Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4648
Peer-review started: January 21, 2023
First decision: March 10, 2023
Revised: March 24, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: July 6, 2023
Edaravone is a widely used treatment for patients with cerebral infarction and, in most cases, edaravone-induced side effects are mild. However, edaravone-related adverse reactions have been receiving increasing attention.
We treated three patients with acute cerebral infarction who died following treatment with edaravone. Edaravone is a widely used treatment for patients with cerebral infarction and, in most cases, edaravone-induced side effects are mild. However, edaravone-related adverse reactions have been receiving increasing attention.
Our cases highlight the importance of educating clinicians regarding the new edaravone-induced clinical syndromes of cerebral infarction as potentially fatal adverse drug reactions. Considering that no laboratory or confirmatory test exists to diagnose edaravone-induced death from cerebral infarction, clinicians’ knowledge is the key element in recognizing this phenomenon.
Core Tip: We report three patients with acute cerebral infarction who died after edaravone treatment. Edaravone is used to scavenge oxygen free radicals in patients with acute ischemic stroke, and most clinicians are only aware of its damage to kidney function. However, the main symptoms in patients treated with intravenous edaravone, such as rapid disease change, coagulation dysfunction, cardiac arrest and other side effects are rarely reported. The purpose of this paper is to share the experience of edaravone therapy in clinical practice.
- Citation: Yang L, Xu X, Wang L, Zeng KB, Wang XF. Edaravone administration and its potential association with a new clinical syndrome in cerebral infarction patients: Three case reports. World J Clin Cases 2023; 11(19): 4648-4654
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4648.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4648
Edaravone is a clinically effective neuroprotective agent and a free radical scavenger that can capture hydroxyl radicals. It was approved for marketing in Japan and China in 2001 and 2005, respectively. Initially indicated for protection against ischemic brain damage, the indications for edaravone have gradually expanded to the treatment of amyotrophic lateral sclerosis[1-5]. However, owing to its widespread clinical application, adverse reactions due to edaravone have also received increasing attention[6-8]. In the earliest randomized controlled trial of edaravone for ischemic stroke treatment, 4 patients in the edaravone and 5 patients in the blank control groups died out of the 125 cases in each group. In the edaravone group, all deaths were caused by cerebral infarction; while in the control group, three patients died of cardiac arrest, pneumonia, and depression suicide, and two patients died of cerebral hernia caused by edema, worsened cerebral infarction, pneumonia, and disseminated intravascular coagulation (DIC) caused by cirrhosis[6]. In 2007, Japanese scholars reported that one patient with cerebral infarction treated with edaravone experienced severe adverse reactions. The patient presented with a sudden disturbance of consciousness and shock 12 d after hospitalization[6]. In 2018, we observed three patients who died of sudden worsening of acute cerebral infarction during treatment with edaravone, which was suspected to be the cause of death.
Case 1: A 63-year-old woman with slurred speech and mental and behavioral abnormalities for ten days was admitted to our hospital.
Case 2: A 65-year-old man was admitted to our hospital due to being unconscious for 11 h.
Case 3: A 71-year-old man was admitted to our hospital due to sudden twisting of the left corner of his mouth for more than five days and difficulty swallowing for two days.
Case 1: A 63-year-old woman with slurred speech and mental and behavioral abnormalities for ten days was admitted to our hospital.
Case 2: A 65-year-old man was admitted to our hospital due to being unconscious for 11 h.
Case 3: A 71-year-old man was admitted to our hospital due to sudden twisting of the left corner of his mouth for more than five days and difficulty swallowing for two days.
Case 1: No significant history of past illness was noted in the patient.
Case 2: The patient had a history of hypertension, diabetes mellitus, and cerebral infarction.
Case 3: The patient had a history of hypertension.
Personal history and family history were unremarkable.
Case 1: On admission, the patient’s vital signs were stable. The patient showed dysarthria, lack of cooperation, level V muscular strength in both limbs, and negative Babinski signs in both limbs.
Case 2: The patient’s temperature was 36.6°C, blood pressure was 175/91 mmHg, heart rate was 80 beats/min, and respiratory rate was 16 breaths/min. The patient was in a superficial coma. The left nasolabial fold was slightly shallower than the contralateral side. Upon pain stimulation, the patient responded with an expression of pain and lifted his left limbs from the bed surface, but not the right limbs. Muscle tone of the right limb was lower than that of the left limb. Tendon reflexes on the right side were weaker than those on the left side.
Case 3: On admission, the patient’s vital signs were stable. The patient was alert and had symmetrical forehead lines and dysarthria. The right nasolabial fold was slightly shallower than its contralateral part, and the patient's tongue deviated to the right when it was protruded. The patient had normal muscular strength and tone in all four limbs and symmetrical sensation and tendon reflexes. Bilateral Babinski signs were negative.
Case 1: Laboratory examinations, including routine blood tests, liver and kidney function tests, and electrolyte and blood coagulation tests were within normal parameters. Electrocardiography revealed normal sinus rhythm.
Case 2: Examination of blood coagulation status, liver and kidney function, and electrolyte levels after admission revealed no obvious abnormalities. Electrocardiography revealed normal sinus rhythm.
Case 3: Laboratory examinations were within normal parameters. Electrocardiography revealed normal sinus rhythm.
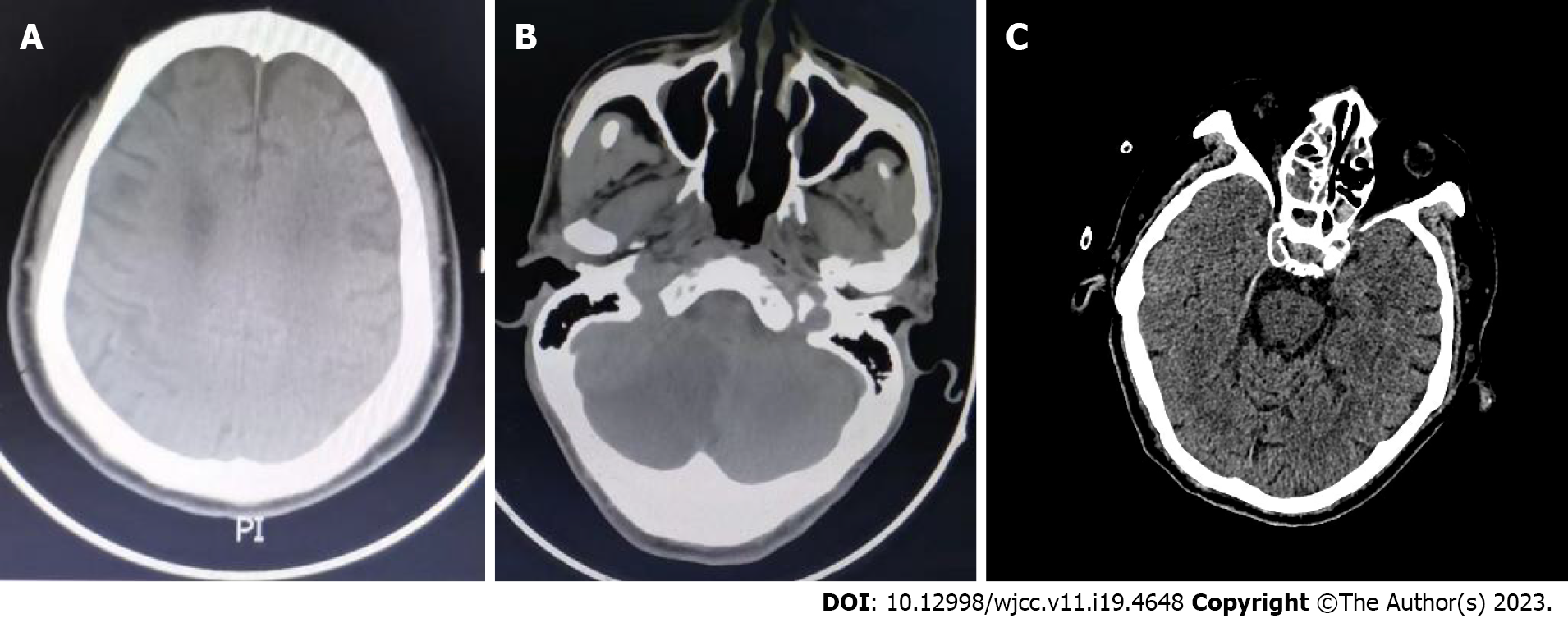
Case 1: Head computed tomography (CT) scan showed cerebral infarction in the bilateral parietal lobes with multiple small ischemic lesions (Figure 1A).
Case 2: The patient’s emergency head CT scan showed cerebral infarction in the left thalamus and right cerebellar hemisphere (Figure 1B).
Case 3: Head CT scan suggested the probability of lacunar infarction in the left brainstem (Figure 1C).
Case 1: Acute cerebral infarction, respiratory failure, and pneumonia.
Case 2: Hypertension, diabetes mellitus, and cerebral infarction.
Case 3: Hypertension, DIC, and cerebral infarction.
Case 1: The following drugs were prescribed: Aspirin (100 mg once a day)[7,8], atorvastatin (20 mg once a day)[8,9], edaravone (30 mg twice a day), and Ginkgo biloba extract[10,11]. On the sixth day after admission, the patient developed dyspnea and showed decreased oxygen saturation. The patient was transferred to the intensive care unit (ICU) for tracheal intubation and mechanical ventilation. Multiple ecchymoses were observed on the patient’s body. DIC was diagnosed based on blood coagulation results, and low-molecular weight heparin (0.2 mL twice a day), tranexamic acid (500 mg once a day), fresh frozen plasma (400 mL), and cryoprecipitate (6 U) were administered to the patient. Blood coagulation status improved gradually after treatment. The patient’s respiratory condition improved after receiving ceftizoxime, and she was released from the ventilator.
Case 2: The patient received aspirin (100 mg once a day)[7,8], atorvastatin (20 mg once a day)[8,9], edaravone (30 mg twice a day), Ginkgo biloba extract[10-13], and ceftizoxime (2 g three times a day) to treat the lung infection. Insulin was pumped intravenously based on increased blood glucose levels.
Case 3: The patient was administered clopidogrel bisulfate (75 mg once a day), atorvastatin (20 mg once a day)[8,9], edaravone (30 mg twice a day), and ozagrel extract.
Case 1: On the tenth day after admission, the patient experienced sudden cardiac and respiratory arrest. After external chest compressions and mechanical ventilation, her heartbeat and breathing were restored. However, two further cardiac arrests occurred, and the patient died after the last attempt at resuscitation failed.
Case 2: On the third day after admission, the patient's consciousness improved into a lethargic state. However, on the fifth day after admission, the patient lapsed into a deep coma and his heart rate gradually decreased. Resuscitation attempts were ultimately unsuccessful.
Case 3: The twisted corner of the mouth rapidly improved. However, sudden cardiac arrest occurred. The heartbeat was restored after external chest compressions, and the patient was alert again. During transfer to the ICU, his heart rate slowed down to 20 beats/min but was restored after external chest compressions. A bedside tracheotomy was performed due to difficulties with orotracheal intubation. Bleeding at the cutting site and urethral bleeding occurred. The coagulation function showed prothrombin time 15.3 s, increased prothrombin time ratio 1.30, increased international normalized ratio 1.31, decreased prothrombin activity 65.2%, activated partial thromboplastin time 32.6 s, thrombin time 18.1 s, and increased D-dimer > 76 mg/L. Fresh frozen plasma (400 mL), cryoprecipitate (6 U), and red blood cell suspension (2 U) were administered to the patient. Compression and carbazochrome sodium sulfonate injection were performed to stop the bleeding, which improved. However, six days after admission, the patient experienced cardiac arrest and died.
Edaravone is a newly developed free radical scavenger, and its administration has been used as a clinical therapeutic option for the management of cerebral infarction[6]. In this case, all three patients had acute cerebral infarction. Therefore, we selected edaravone as the therapeutic option for our patients.
The instructions for edaravone clearly suggest that caution should be exercised when using the drug in cardiac patients due to the risk of death[14]. When edaravone was prescribed to elderly patients with cerebral infarction, some experienced a sudden worsening of their condition or died of cardiac arrest[15]. Therefore, we suspected that edaravone may cause serious adverse reactions, although the reason for this remains unknown. Death in patients with cerebral infarction is commonly caused by cerebral hernias in the acute phase of cerebral edema and organ dysfunction resulting from infection accompanied by deterioration of the underlying heart disease[16,17]. This is a noteworthy aspect that should be emphasized, as the acute ischemic stroke etiology in the patients described may also be due to hematological disease[18]. However, in three cases, the patients’ condition deteriorated suddenly and they passed away soon after, which is different from the common cause of death we mentioned above. All these cases had the following characteristics: (1) Elderly patients (aged > 60-year-old); (2) patients with acute cerebral infarction but with a small infarct size, no increase in intracranial pressure, and no serious infection; (3) no history of coronary heart disease, no atrial fibrillation or heart failure, and no abnormal electrocardiography; (4) treatment with edaravone for 4-10 d; (5) rapid deterioration in the patients’ condition, including coagulation dysfunction, severe hepatic and renal damage, or sudden cardiac arrest; and (6) poor response to treatment and difficulty in recovering.
Edaravone has also been reported to cause coagulation dysfunctions. In Japan, approximately 400000 patients received edaravone within 4 years of its release in 2001. The registered adverse reactions were 477 cases, including hepatobiliary diseases (0.1%), renal urinary diseases (0.05%), and thrombocytopenia/DIC (0.02%)[5,6]. Both cases 1 and 3 showed coagulation dysfunction, which improved with plasma and cryoprecipitate infusion, and edaravone was discontinued. This suggests that coagulation dysfunction may have occurred before and after cardiac arrest in these aggravated conditions. Therefore, early detection and reasonable treatment of coagulation dysfunction are important. Patients showed tracheal hemorrhage after tracheotomy, massive urethral hemorrhage, and an abnormal coagulation index without a significant decline in platelets, which were in line with the serious adverse reactions of edaravone with a high risk of disseminated or diffuse intravascular coagulation.
Cardiac arrest occurred in all three patients during treatment with edaravone. In cases 2 and 3, cardiac arrest was the only manifestation of early exacerbation. Patients 1 and 3 were successfully resuscitated after short-term cardiac arrest, but all three patients ultimately had a worse prognosis. As none of the three patients had prior heart disease, the cardiac arrest could not be attributed to existing heart disease. Based on our clinical observations, other possible causes of the three deaths could be excluded, including concomitant medications such as aspirin, clopidogrel, atorvastatin, Ginkgo biloba extract, ozagrel extract, and ceftizoxime. There were no severe adverse reactions related to sudden cardiac arrest, and there were no warnings for the medications administered for patients with heart disease according to the instructions for these drugs. Therefore, these drugs are unlikely to cause disease progression or cardiac arrest. During this exacerbation, severe hepatic and renal function impairments were observed in case 3, which have been mentioned as severe adverse reactions caused by the edaravone. Therefore, sudden deterioration of the patients may be due to adverse drug reactions caused by edaravone. However, the cause-and-effect relationship between the three patients’ deaths and edaravone’s severe adverse reactions still requires confirmation.
In contrast, experimental animal studies have suggested that edaravone treatment improves hepatic injury following ischemia/reperfusion injury, partial hepatectomy, or endotoxin administration[19]. Other researchers have shown that edaravone has beneficial effects on kidney injury induced by ischemia/reperfusion or cisplatin[20-22]. It is possible that edaravone and edaravone-peroxy radicals, which are metabolic products of edaravone, are responsible for these adverse effects; however, the underlying mechanisms remain unclear. Further studies are required to determine the discrepancies between the protective effects observed in previous animal studies and the adverse effects observed in our patients.
In summary, these patients exhibited similar clinical characteristics. Although the characteristics of edaravone use that may lead to sudden death have not yet been identified, this series of cases represent a new clinical syndrome. Thus, further studies are needed to characterize the pathophysiology of this syndrome and determine the underlying causes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Arboix A, Spain; Mishra AK, United States S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Li LD, Zhou Y, Shi SF. Edaravone combined with Shuxuening versus edaravone alone in the treatment of acute cerebral infarction: A systematic review and meta-analysis. Medicine (Baltimore). 2023;102:e32929. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 2. | Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 546] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 3. | Rosenfeldt F, Wilson M, Lee G, Kure C, Ou R, Braun L, de Haan J. Oxidative stress in surgery in an ageing population: pathophysiology and therapy. Exp Gerontol. 2013;48:45-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Cao S, Wei J, Cai Y, Xiong Z, Li J, Jiang Z, Zhou X, Huang B, Zeng J. Network Pharmacology Prediction and Experimental Verification for Anti-Ferroptosis of Edaravone After Experimental Intracerebral Hemorrhage. Mol Neurobiol. 2023;. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221-3231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 415] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 491] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Dengler R, Diener HC, Schwartz A, Grond M, Schumacher H, Machnig T, Eschenfelder CC, Leonard J, Weissenborn K, Kastrup A, Haberl R; EARLY Investigators. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Wang G, Fang B, Yu X, Li Z. [Interpretation of 2018 guidelines for the early management of patients with acute ischemic stroke]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 9. | Blanco M, Nombela F, Castellanos M, Rodriguez-Yáñez M, García-Gil M, Leira R, Lizasoain I, Serena J, Vivancos J, Moro MA, Dávalos A, Castillo J. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69:904-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Zeng GR, Zhou SD, Shao YJ, Zhang MH, Dong LM, Lv JW, Zhang HX, Tang YH, Jiang DJ, Liu XM. Effect of Ginkgo biloba extract-761 on motor functions in permanent middle cerebral artery occlusion rats. Phytomedicine. 2018;48:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Wu X, Yu Z. Ginkgo leaf extract and dipyridamole injection as adjuvant treatment for acute cerebral infarction: Protocol for systemic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98:e14643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Abe M, Kaizu K, Matsumoto K. A case report of acute renal failure and fulminant hepatitis associated with edaravone administration in a cerebral infarction patient. Ther Apher Dial. 2007;11:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11:1753-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Raymond J, Oskarsson B, Mehta P, Horton K. Clinical characteristics of a large cohort of US participants enrolled in the National Amyotrophic Lateral Sclerosis (ALS) Registry, 2010-2015. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:413-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Jackson C, Heiman-Patterson T, Kittrell P, Baranovsky T, McAnanama G, Bower L, Agnese W, Martin M. Radicava (edaravone) for amyotrophic lateral sclerosis: US experience at 1 year after launch. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:605-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 16. | Zhu L, Huang Z, Sun X, Wu M, Xu Y, Sun Z. The function of mechanical thrombectomy on improving haemodynamics of patients with acute cerebral infarction. Basic & Clinical Pharmacology & Toxicology 2019; 124: 116. Available from: https://www.zhangqiaokeyan.com/journal-foreign-detail/0704028058275.html. . [Cited in This Article: ] |
| 17. | Chen CJ, Wang C, Buell TJ, Ding D, Raper DM, Ironside N, Paisan GM, Starke RM, Southerland AM, Liu K, Worrall BB. Endovascular Mechanical Thrombectomy for Acute Middle Cerebral Artery M2 Segment Occlusion: A Systematic Review. World Neurosurg. 2017;107:684-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Arboix A, Jiménez C, Massons J, Parra O, Besses C. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9:891-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Tsuji K, Kwon AH, Yoshida H, Qiu Z, Kaibori M, Okumura T, Kamiyama Y. Free radical scavenger (edaravone) prevents endotoxin-induced liver injury after partial hepatectomy in rats. J Hepatol. 2005;42:94-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, Sasaki T, Makino H. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003;305:1183-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Koike N, Sasaki A, Murakami T, Suzuki K. Effect of edaravone against cisplatin-induced chronic renal injury. Drug Chem Toxicol. 2021;44:437-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |