Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.104
Peer-review started: October 6, 2022
First decision: October 27, 2022
Revised: November 15, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 6, 2023
Nigeria is one of the thirty high burden countries with significant contribution to the global childhood tuberculosis epidemic. Tuberculosis annual risk for children could be as high as 4% particularly in high tuberculosis (TB) prevalent communities. Isoniazid (INH) Preventive Therapy has been shown to prevent TB incidence but data on its implementation among children are scarce.
To determine the completion of INH among under six children that were exposed to adults with smear positive pulmonary TB in Lagos, Nigeria.
This was a hospital-based retrospective cross-sectional review of 265 medical records of eligible children < 6 years old enrolled for INH across 32 private hospitals in Lagos, Nigeria. The study took place between July and September 2020. Data was collected on independent variables (age, gender, type of facility, TB screening, dose and weight) and outcome variables (INH outcome and proportion lost to follow up across months 1-6 of INH treatment).
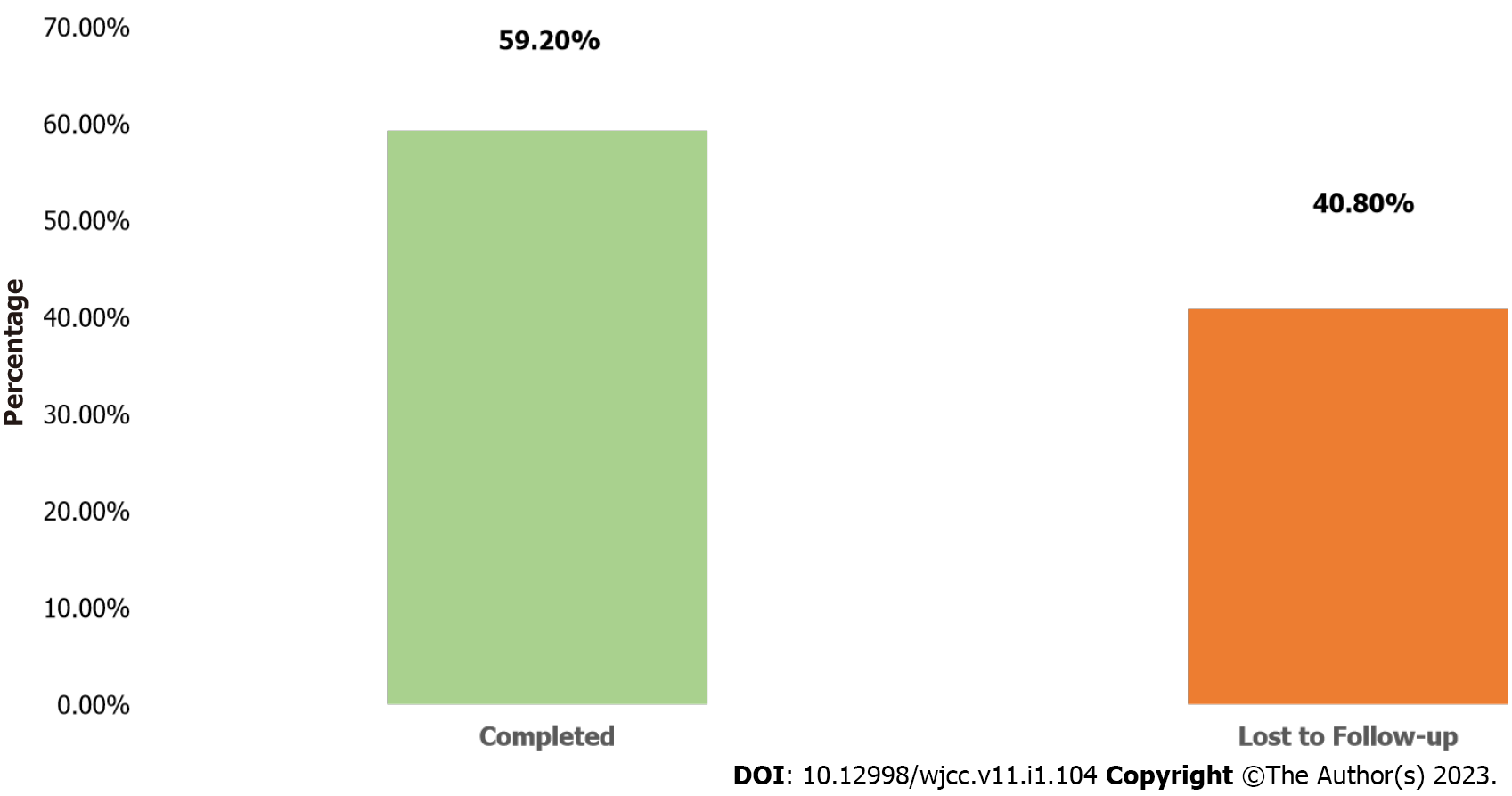
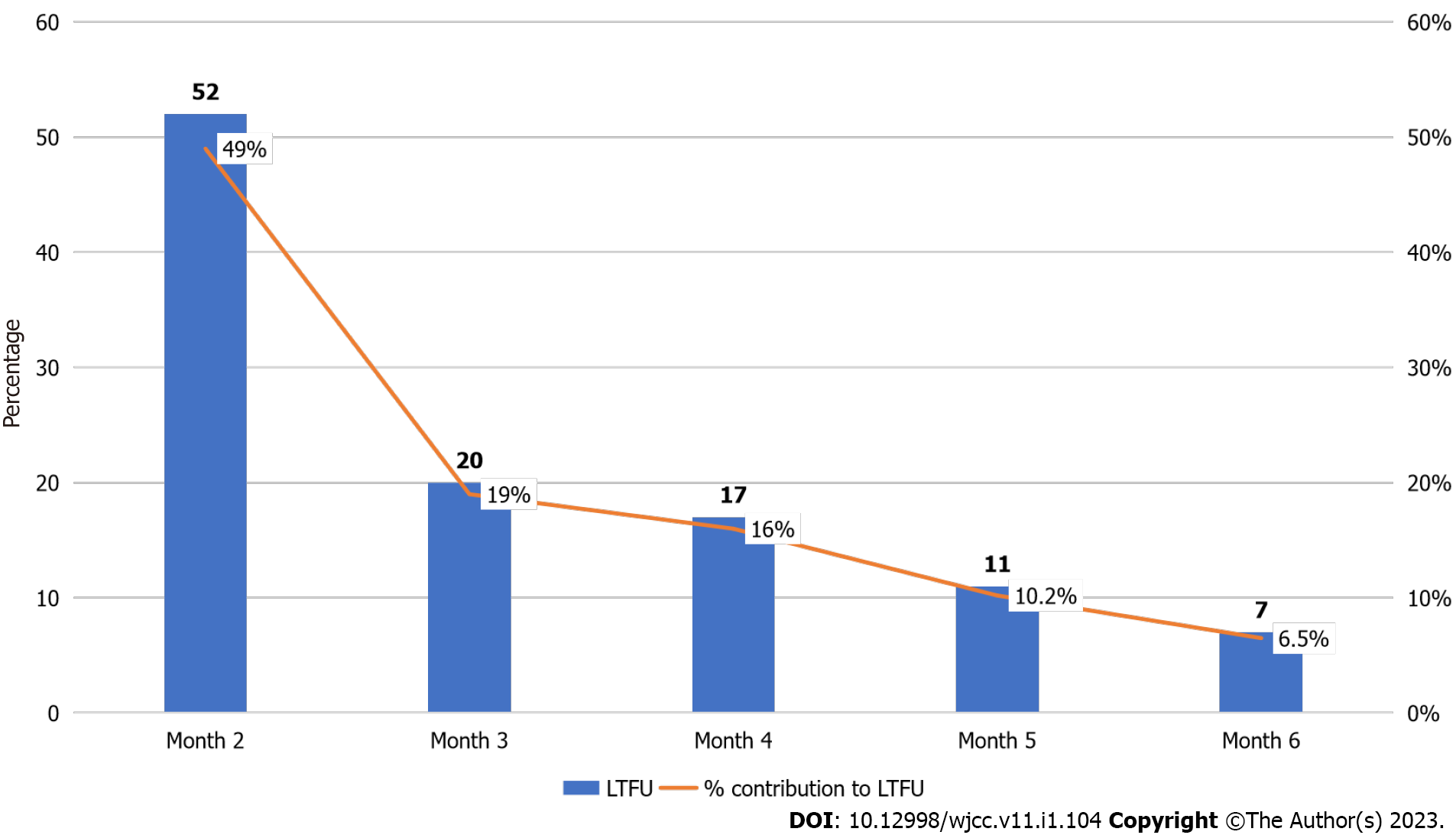
About 53.8% of the participants were female, 95.4% were screened for TB and none was diagnosed of having TB. The participants’ age ranged from 1 to 72 mo with a mean of 36.01 ± 19.67 mo, and 40.2% were between the ages of 1-24 mo. Only 155 (59.2%) of the 262 participants initiated on INH completed the six-month treatment. Cumulatively, 107 (41.0%) children were lost to follow-up at the end of the sixth month. Of the cumulative 107 loss to follow-up while on INH, largest drop-offs were reported at the end of month 2, 52 (49%) followed by 20 (19%), 17 (16%), 11 (10.2%) and 7 (6.5%) at months 3, 4, 5 and 6 respectively. The analysis showed that there was no significant association between age, gender, type of facility and completion of INH treatment (P > 0.005).
This study demonstrated suboptimal INH completion rate among children with only 6 out of 10 children initiated on INH who completed a 6-mo treatment in Lagos, Nigeria. The huge drop-offs in the first 2 mo of INH calls for innovative strategies such as the use of 60-d INH calendar that would facilitate reminder and early engagement of children on INH and their caregivers in care and across the entire period of treatment.
Core Tip: Isoniazid (INH) completion rate among children in Nigeria was suboptimal in this programmatic cohort. The chi-square analysis revealed that age, gender, and type of facility were not determinants of the treatment completion of INH recorded among eligible children initiated in Lagos, Nigeria. The huge drop-offs in the first 2 mo of INH calls for innovative strategies such as the use of 60-day INH calendar that would facilitate reminder and early engagement of children on INH and their caregivers in care and across the entire period of treatment. Targeted interventions such as community initiation and monitoring of INH by healthcare workers, and rapid scale up of shorter tuberculosis preventive therapy regimen are needed to address these drop-offs along the childhood INH cascade. Future studies should therefore qualitatively explore the reasons why some children did not complete the INH.
- Citation: Adepoju VA, Adelekan A, Agbaje A, Quaitey F, Ademola-Kay T, Udoekpo AU, Sokoya OD. Completion of 6-mo isoniazid preventive treatment among eligible under six children: A cross-sectional study, Lagos, Nigeria. World J Clin Cases 2023; 11(1): 104-115
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/104.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.104
Nigeria is one of the 30 high tuberculosis (TB) burden countries which have contributed significantly to the global childhood TB epidemic[1]. TB incidence in Nigeria increased from 338/100000 to 418000 between 2012 and 2017, hence Nigeria has been grouped among high TB burden countries, globally[2,3]. Nigeria is a high burden country not just for drug-sensitive TB but also drug-resistant TB as well as TB/Human Immunodeficiency Virus (HIV) co-infection. The rapid progression of latent TB to active TB in children within 1-2 years has been previously demonstrated[4]. TB annual risk for children could also be as high as 4%, particularly in high TB prevalent communities[5] and the younger children progressed faster from TB infection to TB disease. This may not be unconnected with the relatively lower immunity of the younger children compared to the older ones and the adults. In 2017, an estimated 57000 children developed TB, representing 13.6% of the estimated 418000 new TB cases in Nigeria[6]. Children acquire the disease by inhaling the TB bacteria released by an adult or close family member with open TB[1]. Older children may acquire the disease from close contact in schools[7].
The contribution of isoniazid preventive therapy (IPT) to the decline of TB morbidity and mortality is well known. IPT was first recommended as TB preventive therapy in the 1960s following randomized controlled trials with about 70000 subjects[8]. According to the World Health Organization, all under-5 contacts of bacteriologically positive TB patients should receive at least 6 mo of isoniazid (INH) at a daily dose of 5 mg/kg (maximum 300 mg) for at least six months. INH is a proven intervention that reduced the progression of latent TB to active TB among children when administered for 6 mo[8] and could decrease risk of TB acquisition by 59% in children under the age of 15 years[9].
Although guidelines recommending INH for eligible children aged less than 6 years as well as HIV infected adults without active TB, have been published since 2014, implementation of INH among children has been rarely evaluated in Nigeria and available studies were largely among adult Persons Living with HIV[10,11]. According to the Global Fund Report released in March 2022, less than 30% of eligible under-five children initiated INH, highlighting persistent under performance of this indicator since 2019[12]. Another study from Nigeria observed that between 2015 and 2018, the unscreened proportion amounts to > 95% of missed eligible under-five children in each year[13]. The results reflect huge missed opportunities for INH, with risks for latent TB reactivation in a growing population[13]. In Ethiopia and Kenya, INH uptake of 37% and 53.2% and completion rate of 67.9% and 88% respectively were reported[14,15].
According to guidelines from the National TB Program (NTP) in Nigeria, after excluding the involvement of active TB disease in children under the age of six years who have a history of contact with a patient with bacteriologically confirmed pulmonary TB, INH should be provided for six months[2]. However, INH efficacy relies on 80% or greater adherence to medication[9]. Data showing completion among children could help the National TB Program in evaluating TB control efforts as unfavorable INH outcomes reduce the efficacy of this prevention tool and promote the spread of INH-resistant TB. Even when children commence six months of INH, data evaluating INH completion among child contacts of smear positive adult TB patients are rarely reported in Nigeria. This study aimed to determine the completion of isoniazid preventive treatment among under six children in contact with adults with TB and associated factors under routine programmatic use in Lagos, Nigeria. The outcome of the study will lead to recommendations on how NTP can develop appropriate interventions that could support the successful implementation and completion of INH among children towards minimizing the catastrophic cost associated with latent TB treatment.
This was a retrospective review of routine programmatic INH data of under-6 contacts of bacteriologically positive TB patients. Data was collected across 32 private facilities in Lagos Nigeria. The study took place between July-September, 2020.
The study took place in 32 private facilities (26 private for-profit and six private not-for-profits) spread across 13 Local Government Areas (LGAs) in Lagos, Nigeria. Lagos State is divided into 20 LGAs and has a population of 24 million people[16]. The population of Lagos is about 11% of the total Nigerian population. Each LGA in Lagos is supervised by an LGA TB supervisor who oversees that LGA and responsible for TB surveillance. The delivery of healthcare service in Lagos is also organized at three levels namely primary, secondary, and tertiary. In 2003, the Lagos State TB, Buruli Ulcer, and Leprosy Control Program (LSTBLCP) was established as the sub-national authority responsible for TB surveillance activities at state level. The program was further expanded by engaging the private facilities in 2008. The number of private facilities engaged by LSTBLCP increased from 8 facilities to over 150 private health facilities. Lagos facilities are engaged for TB control under four service schemes, i.e., referral of presumptive TB only, provision of directly observed therapy, short-course treatment only, and provision of microscopy.
The cascade of determining the eligibility of a child contact for INH in Nigeria starts by asking smear-positive adult TB patients about the number of children and age of children residing in the same home. Individuals who respond yes to the presence of children less than 6 years were asked by a healthcare worker to bring them to the hospital or in some cases, visited at home for further evaluation by a community health worker. During home visit or presentation to health facility, healthcare workers conduct routine screening for TB using standardized TB screening tool by asking for symptoms of cough of 2 weeks or more, poor weight gain (defined as weight-for-age less than −3 Z-score, or underweight/ weight-for-age less than −2 Z-score, or confirmed weight loss > 5% since the last visit or growth curve flattening), fever (defined as a body temperature of > 37.5 degree Celsius), or other signs of presumptive TB such as body swelling and lymph node enlargement. Once the above symptoms of TB are excluded, adherence counselling is provided and the healthcare worker offers daily INH at 10 mg/kg/day to a maximum of 300 mg/day for 6 mo[2]. Children screened to have any of the presumptive TB symptoms are offered genexpert test/chest x-ray under the National TB, Buruli Ulcer and Leprosy Control Program guidelines. During INH clinic visits, patients’ caregivers/parents are asked for symptoms of TB, investigated if symptomatic, and assessed for adverse events. HCW then documents the child’s weight and dosage of INH per visit in the INH register/ register for the management of under-6 contacts of TB patients. Lost to follow up has occurred when a child misses 2 mo or more of INH refill visits. Such a child is restarted anytime he or she is back in care. However, INH prescription continues from the last refill without any need to restart for children who miss < 2 mo of refill visits, according to the national guideline. At the end of 6 mo, the INH outcomes are also recorded by the healthcare worker following the case definition by NTBLCP.
Completed INH: A child who received a full course of isoniazid (6 mo/180 doses) in 6-9 mo[2].
Died: A individual on INH who is reported to have died of any cause during INH treatment[2].
Lost to Follow Up: If a patient has taken isoniazid for one or more months, then interrupted for 60 d or more[2].
Stopped INH: An individual for whom the INH has been discontinued/stopped by a healthcare worker due to adverse effects or any other reasons[2].
Developed active TB (Failed): If a person develops active TB disease while on INH[2].
Transferred out: An individual who has been transferred to another facility or region to continue treatment[2].
Non-completion: It was defined as the loss to follow-up, death, developed active TB/failed, transfer out, or stopping INH for any other reason[2].
An index case: It was defined as an individual > 15 years old with a positive smear of sputum for Mycobacteria TB.
Childhood contacts: It were defined as children with 6 years of age or less living and sleeping in the same house or group of clustered houses on the same residential site as the index case for at least 1 mo.
A total of 262 children registered/commenced on INH across the 32 private facilities between July 2015 and May 2019 were included in the study. We calculated sample size using the formula n = a2 × b/d2, where n = sample size, a = Z statistic for a level of confidence, b = prevalence and d = precision or confidence interval. The level of confidence of 95% is conventional at which the value for ‘a’ is 1.96 and ‘d’ is 0.05. A previous study from Brazilian cohort reported INH completion rate of 10%-13% among children of all age-groups[17]. This equates to a ‘b’ value of 0.13. We arrived at an approximate sample size of 200 participants. A total of 262 children under-six were ultimately included in the study.
A four-stage sampling technique (summarized below) was used to select facilities for this study.
Stage 1: Lagos has a total of 20 LGAs out of which purposive sampling technique was used to select seven high TB burden LGAs out of these 20 LGAs. Selected LGAs include Badagry, Ajeromi, Apapa, Ojo, Ifako-Ijaiye, Alimosho, and Oshodi-Isolo.
Stage 2: Facilities providing TB services were first stratified into private and public. Then, private facilities were selected using convenient sampling technique. This was because of less bureaucratic processes in getting access to data from private than public facilities.
Stage 3: Of the 57 facilities across seven selected high TB burden LGAs, thirty-two (32) were proportionately selected. This means that the facilities were selected in proportionate to the number of private facilities in each of the seven LGA.
Stage 4: Eligible child contacts (less than 6 years) initiated on INH were extracted from the facility INH register.
Participants include HIV-negative children less than 6 years who were eligible for INH treatment and placed on INH between July 2015 and May 2019. Eligibility for the study was determined if a child was below age six years, was in contact with a bacteriologically confirmed pulmonary TB (PTB) patient and has lived in the same household for not less than 3 mo before the index TB patient was diagnosed. A bacteriologically confirmed pulmonary TB patient could either be smear positive or has GeneXpert MTB RIF detected. A household contact was defined as a child aged below 6 years of age who lives or has lived within the household of bacteriologically confirmed PTB case. Data collection took place between July and September 2020.
Inclusion criteria: (1) Informed consent by parent/caregiver; (2) Children under 6 years; (3) Children in close contact with or living with a smear-positive adult TB patient; (4) Started on INH at least 6 mo before the study; and (5) Had no symptoms of TB when commencing INH.
Exclusion criteria: (1) The child has received treatment for TB; and (2) Child contacts not living in the same household with the index cases before the diagnosis and child contacts older than 6 years were ineligible for the study and therefore excluded.
INH outcome represents the dependent variable and can either be INH treatment completed or INH non-completed. Independent variables include patient characteristics and facility characteristics. Patient characteristics include age in months, sex (male or female), screened for TB (Yes/No), diagnosis for TB (Yes/No), commenced on INH (Yes/No), date of INH commencement, date, weight and dosage of INH per visit for months 1-6. Facility characteristics include the name of the facility, LGA location, and for-profit status (private not-for-profit vs private for-profit). Descriptive statistics such as frequency and percentage were used to present study variables. Outcome was categorized as INH completed or INH not-completed based on the NTBLCP case definitions. Associations of INH completion with independent variables were assessed using chi-square. The second outcome variable is the proportion of children initiated on INH that dropped off the cascade at months 2, 3, 4, 5 and 6.
Data were extracted from INH register for under-6 children using a standardized excel based data extraction template that mirrors the register. Information such as the age of the patient, sex, INH start date, weight, dosages of INH at months 1-6 as well as health facility-related information such as for-profit status, and LGA were recorded. INH completion was measured using a standard case definition. INH completion was coded as ‘0’ while INH non-completion was coded as 1. For all the Yes/No questions, Yes was assigned code of 1 and No was assigned 0. In this study, children who have taken INH for 6 mo or more were regarded as INH completers, whereas children with documented evidence of < 6 mo of INH intake were regarded as INH non-completers.
Information collected from the INH register was triangulated and compared with that of the INH card to check for correctness and completeness. Where there were discrepancies between the INH card and INH register, the information in the INH card was taken as the correct one and documented in the excel-based data collection template. To minimize bias, the study comprised private for-profit and private not-for-profit facilities across 13 LGAs from both urban and semi-urban populations, as well as large and small-sized facilities.
Data were collected by six trained data clerks using a Microsoft Excel data collection template that was developed for this study. A 3-d training was held on the processes of data collection, entry, validation, and quality assurance. The study tool was piloted in an LGA outside the study location to test the practical knowledge of the trainees and any emerging challenges were addressed. The objectives of the study were concealed from the data clerks. Daily data reviews were held to assure data quality such as missing information and double counting. Collected data were triangulated with another data source, checked by multiple observers, and approved by the supervisor before analysis.
Data were entered into Microsoft Excel and imported into International Business Machine (IBM) Statistical Package for Social Sciences for Windows, Version 21.0. Armonk, NY: IBM Corp for analysis. Data cleaning was done before the analysis. The analysis contains a summary of patient demographic, clinical, and facility characteristics such as age, sex, TB screening status, TB diagnosis status, date of commencing INH, weight, and dose of INH per visit, type of facility, and LGA using frequency and percentage. Bivariate analyses were conducted to determine the relationship between independent and dependent variables (INH completed vs non-completed). None of the explanatory/independent variables was significantly associated with INH completion, i.e., the P value was greater than 0.05 on bivariate analysis for all variables, hence the analysis did not proceed to the multivariate stage.
As the data for this study were collected from routine programmatic data for INH surveillance, no ethical approval was required. Data were de-identified before the analysis for confidentiality and entered into a password-protected Microsoft Excel database. Permission was obtained from the Lagos State TB, Buruli Ulcer and Leprosy Control program. Verbal consent was taken from the parent/caregiver of the children before placing them on INH.
Table 1 shows the characteristics of participants in the study. A little above average (53.8%) of the participants were female, 95.4% were screened for TB and 0.0% was diagnosed of having TB. The participants’ age ranged from 1 to 72 mo with a mean of 36.01 ± 19.67 mo, and 40.2% were between the ages of 1-24 mo.
Table 2 shows the treatment monitoring of the participants. All (100.0%) of the participants were commenced on INH. Across all treatment months, majority weighed 8-15kg i.e., 65.4%, 60.4%, 55.3%, 55.8%, 57.2% and 54.1% in months 1, 2, 3, 4, 5 and 6 respectively. Similarly, and with respect to INH dosage, 150-200 mg dosage was administered to majority i.e., 58.4%, 57.6%, 59%, 60.9%, 61.1% and 63.9% at months 1, 2, 3, 4, 5 and 6 respectively. In the first month, (40.5%) of the participants received less than or equal 100 mg dose of INH, and at the end of the sixth month, 63.9% of the participants received between 150-200 mg dose of INH.
| Variable | N | Percentage (%) | |
| Commenced on | Yes | 262 | 100.0 |
| 1st mo WT (kg) | 1-7 | 23 | 8.8 |
| 8-15 | 170 | 65.4 | |
| 16-23 | 66 | 25.4 | |
| 24-31 | 1 | 0.4 | |
| 1st mo Dose | < 100 mg | 106 | 40.5 |
| 150-200 mg | 153 | 58.4 | |
| 250 mg and above | 3 | 1.1 | |
| 2nd mo WT (kg) | 1-7 | 23 | 11.4 |
| 8-15 | 122 | 60.4 | |
| 16-23 | 56 | 27.7 | |
| 24-31 | 1 | 0.5 | |
| 2nd mo Dose | < 100 mg | 87 | 41.4 |
| 150-200 mg | 121 | 57.6 | |
| 250 mg and above | 2 | 1.0 | |
| 3rd mo WT (kg) | 1-7 | 15 | 7.9 |
| 8-15 | 105 | 55.3 | |
| 16-23 | 69 | 36.3 | |
| 24-31 | 1 | 0.5 | |
| 3rd mo Dose | < 100 mg | 75 | 39.9 |
| 150-200 mg | 111 | 59.0 | |
| 250 mg and above | 2 | 1.1 | |
| 4th mo WT (kg) | 1-7 | 12 | 7.3 |
| 8-15 | 92 | 55.8 | |
| 16-23 | 57 | 34.5 | |
| 24-31 | 4 | 2.4 | |
| 4th mo Dose | < 100 mg | 67 | 38.5 |
| 150-200 mg | 106 | 60.9 | |
| 250 mg and above | 1 | 0.6 | |
| 5th mo WT (kg) | 1-7 | 10 | 6.3 |
| 8-15 | 91 | 57.2 | |
| 16-23 | 53 | 33.3 | |
| 24-31 | 5 | 3.1 | |
| 5th mo Dose | < 100 mg | 62 | 38.3 |
| 150-200 mg | 99 | 61.1 | |
| 250 mg and above | 1 | 0.6 | |
| 6th mo WT (kg) | 1-7 | 6 | 4.1 |
| 8-15 | 79 | 54.1 | |
| 16-23 | 56 | 38.4 | |
| 24-31 | 5 | 3.4 | |
| 6th mo Dose | < 100 mg | 55 | 35.5 |
| 150-200 mg | 99 | 63.9 | |
| 250 mg and above | 1 | 0.6 |
Many (59.2%) of the participants completed INH treatment (Figure 1). Of the cumulative 107 drop-offs while on INH, largest drop-offs were reported at the end of month 2, 52 (49%) followed by 20 (19%), 17 (16%), 11 (10.2%) and 7 (6.5%) at months 3, 4, 5 and 6 respectively (Figure 2).
By using the X2(Chi square) test, the analysis showed that there was no significant relationship between gender and INH completion, [χ2 (1, n = 262) =1.3, P > 0.248], age and completion of treatment [χ2 (2, n = 262) = 4.1, P > 0.128], type of facility and completion of treatment [χ2 (1, n = 262) = 0.03, P > 0.862]. This means that age, gender, and type of facility did not influence the treatment completion of INH recorded among the children (Table 3).
| Treatment Outcome | |||||||
| Completed | Loss to follow up | Total | χ2 | DF | P value | ||
| Gender | Male | 88 (33.6) | 53 (20.2) | 141 (53.8) | 1.336 | 1 | 0.248 |
| Female | 67 (25.6) | 54 (20.6) | 121 (46.2) | ||||
| Total | 155 (59.2) | 107 (40.8) | 262 (100.0) | ||||
| Age | 0-24 | 57 (55.3) | 46 (44.7) | 103 (40.2) | 4.114 | 2 | 0.128 |
| 25-48 | 62 (68.1) | 29 (31.9) | 91 (35.5) | ||||
| 49 and above | 34 (54.8) | 28 (45.2) | 62 (24.2) | ||||
| Total | 153 (59.8) | 103 (40.2) | 256 (100.0) | ||||
| Type of facility | PFP | 103 (39.3) | 70 (26.7) | 173 (66.0) | 0.030 | 1 | 0.862 |
| PNFP | 52 (19.8) | 37 (14.1) | 89 (34.0) | ||||
| Total | 155 (59.2) | 107 (40.8) | 262 (100.0) | ||||
Only 59.2% of the participants completed INH at the end of the sixth month. This was lower than 80.0% reported in Ethiopia[18] but higher than 32.6% recorded in Pakistan[18] , 49.6% in Southern Nigeria and 12.0% from southern Ethiopia[20,21]. In Malawi, only 17.0% of children that participated in a cohort with inactive follow-up concluded six months of INH as opposed to 22.0% of children when the cohort were followed up actively[22]. In Ethiopia, Guinea-Bissau, and Kenya, 67.9%, 76% and 88% of children started on INH completed treatment respectively[14-15,23]. Home delivery of INH by a dedicated field staff was a major facilitator of optimal INH initiation and completion in Guinea-Bissau[15]. Also, in South Africa, adherence to three months of isoniazid and Rifampicin (3RH) was significantly higher (69.6%) when compared with 27.6% adherence among children on six months of isoniazid[24] while in the United States, children on three months of isoniazid and rifapentine (3HP) were more likely to complete therapy compared with those on daily INH for nine months[25]. These findings point to the need for rapid scale up of shorter INH regimen such as 3RH and 3HP for eligible children in Nigeria. Successful implementation of INH and optimal completion are critical to the realization of the goal of reducing childhood incident TB in Nigeria. Differentiated delivery of INH at community level should be embraced by NTBLCP as part of the strategies to address attrition as a result of need for frequent hospital follow-up visits by caregivers when children are taking INH.
Of the cumulative 107 children lost to follow up on INH, largest drop-offs were reported at the end of month two, 52 (49%) followed by 20 (19%), 17 (16%), 11 (10.2%) and 7 (6.5%) at months three, four, five and six respectively. This is similar to study in Ethiopia where majority, 6 (30%) of the 20 children who interrupted INH did so at month two[26] while in Milan, majority (15.2%) of INH drop-offs occurred between the start of treatment and the first follow up visit, although this was lower among those on shorter rifampicin containing regimen[27]. The study observed that the largest patient losses while on INH occurred within the first 2 mo of initiating INH which supports the imperative to target this early period of treatment, particularly by strengthening the initial adherence counseling and caregivers’ education on the importance of remaining adherent to INH for the entire six months of treatment. Although not the focus of this study, several factors could explain this early loss including inappropriate counselling on the benefits of INH in the prevention of TB, stigma, socioeconomic reasons among others. These suggest the need to improve the initial adherence counseling and patient education on the benefits of remaining adherent to INH. Policy makers need to pay attention to human resources needed to ensure accurate and complete documentation of child and caregiver’s contact information during the initial INH visit. Continuous education of healthcare workers on the need to update such information per visit combined with patient reminders are critical for retention of children on INH. Furthermore, early identification of potential psychosocial issues and linking caregivers to useful community-based resources are mechanisms to promote long-term engagement and retention in care. Even though attaining an overall INH completion rate of 59.2% in this study is encouraging when compared with reports from other low- and middle-income countries, there is still a need to explore the factors influencing INH interruption early during the therapy and to address the remaining 40.8% who interrupted INH at the end of the sixth month. Improving adherence to INH treatment among children have multiple dependencies including patient-, provider-, health systems-, caregiver- and community-related factors.
The study used chi-square analysis at 0.05 level of significance to establish the relationship between independent variables (gender, age, type of facility) and dependent variable (completion of INH treatment). The analyses showed that there was no relationship between gender, age, facility type and completion of INH treatment. This means that age, gender, and type of facility did not influence the treatment completion of INH recorded among the children. These findings are similar to reports from Guinea-Bissau and Ethiopia where there was no significant association of the listed risk factors, including sociodemographic variables, on the completion of the full six months of isoniazid preventive treatment[23,26].
The study abstracted routine programmatic data collected over several years across 32 private clinics in Lagos, Nigeria and therefore suitable for evaluating the completion of programmatic INH among eligible under-6 children in a high TB burden country like Nigeria. The study can be generalized in similar population and setting with high TB burden like urban Lagos, Nigeria. That notwithstanding, routinely collected data are subject to limitations, such as incomplete data, which we minimized by triangulating data from multiple sources, including INH register and INH Care card. Confounders like caregiver characteristics (education, HIV status, attitude), facility characteristics (patient volume, INH availability, waiting time), patient characteristics (distance to facility, HIV status) and health workforce attributes such as knowledge of INH, training and attitude were not assessed due to the retrospective nature of the study and limited information available in the INH register.
This study therefore illustrates sub-optimal INH completion rate among under-six children attending private facilities in Lagos State, Nigeria and that INH completion rate was not associated with any of age, gender and type of facility where INH was initiated. Only 6 out of 10 children initiated on INH ultimately completed the 6-mo treatment. The huge drop-offs in the first 2 mo of INH calls for innovative strategies such as the use of 60-d INH calendar that would facilitate reminder and early engagement of children on INH and their caregivers in care and across the entire period of treatment. Targeted interventions such as community initiation and monitoring of INH by healthcare workers, and rapid scale up of shorter TB preventive therapy are some of the interventions with potential to address these drop-offs along the childhood INH cascade. Future studies should therefore explore, through qualitative design, the exact reasons why some children did not complete INH.
Isoniazid (INH) has been proven to be a useful treatment of latent tuberculosis (TB) but its implementation at country level is poor, especially among under-6 children where it is recommended in Nigeria. Even when children are commenced on INH, its completion is rarely investigated.
Completion of INH is necessary to prevent active TB and the development of INH-resistant TB.
This study aimed to determine the completion of isoniazid preventive treatment among under six children in contact with adults with TB and associated factors under routine programmatic use in Lagos, Nigeria.
Retrospective review of INH treatment records of 262 children initiated on a 6-mo INH across 32 private facilities in Lagos, Nigeria.
Only 155 (59.2%) of the 262 participants initiated on INH completed the six-month treatment. Cumulatively, 107 (41.0%) children were lost to follow-up at the end of the sixth month. Of the cumulative 107 loss to follow-up while on INH, largest drop-offs were reported at the end of month 2, 52 (49%) followed by 20 (19%), 17 (16%), 11 (10.2%) and 7 (6.5%) at months 3, 4, 5 and 6 respectively. The analysis showed that there was no significant association between age, gender, type of facility and completion of INH treatment (P > 0.005).
The huge drop-offs in the first 2 mo of INH calls for innovative strategies such as the use of 60-day INH calendar that would facilitate reminder and early engagement of children on INH and their caregivers in care and across the entire period of treatment.
The study added to the body of knowledge on INH completion among eligible children in Nigeria. The findings will alert policy makers on the burden of INH drop-offs and the timing of any interventions that could address retention in care, particularly within the first 2 mo of initiating INH. Future qualitative studies need to unravel actual reasons for these huge loses while on INH.
We wish to thank doctors and nurses from private facilities and the Lagos State TB, Buruli Ulcer and Leprosy Control Program.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nambi G, Saudi Arabia; Yellanthoor RB, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Kanabus, A. Information about Tuberculosis. GHE. [Internet] [accessed 2018]. Available from: http://www.tbfacts.org. [Cited in This Article: ] |
| 2. | Federal Ministry of Health Nigeria (FMOH), Department of Public Health. National Tuberculosis and Leprosy Control Programme (NTBLCP). Workers Manual-Revised. Ed. 5. pp 1- 25, 2015.. [Cited in This Article: ] |
| 3. | World Health Organization. Global tuberculosis report 2018. [Internet] [accessed 2018]. Available from: https://www.who.int/publications/i/item/9789241565646. [Cited in This Article: ] |
| 4. | Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367:348-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 5. | Shanaube K, Sismanidis C, Ayles H, Beyers N, Schaap A, Lawrence KA, Barker A, Godfrey-Faussett P. Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PLoS One. 2009;4:e7749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Nigeria TB. [Internet] [accessed 2017]. Available from: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=NG&LAN=EN&outtype=html. [Cited in This Article: ] |
| 7. | Alex-Hart BA, Paul NI, Ugwu RO. Tuberculosis among School Age (6-18 Years) Children Seen in University of Port Harcourt Teaching Hospital: A Need for Effective School Health Services. Journal of Tuberculosis Research. 2019;7:109-117. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Ferebee SH, Mount FW, Comstock GW. The use of chemotherapy as a prophylactic measure in tuberculosis. Ann N Y Acad Sci. 1963;106:151-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH, Reingold A. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis. 2014;14:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Adepoju AV, Ogbudebe CL, Adejumo OA, Okolie J, Inegbeboh JO. Implementation of Isoniazid Preventive Therapy among People Living with HIV in Northwestern Nigeria: Completion Rate and Predictive Factors. J Glob Infect Dis. 2020;12:105-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ogunsola OO, Ajayi O, Ojo O, Adeyeye O, Akinro Y, Oke O, Adurogbola AA, Olajide O. Improving coverage and completion rate of isoniazid preventive therapy among eligible HIV patients using quality improvement approaches: a case study of State Hospital, Ijebu Ode, Ogun State, Nigeria. Pan Afr Med J. 2019;34:193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Global Fund Grants in the Federal Republic of Nigeria. [Internet] [accessed 2022]. Available from: https://www.theglobalfund.org/media/11864/oig_gf-oig-22-003_report_en.pdf. [Cited in This Article: ] |
| 13. | Umar LW, Chijioke-Akaniro OO. The Status of Tuberculosis Preventive Therapy for Under Five Children: Is Nigeria on the Right Track? The 4th Biennial General Meeting and Scientific Conference of the Nigerian Society for Paediatric Infectious Diseases (NISPID 2019), G-Pinnacle Hotel, Ilorin, Kwara StateAt: ILORIN, NIGERIA. [Cited in This Article: ] |
| 14. | Fentahun N, Wasihun Y, Mamo A, Gebretsadik LA. Contact Screening and Isoniazid Preventive Therapy Initiation for Under-Five Children among Pulmonary Tuberculosis-Positive Patients in Bahir Dar Special Zone, Northwest Ethiopia: A Cross-Sectional Study. Tuberc Res Treat. 2020;2020:6734675. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 15. | Omesa EN, Kathure IA, Masini E, Mulwa R, Maritim A, Owiti PO, Takarinda KC Ogutu O, Kosgei RJ Galgalo T. Uptake of isoniazid preventive therapy and its associated factors among HIV positive patients in an urban health Centre, Kenya. East African Medical Journal. 2016;93:47-54. [Cited in This Article: ] |
| 16. | Lagos State Government. [Internet] [accessed 5 May 2020]. Available from: http://www.lagosstate.gov.ng/pagelinks.php?p. [Cited in This Article: ] |
| 17. | Luciana S, María BA, Alexandra BS, Mariana A, Beatriz B, Caio S, Michael SR, Aline B, Adriana SRM, Jamile GO, Anna CC, Renata S, Marina CF, Solange C, Betina D, José RL, Afrânio LK, Valeria CR, Timothy RS, Marcelo C, Bruno BA. Determinants of losses in the tuberculosis infection cascade of care among children and adolescent contacts of pulmonary tuberculosis cases: A Brazilian multi-centre longitudinal study. The Lancet Regional Health – Americas. 2022;15:100358. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Tadesse Y, Gebre N, Daba S, Gashu Z, Habte D, Hiruy N, Negash S, Melkieneh K, Jerene D, K Haile Y, Kassie Y, Melese M, G Suarez P. Uptake of Isoniazid Preventive Therapy among Under-Five Children: TB Contact Investigation as an Entry Point. PLoS One. 2016;11:e0155525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Jafri R, Abdul Malik A, Hussain H, Hussain S, Khatoon F, Asif K, Amanullah F. IPT uptake among child contacts of TB patients: Experience from the Indus Hospital TB program, Karachi, Pakistan. Int J Mycobacteriol. 2015;4:104-105. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Sharma N, Basu S, Khanna A, Sharma P, Chopra KK, Chandra S. Adherence to Isoniazid Preventive Therapy among children living with tuberculosis patients in Delhi, India: An exploratory prospective study. Indian J Tuberc. 2022;69:100-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Garie KT, Yassin MA, Cuevas LE. Lack of adherence to isoniazid chemoprophylaxis in children in contact with adults with tuberculosis in Southern Ethiopia. PLoS One. 2011;6:e26452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Zachariah R, Spielmann MP, Harries AD, Gomani P, Graham SM, Bakali E, Humblet P. Passive vs active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:1033-1039. [PubMed] [Cited in This Article: ] |
| 23. | Gomes VF, Wejse C, Oliveira I, Andersen A, Vieira FJ, Carlos LJ, Vieira CS, Aaby P, Gustafson P. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: a prospective study from Guinea-Bissau. Int J Tuberc Lung Dis. 2011;15:1637-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | van Zyl S, Marais BJ, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10:13-18. [PubMed] [Cited in This Article: ] |
| 25. | Cruz AT, Starke JR. Completion Rate and Safety of Tuberculosis Infection Treatment With Shorter Regimens. Pediatrics. 2018;141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Seid G, Tsedalu T, Ayele M. Adherence to Isoniazid Preventive Therapy among Under-Five Children in Contact with Adult Bacteriologically Confirmed Pulmonary Tuberculosis Patients: A Mixed-Method Study. Int J Microbiol. 2020;2020:8834806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Villa S, Ferrarese M, Sotgiu G, Castellotti PF, Saderi L, Grecchi C, Saporiti M, Raviglione M, Codecasa LR. Latent Tuberculosis Infection Treatment Completion while Shifting Prescription from Isoniazid-Only to Rifampicin-Containing Regimens: A Two-Decade Experience in Milan, Italy. J Clin Med. 2019;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |