history, we could not exclude diagnosis of propylthiouracil-induced hepatic failure. Moreover, she also suffered from heart failure. Therapeutic plasma exchange (commonly known as TPE) and continuous renal replacement therapy (commonly known as CRRT) were used as life-saving therapy, which resulted in notable improvement of clinical symptoms and laboratory tests.
Combined TPE and CRRT are safe and effective for patients with hyperthyroidism and multiorgan failure.
Core tip: Severe hyperthyroidism accompanied with multiple organ failure has been previously reported but is rare. In this case report, acute liver failure like our patient is a very unusual form of presentation. Considering the patient’s medical history, propylthiouracil-induced hepatotoxicity could not be excluded. Therapeutic plasma exchange combined with continuous renal replacement therapy were performed, and successfully stabilized the patient. We suggest that the early application of blood purification technology is feasible in critically patients with severe hyperthyroidism.
- Citation: Ba JH, Wu BQ, Wang YH, Shi YF. Therapeutic plasma exchange and continuous renal replacement therapy for severe hyperthyroidism and multi-organ failure: A case report. World J Clin Cases 2019; 7(4): 500-507
- URL: https://www.wjgnet.com/2307-8960/full/v7/i4/500.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i4.500
Hyperthyroidism is characterized by elevated levels of thyroid hormones in the circulation[1,2]. The most common causes of hyperthyroidism are Graves’ disease, multinodular toxic goiter, and autonomous hyperfunctioning thyroid nodules. Clinical manifestations of thyroid hyperfunction are usually mild or moderate. Severe hyperthyroidism is accompanied by more symptoms, particularly, asthenia followed by nervousness, dyspnea, and loss of weight. Severe hyperthyroidism is rare but life-threatening and can lead to irreversible multiorgan failure and high mortality, especially for older people or patients with cardiovascular disease[3]. Thus, a clinically euthyroid state should be achieved as soon as possible.
Traditional medical management has focused on supportive treatment and medication that halt the synthesis, release and peripheral effects of thyroid hormones[4]. However, these measures are limited because of adverse effects or failure to relieve the critical condition quickly. As therapeutic plasma exchange (TPE) is able to remove large amounts of serum protein-bound thyroid hormones from the circulation, plasmapheresis has been used as one of the effective alternative treatments since the 1970s[5]. Continuous renal replacement therapy (CRRT) can eliminate toxic substances and regulate water–electrolyte and acid–base balance. The combined use of blood purification technology can replace some metabolic functions, thereby effectively improving metabolic disorders.
We report herein a case of severe hyperthyroidism with multiorgan failure, for which antithyroid therapy was contraindicated, and TPE combined with CRRT successfully stabilized the condition of the patient.
Chest discomfort, palpitations, anorexia, and bloated legs for a week.
About 1 year ago, the patient suffered from palpitations and hyperhidrosis. However, she paid no attention to these clinical signs and did not seek medical help. Seven days prior to presentation to a local hospital, she began to experience chest discomfort, palpitations, nausea, vomiting, abdominal pain with diarrhea, lack of energy, anorexia, and bloated legs. Upon admission, the levels of free thyroxin (FT4, > 100 pmol/L, normal range: 11.5-22.7 pmol/L) and free tri-iodothyronine (FT3, 26.33 pmol/L, normal range: 3.5-6.5 pmol/L) were significantly elevated, and the patient was diagnosed with hyperthyroidism. Blood test for liver function showed that alanine aminotransferase (ALT) was 65 U/L (normal range: 3-35 U/L) and aspartate aminotransferase (AST) was 60 U/L(normal range: 13-35 U/L). After taking a small dose of propylthiouracil (PTU, 200 mg), her symptoms worsened and liver function deteriorated abruptly within 1 d, which was reflected in the level of ALT at 4597 U/L and AST at 7245 U/L (Table 1). Consequently, she was transferred to our hospital.
| Laboratory test | FT3 pmol/L | FT4 pmol/L | TSH μIU/L | ALT U/L | AST U/L | TB μmol/L | DB μmol/L |
| Normal range | 3.5-6.5 | 11.5-22.7 | 0.55-4.78 | 3-35 | 13-35 | 4.0-23.9 | 0-6.8 |
| Day 1 | 26.33 | > 100 | < 0.005 | 65 | 60 | 34.6 | 19.1 |
| Day 2 | - | 55.8 | 0.072 | 4597 | 7254 | 113.5 | 70.2 |
The patient denied history of hypertension, diabetes mellitus, or exposure to viral hepatitis or tuberculosis. She also denied history of operation, trauma or blood transfusion. She had no known drug or food allergies.
Personal history: (1) Use for drugs: No medications were used daily. (2) Use of alcohol: No drinking. (3) Use of tobacco: No smoking.
Family history: (1) Health status or cause of death of parents, siblings and children: All are healthy. (2) Specific diseases related to problems identified in the chief complaint or history of the present illness and/or system review: None. (3) Diseases of family members which may be hereditary or place the patient at risk: No hereditary diseases.
Temperature: 36.5 °C, Heart rate: 98 beats/min; Respiratory rate: 30 breaths/min; Blood pressure: 110/80 mmHg. Acutely ill-looking, showing agitation and dyspnea. Slightly jaundiced skin and sclerae. Ocular proptosis, bilateral thyroid gland swelling. Rales heard in bilateral lungs. Heart rate of 98 bpm, with normal rhythm. No extra or abnormal heart sound, murmurs or pericardium friction sound. Abdomen flat and soft, marked pitting edema in lower extremities.
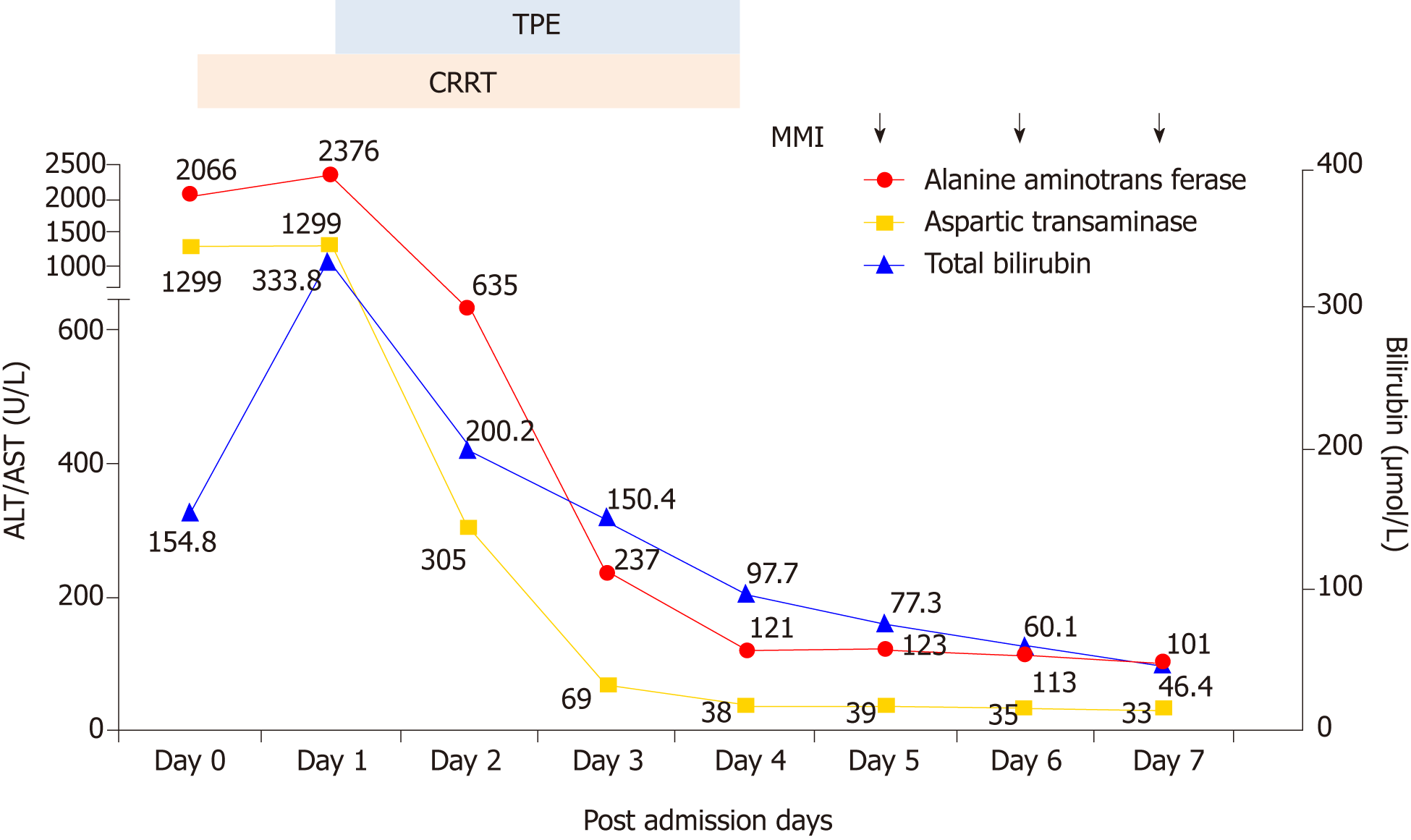
Liver function test results: ALT 2066 U/L, AST 1229 U/L, total bilirubin 154.8 μmol/L (normal range: 4.0-23.9 μmol/L), direct bilirubin 94 μmol/L (normal range: 0-6.8 μmol/L).
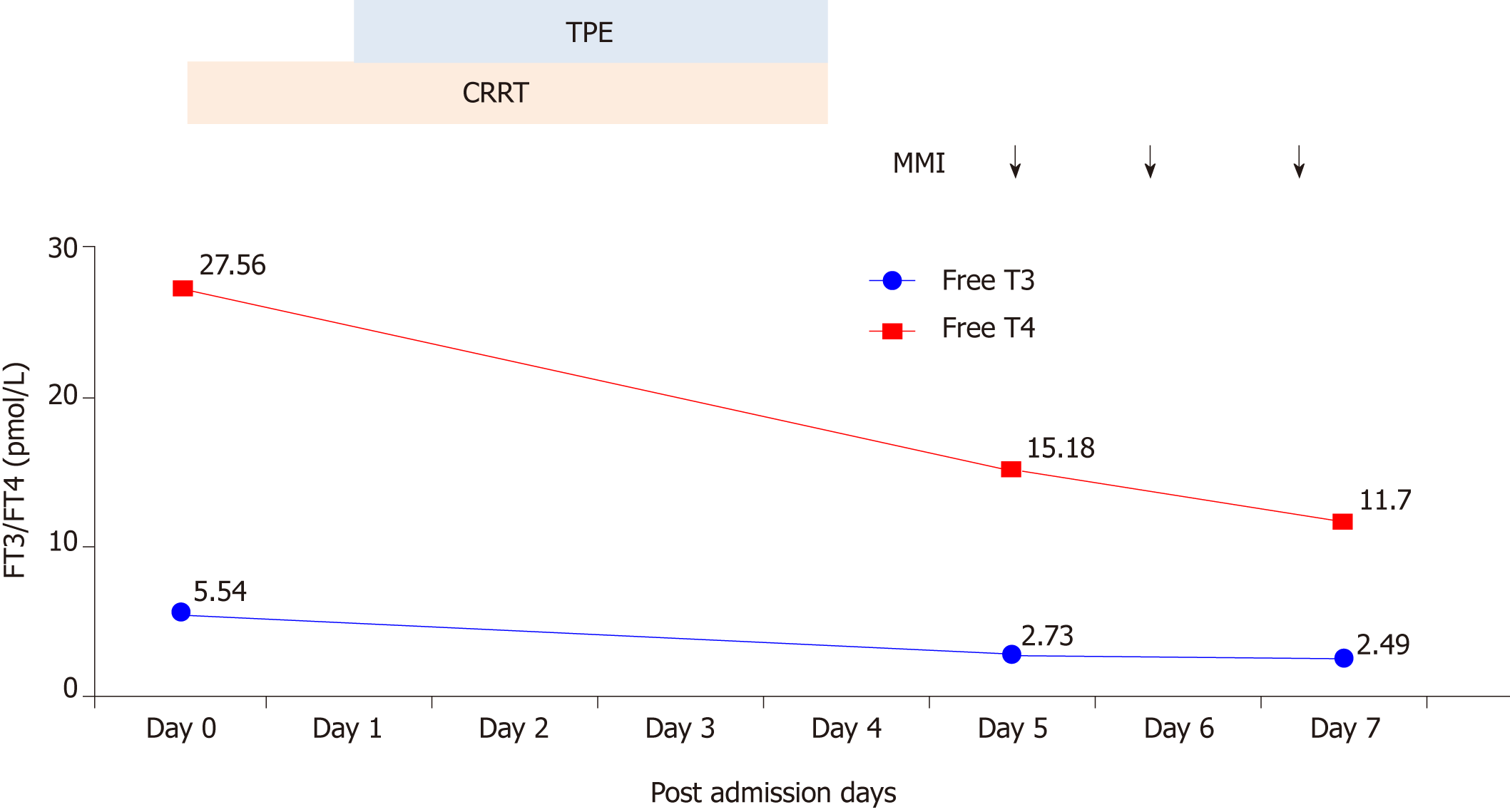
Thyroid function test results: FT4 27.56 pmol/L, FT3 5.54 pmol/L, thyroid-stimulating hormone 0.01 μU/mL (normal range: 0.55-4.78 μU/mL). Thyrotrophin receptor antibody: Positive.
Coagulation test results: Prothrombin time of 39.3 s (normal range: 11.0-14.5 s), prothrombin activity 17% (normal range: 70%-120%).
Brain natriuretic peptide: 2219 pg/mL (normal range: < 100 pg/mL).
Test for hepatitis virus A, B, C and E, Epstein–Barr virus, cytomegalovirus: Nega-tive. Immunological tests: Negative.
Ultrasound: Diffuse enlargement of the thyroid, no cholestasis or extrahepatic obstruction.
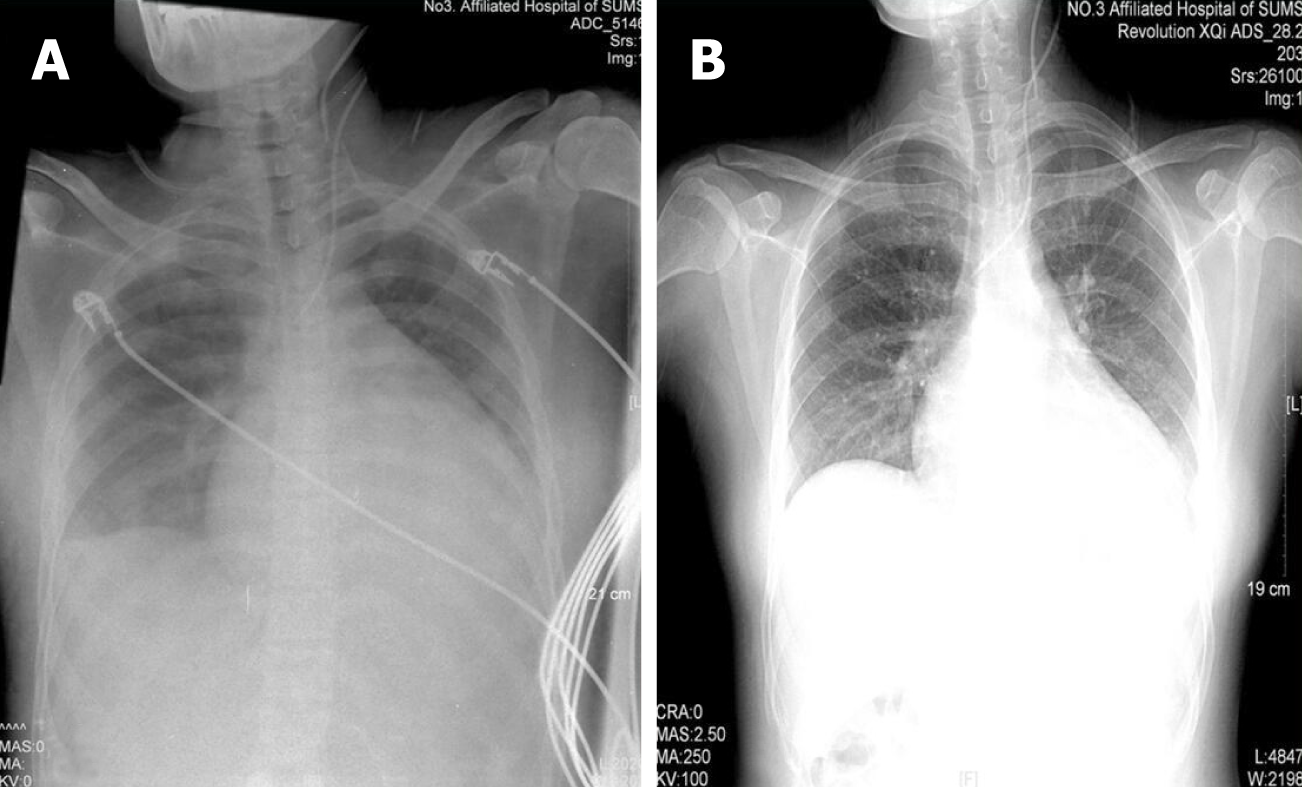
Chest radiography: Left atrium enlargement (Figure 1).
Echocardiography: Left ventricular ejection fraction of 46% with a pericardial and estimated pulmonary arterial systole pressure of 36 mmHg.
Burch and Wartofsky score was used to assess the probability of thyrotoxicosis; the patient’s Burch and Wartofsky score was 40. According to clinical presentation and laboratory results, the diagnoses of GD, hyperthyroid heart disease, and multiorgan dysfunction was confirmed.
On the day of admission, we intended to initiate TPE; however, considering the patient’s heart failure and severe edema, CRRT was administered with citrate anticoagulation. Continuous venovenous hemodiafiltration was performed and her blood flow rate was maintained at 150-200 mL/min based on target ultrafiltration. Replacement solutions were infused with post-displacement liquid at 1.5 L/h and dialysate liquid at 1.5 L/h. After treatment with CRRT, the vital signs began to stabilize and the agitated mental status was relieved, but the serum bilirubin remained dangerously elevated, which was reflected in the level of total bilirubin at 333.8 μmol/L. Therefore, TPE using 2 L fresh frozen plasma was performed in the following 4 d.
Following these procedures, the clinical signs were relieved almost completely. Chest radiography showed moderate improvement of cardiac enlargement (Figure 1). Upon further investigation, PTU-induced hepatic failure could not be excluded, so no antithyroid drugs were administered during this period. Liver biopsy was refused by the patient’s parents. On the 5th day, when the laboratory test results were improved (Figures 2 and 3), the patient tried taking methimazole (MMI, 20 mg/d) on the recommendation of an endocrinologist. On the 10th day, her transaminase levels and coagulation parameters were close to normal limits. She was then transferred to the Endocrinology Department for follow-up care.
The patient was discharged from our hospital after normal liver and thyroid function tests were obtained 1 mo later. Upon regular follow-up in the endocrinology clinic, her thyroid function status was normal and MMI was gradually reduced to 5 mg/d.
Hyperthyroidism can induce abnormal liver function, especially in the setting of congestive heart failure or thyroid storm, since excessive thyroid hormone levels augment cardiac output without an increase in hepatic blood flow[6], which leads to increased oxygen consumption and decreased liver perfusion that eventually lead to tissue hypoxia[7,8]. A previous report cited that mild derangements in liver function tests are common even in patients with subclinical hyperthyroidism but hepatic failure is rare[9].
An unusual feature of our patient was that our patient’s transaminases were slightly abnormal at the beginning. On commencing treatment with PTU for 1 d in the local hospital, her liver function deteriorated sharply. PTU was withdrawn and transaminases decreased but remained dangerously elevated. Other causes of hepatic injury, such as viral hepatitis, autoimmune disorders, alcohol consumption, and hepatotoxicity induced by other drugs were excluded. Acute liver failure in this patient was likely to be attributed to hepatocellular injury caused by insufficient oxygen supply as a result of decreased cardiac output. This was compounded by heart failure.
Furthermore, a possible relationship between liver dysfunction and PTU should be considered. However, hepatitis secondary to drug intake is difficult to diagnose. Hanson proposed the criteria to assist with diagnosis of drug-induced hepatitis. Only histological confirmation of hepatocellular injury and drug rechallenge could establish the diagnosis, but it is ethically unusual and only possible practically[10]. In our case, the liver biopsy sample was not obtained owing to her parents’ refusal.
In fact, transaminases in most patients with thyroid storm are only mildly elevated. An elevation of transaminases with AST levels > 1000 U/L is uncommon[11], and severe acute liver failure, as in the present patient, is an unusual presentation[12]. In most reported cases, liver failure occurs a few days or weeks after treatment by PTU[13]. Carhill et al[4] reported a 27-year-old patient with heart failure whose transaminases were elevated significantly after initiation of PTU. The PTU was replaced with MMI, and transaminases then decreased. Both Eisen and Lock reported severe hepatotoxicity after using 300 mg PTU for just 1 d[14,15]. PTU-related liver failure can occur at any time over the course of therapy. Unlike MMI, the adverse effects of PTU are not dose related. When PTU-related liver failure occurs, the onset is sudden, and the course is rapidly progressive[16].
TPE is an extracorporeal blood purification technique designed for rapid removal of harmful plasma constituents or to decrease the concentrations of antibodies and immune complexes[14,17]. It is a well-established and effective therapeutic option in many diseases and has been used successfully to treat severe hyperthyroidism in recent years[18,19]. The mechanism of this approach is based on the fact that approximately 99% of the circulating thyroid hormones are bound to serum proteins (thyroxin-binding globulin, transthyretin, and albumin), and these protein-bound thyroid hormones can be cleared during TPE. TPE can supplement unbound globulins from fresh frozen plasma to provide new binding sites for free thyroid hormones, which can be removed during the next TPE procedure[20]. In addition, TPE provides a particular benefit to patients with coagulopathy of liver disease by supplying coagulation factors. In patients with fulminant hepatic failure, TPE can improve survival of those with sufficient residual hepatic capacity for generation[15]. However, TPE alone was not adequately effective in the present patient, which indicated that she needed more urgent therapy.
It is well known that CRRT is conducive to regulation of acid–base balance, electrolytes, and fluid balance to maintain cellular metabolism and homeostasis. Thus, we performed CRRT initially to alleviate symptoms of heart failure and improve life-threatening conditions. CRRT involves continuous dialysis and filtration to facilitate the slower removal of fluids and solutes, which is well tolerated and results in fewer metabolic changes in critically ill patients[21]. This approach has advantages in terms of cardiovascular stability, which is suggested as a therapeutic measure in patients with heart failure. Furthermore, it can continually eliminate harmful molecular substances, blood ammonia, and other toxic substances such as lactic acid, which is also beneficial to patients with liver failure.
Our patient responded well to CRRT and TPE. However, approximately 25% of the thyroid hormones are present in the intravascular compartment, and each plasma exchange decreases FT3 and FT4 levels by 30%-50%. The influx of extravascular thyroid hormones into the circulation cannot be ignored[22]. Therefore, co-administration of antithyroid drugs is still necessary to maintain a long-term clinical stabilization. Some patients are treated with radioiodine, surgery or MMI after discontinuation of PTU. In recent years, the use of radioiodine in Graves’ disease has increased in Asia. In contrast, there is a decreasing trend of radioiodine therapy in the United States and Europe, which may be related to reports of higher rates of radiation-induced malignancies after radioiodine treatment[23]. In the present case, PTU was replaced with MMI 20 mg/d orally. Thyroid hormone and transaminases levels were stable. During follow-up of > 2 years, the thyroid and liver function tests were normal.
In conclusion, severe hyperthyroidism with high mortality requires multidisciplinary therapeutic measures in the intensive care setting. TPE combined with CRRT should be considered as a reasonably safe alternative when conventional treatment has failed. Early application of blood purification is feasible in serious clinical situations. Later, conventional medicine can be administered at an appropriate time. This sequential treatment procedure is worth considering in critically ill patients.
If the patient is on antithyroid therapy, and other contributors to liver damage have been excluded, drug-induced hepatotoxicity should be kept in mind. Propylthiouracil-induced liver injury may be very severe, even leading to acute liver failure. It can occur at any time over the course of therapy and is not dose related. TPE combined with CRRT in severe hyperthyroidism patients should be considered as a reasonably safe alternative in serious clinical situations.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sureshkumar K; Akoh JA; Mogulkoc R S- Editor: Dou Y L- Editor: Filipodia E- Editor: Tan WW
| 1. | Ezer A, Caliskan K, Parlakgumus A, Belli S, Kozanoglu I, Yildirim S. Preoperative Therapeutic Plasma Exchange In Patients With Thyrotoxicosis. J Clin Apher. 2009;24:111-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Keklik M, Kaynar L, Yilmaz M, Sivgin S, Solmaz M, Pala C, Aribas S, Akyol G, Unluhizarci K, Cetin M, Eser B, Unal A. The Results Of Therapeutic Plasma Exchange In Patients With Severe Hyperthyroidism: A Retrospective Multicenter Study. Transfus Apher Sci. 2013;48:327-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Iglesias P, DÉVora O, GarcÍA J, Tajada P, GarcÍA-ArÉValo C, DÍEz JJ. Severe Hyperthyroidism: Aetiology, Clinical Features And Treatment Outcome. Clin Endocrinol (Oxf). 2010;72:551-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Carhill A, Gutierrez A, Lakhia R, Nalini R. Surviving The Storm: Two Cases Of Thyroid Storm Successfully Treated With Plasmapheresis. BMJ Case Rep. 2012;2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ashkar FS, Katims RB, Smoak WM, Gilson AJ. Thyroid Storm Treatment With Blood Exchange And Plasmapheresis. JAMA. 1970;214:1275-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | De Campos Mazo DF, De Vasconcelos GB, Pereira MA, De Mello ES, Bacchella T, Carrilho FJ, Cançado EL. Clinical Spectrum And Therapeutic Approach To Hepatocellular Injury In Patients With Hyperthyroidism. Clin Exp Gastroenterol. 2013;6:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | EISEN MJ. Fulminant Hepatitis During Treatment With Propylthiouracil. N Engl J Med. 1953;249:814-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Barzilay-Yoseph L, Shabun A, Shilo L, Hadary R, Nabriski D, Kitay-Cohen Y. Thyrotoxic Hepatitis. Isr Med Assoc J. 2011;13:448-450. [PubMed] [Cited in This Article: ] |
| 9. | Elias RM, Dean DS, Barsness GW. Hepatic Dysfunction In Hospitalized Patients With Acute Thyrotoxicosis: A Decade Of Experience. ISRN Endocrinol. 2012;2012:325092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Livadas S, Xyrafis X, Economou F, Boutzios G, Christou M, Zerva A, Karachalios A, Palioura H, Palimeri S, Diamanti-Kandarakis E. Liver Failure Due To Antithyroid Drugs: Report Of A Case And Literature Review. Endocrine. 2010;38:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Chong HW, See KC, Phua J. Thyroid Storm With Multiorgan Failure. Thyroid. 2010;20:333-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Sousa DomÍNguez A. Severe Acute Liver Failure And Thyrotoxicosis: An Unusual Association. Rev Esp Enferm Dig. 2015;107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Akmal A, Kung J. Propylthiouracil, And Methimazole, And Carbimazole-Related Hepatotoxicity. Expert Opin Drug Saf. 2014;13:1397-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Garla V, Kovvuru K, Ahuja S, Palabindala V, Malhotra B, Abdul Salim S. Severe Hyperthyroidism Complicated By Agranulocytosis Treated With Therapeutic Plasma Exchange: Case Report And Review Of The Literature. Case Rep Endocrinol. 2018;2018:4135940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Aydemir S, Ustundag Y, Bayraktaroglu T, Tekin IO, Peksoy I, Unal AU. Fulminant Hepatic Failure Associated With Propylthiouracil: A Case Report With Treatment Emphasis On The Use Of Plasmapheresis. J Clin Apher. 2005;20:235-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, Mcdougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN. The Role Of Propylthiouracil In The Management Of Graves' Disease In Adults: Report Of A Meeting Jointly Sponsored By The American Thyroid Association And The Food And Drug Administration. Thyroid. 2009;19:673-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Jha S, Waghdhare S, Reddi R, Bhattacharya P. Thyroid Storm Due To Inappropriate Administration Of A Compounded Thyroid Hormone Preparation Successfully Treated With Plasmapheresis. Thyroid. 2012;22:1283-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Pasimeni G, Caroli F, Spriano G, Antonini M, Baldelli R, Appetecchia M. Refractory Thyrotoxicosis Induced By Iodinated Contrast Agents Treated With Therapeutic Plasma Exchange. A Case Report. J Clin Apher. 2008;23:92-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Muller C, Perrin P, Faller B, Richter S, Chantrel F. Role Of Plasma Exchange In The Thyroid Storm. Ther Apher Dial. 2011;15:522-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Min SH, Phung A, Oh TJ, Han KS, Kim MJ, Kim JM, Lee JH, Park YJ. Therapeutic Plasmapheresis Enabling Radioactive Iodine Treatment In A Patient With Thyrotoxicosis. J Korean Med Sci. 2015;30:1531-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Park HS, Kwon SK, Kim YN. Successful Treatment Of Thyroid Storm Presenting As Recurrent Cardiac Arrest And Subsequent Multiorgan Failure By Continuous Renal Replacement Therapy. Endocrinol Diabetes Metab Case Rep. 2017;2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kokuho T, Kuji T, Yasuda G, Umemura S. Thyroid Storm-Induced Multiple Organ Failure Relieved Quickly By Plasma Exchange Therapy. Ther Apher Dial. 2004;8:347-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Burch HB, Burman KD, Cooper DS. A 2011 Survey Of Clinical Practice Patterns In The Management Of Graves' Disease. J Clin Endocrinol Metab. 2012;97:4549-4558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |