Published online Jul 26, 2022. doi: 10.12998/wjcc.v10.i21.7376
Peer-review started: November 18, 2021
First decision: December 27, 2021
Revised: February 21, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: July 26, 2022
Safe and effective analgesia strategy remains one of the priorities for pediatric inguinal hernia treatment.
To explore safety and efficacy of dexmededomidine monotherapy for postope
This randomized single-center controlled trial included 390 children (aged 1-3 years, ASA grade I-II), randomly divided into a dexmededomidine group (D group), a dexmededomidine + sufentanil group (DS group), and a sufentanil group (S group). The primary endpoint was percentage of children with the Face, Legs, Activity, Cry, and Consolability (FLACC) score ≤ 3 points 2 h after surgery.
The comparisons of the FLACC scores at 2, 4, 6, 8, 12, and 24 h were not significantly different among the three groups (P > 0.05). The sedative effects in the D group were significantly better than those in the S group (P > 0.05), but not significantly different from those in the DS group. The incidence of nausea and vomiting was significantly lower in the D group than in the S group and DS group (P > 0.05).
Analgesic effects of dexmededomidine monotherapy are comparable to those of sufentanil alone or in combination with dexmededomidine for children who underwent laparoscopic unilateral internal inguinal ring ligation, with better sedative effects and a lower incidence of adverse events.
Core Tip: This randomized controlled trial aimed to evaluate the safety and efficacy of dexmededomidine monotherapy in children who underwent laparoscopic unilateral internal inguinal ring ligation. A total of 390 children were included and randomly divided into a dexmededomidine group, a dexmededomidine + sufentanil group, and a sufentanil group. Our study suggested that the analgesic effects of dexmededo
- Citation: Liu G, Zhang L, Wang HS, Lin Y, Jin HQ, Wang XD, Qiao WN, Zhang YT, Sun JQ, Liu ZN. Dexmededomidine in pediatric unilateral internal inguinal ring ligation. World J Clin Cases 2022; 10(21): 7376-7385
- URL: https://www.wjgnet.com/2307-8960/full/v10/i21/7376.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i21.7376
Pediatric inguinal hernia is not uncommon in children, with incidence rates ranging from 0.8% to 6.62%[1-3], occurring approximately in 5% of full-term and 30% of preterm infants, more frequently in boys[2,3]. Internal inguinal ring ligation is a common surgery in the Pediatric Department, and is a preferred method for the treatment of indirect inguinal hernia and hydrocele of the tunica vaginalis in children[4,5]. This procedure, considered simple enough in adults, is greatly complicated by early age, as children are generally in agitation due to the incision pain, which leads to an increased risk of incision bleeding and difficulties in nursing[4,6]. Because of that, search for safe and effective analgesia strategy is one of the priorities for inguinal hernia treatment in pediatric practice.
Opioids are the commonly used drugs for postoperative analgesia in children[7]. Sufentanil, a highly selective μ opioid receptor agonist, has a relatively good analgesic effect, but high doses of sufentanil are accompanied with a relatively high risk of nausea and vomiting[8]. On the other hand, dexme
Based on the above, this randomized controlled trial aimed to compare the safety and effectiveness between dexmededomide monotherapy and the commonly used strategy of sufentanil combined with dexmededomide for postoperative analgesia in children who received laparoscopic unilateral internal inguinal ring ligation, in order to provide evidence for clinical application of this method.
This randomized controlled trial (registration number: ChiCTR2000034105) included 390 children who received internal inguinal ring ligation at the Baoding Children’s Hospital between March 2019 and May 2019. This study was conducted in agreement with the GCP standards and Declaration of Helsinki, and approved by the Ethics Committee of the Baoding Children’s Hospital (approval number: 201829). The parents of all children provided informed consent.
The inclusion criteria were: (1) Children aged 1-3 years and weighing 10-20 kg; (2) Received elective laparoscopic unilateral internal inguinal ring ligation; (3) American Society of Anesthesiologists (ASA) scores of I-II; (4) Usage of a continuous constant speed intravenous analgesia pump (CCIA) immediately after surgery; and (5) In agreement with the ethics requirements, children volunteered to participate in the study, and their parents provided informed consent. The exclusion criteria were: (1) Neurological disorders or cognitive impairments, such as epilepsy, depression, and dementia; (2) History of using psychotropic drugs; (3) Vital organ (such as the liver, heart, and kidney) dysfunction; (4) Vasoactive drugs received during the surgery; (5) Allergy to sufentanil or dexmededomide; and (6) Participated in other clinical trials within the last 3 mo.
The randomization strategy was designed by an independent third-party that has not participated in this study. According to the random number table generated with SPSS 22.0 software (SPSS Inc., Chicago, IL), the 390 children were 1:1:1 randomized into a dexmededomide group (D group), a dexmededomide + sufentanil group (DS group), and a sufentanil group (S group). The protocol of randomization was also managed by an independent third-party that has not participated in this study. The personnel in charge of drug preparation confirmed the randomization results of the children, then labeled the corresponding syringes with the number 1, 2, or 3 according to the protocols, and after that contacted the investigator in charge of the randomization to pass the ID numbers and names of the children. Then, the investigator in charge of the randomization informed the personnel in charge of drug preparation regarding the randomization numbers and groups of children. After confirming the groups of children, the drugs were prepared according to the study protocols, and the randomization numbers of the children were attached to the corresponding syringes.
Double-blind approach was adopted in this study, in which children (guardians), personnel in charge of drug preparation, and data collectors were independent from each other. All the children (guardians) and data collectors were masked from the randomization results, and the only personnel in charge of drug preparation knew the groups of the children. After the surgery of internal inguinal ring ligation was completed, the anesthesia was stopped, and the children were equipped with a CCIA. The packages of all the analgesics were masked by a white envelope. After the CCIA was equipped and the child restored satisfactory autonomous respiration, the laryngeal mask was removed and the child was transferred to the recovery room, and then transferred back to the ward after awakening. The postoperative data were collected by an investigator not participating in drug management and blinded to the randomization results. The anesthetists were strictly isolated from the data collector in the study. In addition, the statistical analyses of data were also performed by statisticians from an independent third-party that has not participated in this study. The preparation and management of all the drugs in this study were performed by the same personnel. All the surgeries were performed by the same group of surgeons. The study processes were supervised by two supervisors.
The children were routinely fasted from food for 8 h, and not allowed to drink water for 2 h. After transfer to the operating room, the vital signs of the children were monitored. The induction strategy included 3 mg/kg propofol, 0.1 mg/kg cisatracurium besylate, and 2 μg/kg remifentanil. After the requirements of tracheal intubation were met, the laryngeal mask was applied and the mechanical ventilator was connected to manage the respiration. The respiratory parameters were adjusted to maintain the EtCO2 at 35-40 mmHg. Afterwards, 0.35 μg/kg/min remifentanil and 2% sevoflurane was applied for anesthesia maintenance. After the surgery ended, the anesthesia was stopped, and the children were equipped with a CCIA. According to the randomization results, the drugs were prepared as follows: (1) D group: Normal saline was added to 1.5 μg/kg dexmededomide until the volume was 100 mL. The background infusion rate was 2 mL/h, and the drug was pumped in the CIAA for 48 h; (2) DS group: Normal saline was added to 1.5 μg/kg dexmededomide and 1.5 μg/kg sufentanil until the volume was 100 mL. The background infusion rate was 2 mL/h, and the drug was pumped in the CIAA for 48 h; and (3) S group: Normal saline was added to 1.5 μg/kg sufentanil until the volume was 100 mL. The background infusion rate was 2 mL/h, and the drug was pumped in the CIAA for 48 h.
The study was completed 48 h after surgery. After the data collection, for the children in D group or S group with a Face, Legs, Activity, Cry, and Consolability (FLACC) pain score ≥ 7 points, one other analgesic drug was added to combination for alleviating the pain.
General characteristics, including age, sex, height, body weight, vital signs, diagnosis, previous histories, allergic histories, pre-operative examination results, and combined drug therapies, were collected.
The primary endpoint was the percentage of children with an FLACC analgesia score ≤ 3 points at 2 h after surgery. The secondary endpoints included the FLACC analgesia score, pediatric anesthesia emergence delirium score, Ramsay sedation score, Cole 5-point scale score, and safety treatment.
The FLACC scale was used for the evaluation of analgesia success. The FLACC score is a useful tool for the evaluation of postoperative pain in children aged from 2 mo to 7 years, which includes five items. The data collectors were required to observe the children for 1-15 min, and then scores were assigned for the children according to the findings and the descriptions in the scale. The scores for each item ranged from 0-2 points, and the scores of each item were added to acquire the total score, which ranged from 0-10 points. The score of 0 indicated relaxed and comfort, 1-3 points indicated mild discomfort, 4-6 points indicated moderate pain, and 7-10 points indicated severe pain, discomfort, or both. In this study, FLACC scale evaluation was performed at 2, 4, 6, 8, and 12 h after the equipment of CIAA, and the corresponding data were collected.
Sedation evaluation was evaluated using the PAED scale, Ramsay sedation score, and Cole 5-point scale score (CPS). PAED is a scale for the evaluation of agitation in the recovery period that has been proven effective, which could be used to evaluate the agitation of children after surgery. This scale evaluates the agitation from the following five aspects: Eye contact; aware of the surroundings; purposeful actions; restless, confused, or delirium; and inconsolable crying. The score for each item ranges from 0-4, and the total score of the scale is 20. Children with a score > 12 points were considered with agitation in recovery period. Higher scores indicated more severe agitation of children. The Ramsay sedation score is commonly used for patients receiving continuous intravenous sedation, with a total score ranging from 1 to 6 points: 1 point = restless and agitated; 2 points = co-operative and tranquil; 3 points = asleep and responding to commands; 4 points = asleep, and could be waked; 5 points = asleep, with sluggish responses; and 6 points = could not be waked. The scores of 2-3 indicated satisfactory sedation. The total score of CPS is 5 points, with 1 point indicating asleep, 2 indicating awake and tranquil, 3 indicating agitated and crying, 4 indicating inconsolable and crying, and 5 indicating severe agitation and disorientation. The scores of 2 points or higher indicated satisfactory sedation. In this study, the sedation scale evaluation was performed at 2, 4, 6, 8, 12, and 24 h after the equipment of CCIA, and the corresponding data were collected.
This study was a non-inferiority designed randomized controlled trial, which aimed to investigate whether or not dexmededomide monotherapy is not inferior to the combined use of sufentanil and dexmededomide for postoperative analgesia in children who received laparoscopic unilateral internal inguinal ring ligation. According to the results of preliminary experiments and previous studies, the percentage of children with an FLACC analgesia score ≤ 3 points at 3 h after surgery was 80% after the combined application of sufentanil and dexmededomide. We assumed that the effects of using dexmededomide alone are not inferior to the combined use of sufentanil and dexmededomide. The non-inferiority margin (δ) of this study was set at 15%, α was 0.025 (one-sided), and power (1-β) was 0.8. The sample sizes of the three groups (N1 = N2 = N3) were equal. PASS 11 software showed that the sample size was 112 for all the three groups. After the drop-off rate of 20% was considered, 130 children were planned to be included in each group.
All the data were managed with Epidata3.0 software. The data input was performed independently by two investigators, with following checks and modifications to ensure the accuracy of the data. SAS 9.4 (SAS Institute Inc, Cary, North Carolina) was used for the statistical analyses of data. The baseline data, including age, sex, body weight, vital signs, surgeries, initiate sedation, and sedation scores, were compared by one-way analysis of variances (ANOVA) or non-parametric tests, and are described as the mean ± SD. Paired t-test was performed for the comparisons within groups, while for the data not following a normal distribution or with unequal variances, non-parametric test was performed. One-way ANOVA or non-parametric test was performed for the comparison of data among the three groups, and LSD test was further performed for the pair-wise comparison. P ≤ 0.05 was considered statistically significant.
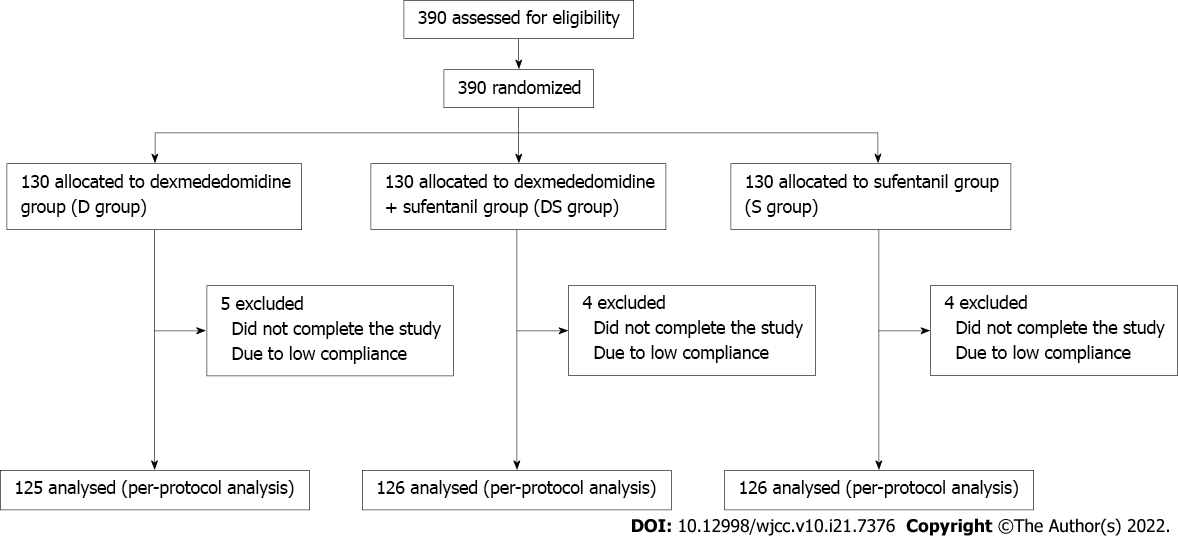
A total of 390 children satisfied the inclusion criteria in this study, of which nine did not complete the study, and four were excluded due to low compliance. Finally, 377 children were included, of which 125, 126, and 126 were in the D group, DS group, and S group, respectively (Figure 1). Statistical analyses showed that the three groups were comparable with regard to baseline data, including age, sex, body weight, vital signs, initial sedation, and sedation scores (P > 0.05) (Table 1).
| Variable | D group (n = 125) | DS group (n = 126) | S group (n = 126) | P |
| Sex (male), n (%) | 119 (95.2) | 117 (92.9) | 118 (93.7) | 0.85 |
| Age (yr) | 12.62 ± 1.88 | 13.07 ± 2.24 | 12.60 ± 1.78 | 0.26 |
| Body weight (kg) | 83.9 ± 2.02 | 84.1 ± 1.96 | 84.1 ± 1.99 | 0.83 |
| Blood pressure (mmHg) | 49.3 ± 3.64 | 49.3 ± 3.39 | 49.3 ± 3.44 | 0.98 |
| Respiration (times/min) | 24.5 ± 2.01 | 24.1 ± 1.97 | 24.6 ± 1.87 | 0.21 |
| Heart rate (beats/min) | 113.2 ± 5.03 | 112.4 ± 5.16 | 113.2 ± 4.79 | 0.37 |
| FLACC score (points) | 5.0 ± 0.48 | 5.0 ± 0.52 | 4.9 ± 0.52 | 0.30 |
| PAED score (points) | 10.0 ± 1.00 | 9.9 ± 1.42 | 9.8 ± 1.00 | 0.22 |
| Ramsay score (points) | 1.2 ± 0.37 | 1.2 ± 0.39 | 1.2 ± 0.37 | 0.80 |
| CPS score (points) | 3.0 ± 0.53 | 3.0 ± 0.56 | 2.9 ± 0.48 | 0.81 |
The FLACC pain scores of children at 2, 4, 6, 8, 12, and 24 h after surgery are shown in Table 2. The percentages of children with an FLACC analgesia score ≤ 3 points at 2 h after surgery were not different between groups (P > 0.05). The comparisons of the data in the three groups showed that the FLACC scores at different time points were not significantly different among the three groups (P > 0.05).
| Time | D group (n = 125) | DS group (n = 126) | S group (n = 126) | P |
| Postoperative 2 h | 3.6 ± 0.63 | 3.4 ± 0.72 | 3.4 ± 0.76 | 0.143 |
| Postoperative 2 h (≤ 3), n (%) | 48 (38.4) | 59 (46.8) | 58 (46.0) | 0.332 |
| Postoperative 4 h | 1.9 ± 0.64 | 1.9 ± 0.70 | 1.8 ± 0.76 | 0.558 |
| Postoperative 6 h | 0.1 ± 0.45 | 0.2 ± 0.53 | 0.2 ± 0.57 | 0.350 |
| Postoperative 8 h | 0.0 ± 0.18 | 0.0 ± 0.00 | 0.0 ± 0.35 | 0.608 |
| Postoperative 12 h | 0.0 ± 0.18 | 0.0 ± 0.00 | 0.0 ± 0.35 | 0.608 |
| Postoperative 24 h | 0.0 ± 0.18 | 0.0 ± 0.00 | 0.0 ± 0.35 | 0.608 |
The pair-wise comparison of PAED scores among the three groups showed that the PAED score in the D group was significantly lower than that in the S group at 4 and 6 h after surgery (P < 0.05). The PAED score was also significantly lower in the DS group than in the S group at 2 and 4 h after surgery (P < 0.05), and significantly lower in the D group than in the DS group at 6 h after surgery (P < 0.05) (Table 3).
| Time | D group (n = 125) | DS group (n = 126) | S group (n = 126) | P |
| Postoperative 2 h | 8.1 ± 1.56 | 7.6 ± 1.64 | 8.3 ± 1.53b | 0.005 |
| Postoperative 4 h | 2.1 ± 1.79 | 2.0 ± 1.76 | 4.4 ± 3.08a,b | < 0.001 |
| Postoperative 6 h | 0.5 ± 1.16 | 1.3 ± 1.01a | 1.4 ± 1.07a | < 0.001 |
| Postoperative 8 h | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | > 0.999 |
| Postoperative 12 h | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | > 0.999 |
| Postoperative 24 h | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | > 0.999 |
Statistical analysis of the satisfactory degree to Ramsay sedation score at different time points (sedation scores of 2 or 3 points were considered satisfactory) showed that more children in the D group and DS group were satisfactory to the sedation at 2-6 h after surgery than those in the S group (P < 0.05); however, the difference was not statistically significant between the D group and DS group (P > 0.05) (Table 4).
| Time | D group (n = 125) | DS group (n = 126) | S group (n = 126) | P |
| Postoperative 2 h | 107 (85.6) | 105 (83.3) | 87 (69.0)a,b | 0.005 |
| Postoperative 4 h | 127 (100.0) | 122 (96.8) | 106 (84.1)a,b | < 0.001 |
| Postoperative 6 h | 127 (100.0) | 127 (100.0) | 120 (95.2)a,b | 0.001 |
| Postoperative 8 h | 127 (100.0) | 127 (100.0) | 126 (100.0) | > 0.999 |
| Postoperative 12 h | 127 (100.0) | 127 (100.0) | 126 (100.0) | > 0.999 |
| Postoperative 24 h | 127 (100.0) | 127 (100.0) | 126 (100.0) | > 0.999 |
The pair-wise comparison of satisfactory degree to CPS sedation score (CPS score > 2 points indicated satisfactory) at different time points after surgery among the three groups showed that more children in the D group and DS group were satisfactory to the sedation at 2 h after surgery than those in the S group (P < 0.05); however, the difference was not statistically significant between the D group and DS group (P > 0.05) (Table 5).
| Time | D group (n = 125) | DS group (n = 126) | S group (n = 126) | P |
| Postoperative 2 h | 107 (85.6) | 109 (86.5) | 87 (69.0)a,b | 0.001 |
| Postoperative 4 h | 65 (52.0) | 56 (44.4) | 68 (54.0) | 0.299 |
| Postoperative 6 h | 106 (84.8) | 105 (83.3) | 95 (75.4) | 0.149 |
| Postoperative 8 h | 127 (100.0) | 127 (100.0) | 126 (100.0) | > 0.999 |
| Postoperative 12 h | 127 (100.0) | 127 (100.0) | 126 (100.0) | > 0.999 |
| Postoperative 24 h | 127 (100.0) | 127 (100.0) | 126 (100.0) | > 0.999 |
The comparison of postoperative incidence of nausea and vomiting showed that the incidence was significantly lower in the D group than in the S group and DS group (P < 0.05). Four (3.1%), 18 (14.3%), and 10 (7.9%) of children in the D group, S group, and DS group, respectively, demonstrated symptoms of nausea after surgery. In addition, 0, 8 (6.3%), and 2 (1.6%) of children in the D group, S group, and DS group, respectively, demonstrated symptoms of vomiting after surgery (Table 6).
| D group (n = 125) | DS group (n = 126) | S group (n = 126) | |
| Nausea | 4 (3.2) | 18 (14.3) | 10 (7.9) |
| Vomit | 0 (0.0) | 8 (6.3) | 2 (1.6) |
Postoperative pain in children could induce drastic stresses, lead to physiological and psychological disorders, influence the development of the central nervous system, and even cause cognitive impairment[20]. The conventional analgesia mainly involves single opioid use (such as sufentanil), with risks of nausea, vomiting, and respiration inhibition. Our results showed that for children who underwent laparoscopic unilateral internal inguinal ring ligation, the analgesic effects of dexmede
Dexmededomide, a highly selective α2 adrenergic receptor agonist, previously demonstrated several advantages, such as short half-life and short action time[9]. In addition, in a certain range of doses, the sedative effects are linearly correlated with the doses, and the sedation degree is easy to be modulated, thus the anticipated sedation score could be rapidly achieved[16]. In this study, the postoperative analgesic effects of using dexmededomidine alone were not inferior to those of the combined application of dexmededomidine and sufentanil, indicating that dexmededomidine has substantial analgesic effects. The analgesic effects are mainly explained by the effects of dexmededomidine on the α2 adrenergic receptors on the presynaptic membrane of the posterior horn of the spinal cord and subsynaptic membrane of interneurons, which consequently induce the hyperpolarization of cell membrane and thus inhibit the transduction of pain signals to the brain[9,10]. In addition, dexmededomidine could also exert analgesic effects at the central nervous level. For instance, dexmededomidine could bind to the α2 receptors at the locus ceruleus of the brainstem, thus terminating the transduction of pain signals[16]. Dexmededomidine could also inhibit the release of P substances and other peptides from the presynaptic membrane of the noradrenergic pathway of the downward medulla oblongata and spinal cord, and consequently exert analgesic effects[11]. Therefore, using dexmededomidine as assistance could reduce the doses of other anesthetics. Various studies have demonstrated that using dexmededomidine for postoperative analgesia could reduce the doses and adverse reactions of opioids, including morphine[21] and tramadol[22]. Studies have also demonstrated that the effects of postoperative analgesia by dexmededomidine are better than intravenous anesthetics, such as ketamine[23] and midazolam[24].
The findings of this study confirmed that using dexmededomidine monotherapy for sedation and analgesia significantly reduce the incidence of nausea and vomiting, which could be the result of the change in medication volume, as the application of opioids could increase the incidence of those side effects[8]. It was previously reported that dexmededomidine has synergistic effects with opioids, thus using dexmededomidine during or after surgery could reduce the doses, increase the action time, and reduce the incidence and severity of adverse reactions of opioids[25]. Our study shown that dexmededomidine monotherapy could achieve analgesia non-inferior to the dexmededomidine plus sufentanil combination, with a significantly lower incidence of side effects, such as nausea and vomiting.
Reducing the use of opioids is one of the important areas of pediatric surgical practice which is currently being actively discussed[26]. For instance, some recent studies demonstrated the successful use of non-steroidal anti-inflammatory drugs (NSAIDs) as an additional postoperative treatment, reporting that ibuprofen plus propacetamol immediately following laparoscopic hernia repair surgery in children resulted in the reduced use of an opioid drug compared with the use of propacetamol alone[27]. On the other hand, dexmedetomidine alone demonstrated comparable duration of postoperative analgesia, with no significant side effects[28]. Although our study excluded the use of NSAIDs or any other medication during 48 h period after surgery, searching for suitable combinations or alternatively proving the superiority of monotherapy is one of the potential directions for future research.
Our study has some limitations. One of them is the choice of the pain assessment tool. As none of the available pain scales have yet demonstrated clear superiority in pediatric practice, we decided to use the FLACC scale, which provides a framework for quantifying pain behaviors in children who may not be able to verbalize the presence or severity of pain. In our experience, the FLACC scale shows satisfactory results in accessing pain levels in both newborns and infants, and is easy enough to use in clinical practice. However, our results cannot be directly compared to the studies which adopted other ways to assess pain. Second, this is a single center study, and whereas all surgery operations being performed by the same team was necessary to compare the results, the analgesia effect of different dexmededomidine doses depending on the type of surgery is still of interest for future studies.
In conclusion, this study adds to the recent search for the safe and efficient strategies for combating pain in pediatric practice. The subjects in this study were all children aged 1-3 years. The children were continuously observed for 24 h after surgery, and the findings showed that after using dexmede
Pediatric inguinal hernia is common in children. Safe and effective analgesia strategy remains one of the priorities for pediatric inguinal hernia treatment.
Search for safe and effective analgesia strategy is one of the priorities for inguinal hernia treatment in pediatric practice.
Our aim was to explore the safety and efficacy of dexmededomidine monotherapy for postoperative analgesia in children who received laparoscopic unilateral internal inguinal ring ligation.
This randomized single-center controlled trial included children (aged 1-3 years, ASA grade I-II), randomly divided into the D group, DS group, and S group. The analgesia effect, sedative effects, and complications were compared.
Finally, 377 children were included, of which 125, 126, and 126 were in a dexmededomidine group (D group), a dexmededomidine + sufentanil group (DS group), and a sufentanil group (S group), respectively. The analgesia effect showed no difference among the three groups. The sedative effects were significantly better in the D group than in the S group, but not significantly different from that in the DS group. The incidence of nausea and vomiting was significantly lower in the D group than in the S group and DS group.
Analgesic effects of dexmededomidine monotherapy were comparable with those of sufentanil alone or in combination with dexmededomidine for children who underwent laparoscopic unilateral internal inguinal ring ligation, with better sedative effects and a lower incidence of adverse events.
Dexmededomidine for analgesia and sedation is worth to be applied in pediatric inguinal hernia treatment. Pediatric inguinal hernia is common in children.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poddighe D, Kazakhstan; Zavaleta MJC, Peru A-Editor: Liu X, China S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Wu YXJ
| 1. | Liu J, Wu X, Xiu W, Hao X, Zhao J, Wei B, Dong Q. A comparative study examining laparoscopic and open inguinal hernia repair in children: a retrospective study from a single center in China. BMC Surg. 2020;20:244. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Zhu LL, Xu WJ, Liu JB, Huang X, Lv ZB. Comparison of laparoscopic hernia repair and open herniotomy in children: a retrospective cohort study. Hernia. 2017;21:417-423. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Chen YH, Wei CH, Wang KK. Children With Inguinal Hernia Repairs: Age and Gender Characteristics. Glob Pediatr Health. 2018;5:2333794X18816909. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Geiger S, Bobylev A, Schädelin S, Mayr J, Holland-Cunz S, Zimmermann P. Single-center, retrospective study of the outcome of laparoscopic inguinal herniorrhaphy in children. Medicine (Baltimore). 2017;96 e9486 [PMID:29384943 DOI: 10.1097/MD.0000000000009486. [Cited in This Article: ] |
| 5. | Ozgediz D, Roayaie K, Lee H, Nobuhara KK, Farmer DL, Bratton B, Harrison MR. Subcutaneous endoscopically assisted ligation (SEAL) of the internal ring for repair of inguinal hernias in children: report of a new technique and early results. Surg Endosc. 2007;21:1327-1331. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Namgoong JM, Choi WY. Reliability of Preoperative Inguinal Sonography for Evaluating Patency of Processus Vaginalis in Pediatric Inguinal Hernia Patients. Int J Med Sci. 2019;16:247-252. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Ziesenitz VC, Vaughns JD, Koch G, Mikus G, van den Anker JN. Correction to: Pharmacokinetics of Fentanyl and Its Derivatives in Children: A Comprehensive Review. Clin Pharmacokinet. 2018;57:393-417. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Lundeberg S, Roelofse JA. Aspects of pharmacokinetics and pharmacodynamics of sufentanil in pediatric practice. Paediatr Anaesth. 2011;21:274-279. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56:893-913. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457-461. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother. 2007;41:245-252. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Paediatr Drugs. 2008;10:49-69. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Mason KP, Lerman J. Review article: Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113:1129-1142. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Sottas CE, Anderson BJ. Dexmedetomidine: the new all-in-one drug in paediatric anaesthesia? Curr Opin Anaesthesiol. 2017;30:441-451. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72:323-330. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899-1904. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Mondardini MC, Amigoni A, Cortellazzi P, Di Palma A, Navarra C, Picardo SG, Puzzutiello R, Rinaldi L, Vitale F, Zito Marinosci G, Conti G. Intranasal dexmedetomidine in pediatrics: update of current knowledge. Minerva Anestesiol. 2019;85:1334-1345. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Burns J, Jackson K, Sheehy KA, Finkel JC, Quezado ZM. The Use of Dexmedetomidine in Pediatric Palliative Care: A Preliminary Study. J Palliat Med. 2017;20:779-783. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | De Zen L, Marchetti F, Barbi E, Benini F. Off-label drugs use in pediatric palliative care. Ital J Pediatr. 2018;44:144. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Kortesluoma RL, Nikkonen M, Serlo W. "You just have to make the pain go away"--children's experiences of pain management. Pain Manag Nurs. 2008;9:143-149, 149.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Lin TF, Yeh YC, Lin FS, Wang YP, Lin CJ, Sun WZ, Fan SZ. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102:117-122. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Sitilci AT, Ozyuvacı E, Alkan Z, Demirgan S, Yiğit O. [The effect of perioperative infused dexmedetomidine on postoperative analgesic consumption in mastoidectomy operations]. Agri. 2010;22:109-116. [PubMed] [Cited in This Article: ] |
| 23. | Lee W, Shin JD, Choe K, Kim MH. Comparison of dexmedetomidine and ketamine for the analgesic effect using intravenous patient-controlled analgesia after gynecological abdominal surgery. Korean J Anesthesiol. 2013;65:S132-S134. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Karaaslan K, Yilmaz F, Gulcu N, Colak C, Sereflican M, Kocoglu H. Comparison of dexmedetomidine and midazolam for monitored anesthesia care combined with tramadol via patient-controlled analgesia in endoscopic nasal surgery: A prospective, randomized, double-blind, clinical study. Curr Ther Res Clin Exp. 2007;68:69-81. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Shu-Min JI, Wang QX, Anesthesiology D, Hospital E, University T. Research progress of dexmedetomidine in reducing postoperative nausea and vomiting. Journal of Tongji University (Medical Science). 2018;. [Cited in This Article: ] |
| 26. | Lee HM, Park JH, Park SJ, Choi H, Lee JR. Comparison of Monotherapy Versus Combination of Intravenous Ibuprofen and Propacetamol (Acetaminophen) for Reduction of Postoperative Opioid Administration in Children Undergoing Laparoscopic Hernia Repair: A Double-Blind Randomized Controlled Trial. Anesth Analg. 2021;133:168-175. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Poddighe D, Brambilla I, Licari A, Marseglia GL. Ibuprofen for Pain Control in Children: New Value for an Old Molecule. Pediatr Emerg Care. 2019;35:448-453. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Varsha R, Desai SN, Mudakanagoudar MS, Annigeri VM. Comparison between caudal epidural and ultrasound-guided ilioinguinal-iliohypogastric block with bupivacaine and dexmedetomidine for postoperative analgesia following pediatric inguinal hernia surgeries: A prospective randomized, double-blind study. J Anaesthesiol Clin Pharmacol. 2021;37:389-394. [PubMed] [DOI] [Cited in This Article: ] |