Published online Jul 24, 2014. doi: 10.5410/wjcu.v3.i2.144
Revised: February 14, 2014
Accepted: March 11, 2014
Published online: July 24, 2014
AIM: To investigate the relationship between prostate-specific antigen (PSA) levels and (1) bladder outlet obstruction (BOO) and (2) the severity of prostate inflammation.
METHODS: Two hundred and twenty-two consecutive patients undergoing transurethral resection of the prostate (TURP) were prospectively included. Patients with proven urinary tract infection and/or known prostate cancer were excluded. PSA levels, International Prostate Symptoms Score (IPSS), prostate weight, post residual volume and pressure flow parameters were determined. A histopathological assessment of the presence and severity of inflammation was also performed.
RESULTS: Patients had a mean age of 69.1 ± 8.6 years (45-90 years), with mean preoperative PSA levels of 4.7 ± 5.4 ng/mL (0.2-32.5 ng/mL) and IPSS of 15.7 ± 6.9 (0-32). Mean PdetQmax was 96.3 ± 34.4 cmH2O (10-220 cmH2O). The mean resected prostate weight was 39.4 ± 27.3 g (3-189 g). Correlations were observed between PSA (logarithmic) and resected prostate weight (r = 0.54; P < 0.001), PSA (logarithmic) and PdetQmax (r = 0.17; P = 0.032), and resected prostate weight and PdetQmax (r = 0.39; P < 0.001). Furthermore, low correlations were observed between PSA (logarithmic) and active (r = 0.21; P < 0.0001) and chronic (r = 0.19; P = 0.005) inflammation.
CONCLUSION: In this study we showed a correlation between BOO (PdetQmax) and PSA (logarithmic). Furthermore, we demonstrated a weak correlation between PSA (logarithmic) and active as well as chronic prostatic inflammation.
Core tip: The goal was to investigate the relationship between prostate-specific antigen (PSA) levels and (1) bladder outlet obstruction (BOO) and (2) the severity of prostate inflammation. We performed a prospective study on 222 consecutive patients undergoing transurethral resection of the prostate. Patients with proven urinary tract infection and/or known prostate cancer were excluded. PSA levels, International Prostate Symptoms Score, prostate weight, post residual volume and pressure flow parameters were determined. A histopathological assessment of the presence and severity of inflammation was also performed. In this study we showed a correlation between BOO (PdetQmax) and PSA (logarithmic). Furthermore, we demonstrated a weak correlation between PSA (logarithmic) and active as well as chronic prostatic inflammation.
- Citation: Renterghem KV, Rosette JL, Thijs H, Wisanto E, Achten R, Ory JP, Koeveringe GV. Alternative mechanisms for prostate-specific antigen elevation: A prospective analysis of 222 transurethral resections of prostate patients. World J Clin Urol 2014; 3(2): 144-151
- URL: https://www.wjgnet.com/2219-2816/full/v3/i2/144.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i2.144
Since its introduction, prostate-specific antigen (PSA) has played a key role in prostatic evaluation[1]. Elevated or rising PSA levels might indicate prostate cancer, a highly prevalent cancer in men with an incidence rate surpassing 20%[2]. The downside of PSA testing is that it is not cancer specific but merely organ specific.
Consequently, elevated PSA levels are a challenging problem for urologists in assessing or excluding potential life-threatening prostate cancer. However, to confirm the diagnosis of prostate cancer, an additional histological evaluation from prostate biopsies is still required. These biopsies can be falsely negative and cannot be repeated infinitely[3]. Furthermore, taking a prostate biopsy is a moderately invasive examination that has, although infrequently, potential life threatening complications[4].
Therefore, a better understanding of other possible mechanisms causing PSA elevation is of utmost importance as this may help to avoid unnecessary biopsies and prevent patient anxiety. Furthermore, patients do not have to be bothered with other, sometimes expensive diagnostic tests, including magnetic resonance imaging[5], advanced transrectal ultrasound imaging[6] or molecular diagnostics[7].
Benign prostate hyperplasia (BPH) is a very common condition in ageing males. As prostatic enlargement can be asymptomatic, the exact incidence of BPH is unknown, ranging between 28% and 60% of the population[8,9]. Additionally, BPH seems to be the second most common reason for surgery in men over 60[10]. Currently, the exact pathogenesis of BPH is not fully understood. Amongst other factors, including hormonal influence, prostatic inflammation could stimulate prostatic growth. Moreover, data on the association between inflammation, prostatic volume, PSA levels and acute urinary retention risk have been published[11].
Therefore, the aim of this study was to investigate the relationship between PSA and the degree of prostate inflammation and to investigate whether PSA levels can be used as a biomarker for bladder outlet obstruction (BOO).
The study was approved by the hospital’s Ethics Committee (07.58/uro07.02) and was conducted according to the established GCP criteria. In this prospective study, 222 consecutive patients undergoing transurethral resection of the prostate (TURP) between May 2008 and June 2010 were included. A single high volume surgeon in a non-academic referral center operated on all patients. As indicated in the EAU guidelines[12], surgery was performed only on patients with a clear indication for TURP, including patients who did not improve after medical therapy, patients with (recurrent) acute urinary retention, high post void residual volume, obstruction characterised by pressure flow analysis or post renal kidney insufficiency. However, patients who were treated with 5ARIs were excluded because of the possible impact on PSA values. Patients with a proven urinary tract infection were excluded, except for patients with catheters, which are colonised by definition. Additionally, patients with known prostate cancer were excluded in order to prevent influence on PSA by cancer cells.
Before surgery, PSA levels were determined for all patients by GPs. The International Prostate Symptom Score (IPSS) was determined. Full urodynamic studies were performed in 154 patients using Laborie Medical Technologies INC/UDS-64-IIs and were evaluated by PIs. Urodynamic studies were not performed in cases of acute urinary retention or high post-residual volume. All patients were treated with low-dose quinolone prophylaxis for 48 hours, starting the day before urodynamic testing. Filling was done standing with a filling speed of 35 mL/minute, using a 6F-filling catheter (double lumen). When indicated, pressure flow analysis was performed according to the International Continence Society criteria[13]. Endoscopic procedures were performed under loco-regional anesthesia using an Olympus resectoscope 26 (6%) or 28 Charrière (94%), depending on the estimated prostate volume. The resected prostate specimens were weighed and the fragments were embedded until four cassettes, each containing 2 g of tissue, were filled. Each additional 10 g of prostate tissue was used to fill an extra cassette[14]. The tissue was fixed in formalin and embedded in paraffin. One slide was made from every paraffin block and examined after staining by hematoxylin and eosin. The inflammatory infiltrate was first divided into an active (mixed infiltrate of lymphocytes, plasma cells and polynuclear cells) and chronic (mononuclear infiltrate of lymphocytes and plasma cells) component, after which the density of both components was scored 0-3 according to a semi-quantitative scoring system: 0 being no infiltrate and 3 severe infiltrate (Figure 1). Two senior pathologists analyzed all tissue sections of each patient independently and were blinded for clinical data. They scored the mean value of the infiltrate, considering all tissue sections.
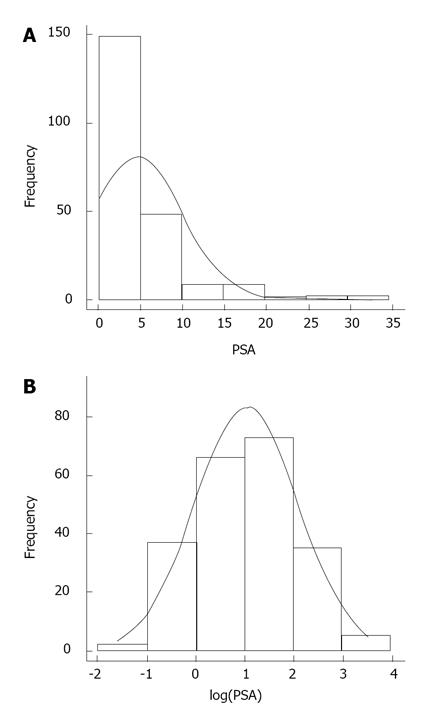
In an initial step, data were analyzed with descriptive statistics and Pearson correlation was used to investigate simple correlations between different variables. Appropriate linearity tests and lack of fit tests were performed to ensure that these relationships were indeed linear and adequate. Secondly, in a more in depth analysis the relationship between PSA levels and the different potential covariates was analyzed with a multiple regression model. Since the histogram of PSA is severely skewed due to the prevalence of lower PSA values in a normal population (Figure 2), PSA values were transformed with a natural log transformation, resulting in a more realistic normality assumption of the log PSA than the original PSA scale. Selection of the variables included in the model for log PSA was based on the AIC criterion and the adjusted r-square. With respect to the variable active inflammation, it might not seem realistic from a clinical point of view to assume that the difference between any consecutive levels of the active inflammation was the same or the increase was linear in nature. For this reason, the choice was made to treat inflammation as a categorical rather than a continuous variable. Furthermore this choice resulted in a better fit of the model.
The patients were between 45 years and 90 years old (mean age: 69.1 ± 8.6 years) (Table 1) and had an indication for TURP. Mean preoperative PSA value was 4.7 ± 5.4 ng/mL (0.2 to 32.5 ng/mL). Mean IPSS was 15.7 ± 6.9 (0 to 32). Mean peak flow was 10.7 ± 6.5 mL/s (1 to 47 mL/s). Post residual volume ranged from 0 to 890 mL with a mean value of 83.8 ± 130.3 mL. When indicated (n = 154), pressure flow analysis was executed and showed a mean value of PdetQmax of 96.3 ± 34.4 cmH2O (10 to 220 cmH2O). The mean resected prostate weight was 39.4 ± 27.3 g (3 to 189 g). Mean operating time was 31.1 ± 11.9 min (10 to 110 min).
| n | Mean | SD | Min | Max | |
| PSA (ng/mL) | 218 | 4.7 | 5.4 | 0.2 | 32.5 |
| Age (yr) | 222 | 69.1 | 8.6 | 45 | 90.0 |
| UF (mL/s) | 204 | 10.7 | 6.5 | 1 | 47.2 |
| IPSS | 222 | 15.7 | 6.9 | 0 | 32.0 |
| PRV (mL) | 208 | 83.8 | 130.3 | 0 | 890 |
| P/F (cm H2O) | 154 | 96.3 | 34.4 | 10 | 220 |
| Operation time (min) | 222 | 31.1 | 11.9 | 10 | 110 |
| TURP weight (g) | 222 | 39.4 | 27.3 | 3 | 189 |
We investigated the relationship between log PSA and several parameters, including IPSS, maximum uroflow, post voided residual volume, pressure flowmetry parameter PdetQmax and the weight of the resected prostate tissue (Table 2). A significant correlation between log PSA and the resected prostate weight was found (r = 0.54; P < 0.001). Furthermore, log PSA was correlated with PdetQmax (r = 0.17; P = 0.032). A negative correlation was encountered between log PSA and IPSS (r = -0.14; P = 0.04). No correlation was observed between log PSA and peak flow on uroflowmetry (r = 0.00; P = 0.99). The correlation between log PSA and the post residual volume (r = 0.08; P = 0.28) was not significant. Additionally, we did not find any correlation between IPSS and PdetQmax (r = 0.051; P = 0.53). Last but not least, a significant correlation between the weight of the resected tissue and PdetQmax (r = 0.39; P < 0.001) was observed. These relationships were confirmed in a multiple regression model (Table 3).
| Variable | Linearity(P value) | Significance(P value) | Correlation(r) |
| IPSS | 0.488 | 0.04 | -0.14 |
| Age (decades) | 0.563 | < 0.0001 | 0.29 |
| UF (mL/s) | 0.929 | 0.986 | 0.00 |
| PRV (mL) | 0.766 | 0.276 | 0.08 |
| P/F (cm H2O) | 0.443 | 0.032 | 0.17 |
| TURP weight (g) | 0.504 | < 0.0001 | 0.54 |
| Effect | Estimate(st.err) | P value |
| Age (decades) | 0.18 (0.069) | 0.010 |
| IPSS | -0.02 (0.008) | 0.008 |
| Active_0 | -1.58 (0.47) | 0.001 |
| Active_1 | -0.96 (0.53) | 0.073 |
| Active_2 | -0.78 (0.54) | 0.146 |
| Active_3 | -0.80 (0.55) | 0.150 |
| TURP weight | 0.034 (0.006) | < 0.0001 |
| R2 adj | 0.377 |
We also investigated the relationship between inflammation (active and chronic) and log PSA, IPSS, age and the resected prostate tissue weight (Tables 4 and 5). A significant correlation between log PSA and active (r = 0.21; P < 0.0001) and chronic (r = 0.19; P = 0.005) inflammation was observed. Age was related to active (r = 0.24; P < 0.0001) but not to chronic (r = 0.09; P = 0.08) inflammation. Similarly, the weight of the resected prostate tissue was related to active inflammation (r = 0.13; P = 0.011) but not to chronic inflammation (r = 0.1; P = 0.34). However, IPSS was not correlated with active inflammation (r = 0.03; P = 0.6) or chronic inflammation (r = - 0.03; P = 0.91). Additionally, categorization of IPSS into 3 categories did not result in any association with active and chronic inflammations (Table 6).
| Active inflammation | P value | Correlation, r | ||||
| 0 | 1 | 2 | 3 | |||
| Log PSA, n | 25 | 91 | 88 | 14 | ||
| Mean (SD) | 1.4 (1.6) | 4.4 (4.3) | 5.9 (6.4) | 4.6 (6.6) | <0.0001 | 0.21 |
| IPSS, n | 25 | 94 | 88 | 15 | ||
| Mean (SD) | 14.4 (6.3) | 16.2 (7.0) | 15.3 (7.0) | 16.6 (7.1) | 0.595 | 0.03 |
| Age, n | 25 | 94 | 88 | 15 | ||
| Mean (SD) | 59.7 (5.4) | 70.1 (8.1) | 70.8 (8.2) | 68.4 (9.5) | <0.0001 | 0.24 |
| PRV, n | 25 | 90 | 81 | 15 | ||
| Mean (SD) | 75.0 (103.9) | 83.8 (110.6) | 68.9 (110.0) | 202.8 (303.2) | 0.001 | 0.09 |
| TURP weight, n | 25 | 94 | 88 | 15 | ||
| Mean (SD) | 24.7 (10.7) | 39.5 (27.4) | 44.2 (30.4) | 32.6 (14.0) | 0.011 | 0.13 |
| Chronic inflammation | P value | Correlation, r | ||||
| 0 | 1 | 2 | 3 | |||
| Log PSA, n | 4 | 109 | 91 | 14 | ||
| Mean (SD) | 1.0 (0.7) | 4.0 (5.0) | 5.7 (5.9) | 4.2 (4.4) | 0.005 | 0.19 |
| IPSS, n | 4 | 110 | 93 | 15 | ||
| Mean (SD) | 14.8 (7.9) | 16.0 (6.5) | 15.3 (7.2) | 15.7 (8.3) | 0.91 | -0.03 |
| Age, n | 4 | 110 | 93 | 15 | ||
| Mean (SD) | 61.5 (5.2) | 68.4 (8.75) | 70.5 (8.31) | 67.6 (9.3) | 0.08 | 0.09 |
| PRV, n | 4 | 105 | 86 | 13 | ||
| Mean (SD) | 68.5 (137.0) | 80.3 (129.5) | 83.8 (121.8) | 116.9 (191.4) | 0.88 | 0.05 |
| TURP weight, n | 4 | 110 | 93 | 15 | ||
| Mean (SD) | 25.0 (5.2) | 36.8 (25.4) | 42.5 (28.9) | 40.1 (31.6) | 0.34 | 0.1 |
| IPSS Categorized | P value | ||||
| Mild | Moderate | Severe | |||
| Active inflammation | 0 | 4 | 16 | 5 | 0.68 |
| 1 | 11 | 47 | 36 | ||
| 2 | 15 | 43 | 30 | ||
| 3 | 2 | 7 | 6 | ||
| Chronic inflammation | 0 | 1 | 1 | 2 | 0.13 |
| 1 | 10 | 65 | 35 | ||
| 2 | 18 | 41 | 34 | ||
| 3 | 3 | 6 | 6 | ||
We also evaluated the influence of pre-operatively placed suprapubic catheters, trans-urethral catheters or previous prostate puncture biopsies and TURP on the relationship with active and chronic inflammation (Table 7). Only a weak negative correlation was found (r = -0.16; P = 0.003) between active inflammation and the presence of a suprapubic catheter. No significant correlation was observed with the other variables (Table 7). However, for some categories of degree of inflammation no data were obtained, complicating comparisons.
PSA measurement is one of the cornerstones in prostate evaluation. Besides BPH and acute prostatitis, prostate cancer can be one of the reasons for PSA elevation, sensitizing and alarming many patients and care givers. Dealing with a patient with elevated/rising PSA levels is always a challenge, especially in ruling out potential life-threatening prostate cancer.
Multiple papers have been published indicating the relationship between PSA levels and BOO. In patients with clinical BPH, PSA levels are shown to be positively correlated with the five year cumulative risk of invasive BPH treatment[15]. Additionally, in men with BPH, prostate volume and PSA have been evaluated as predictors of acute urinary retention[16,17]. Consequently, elevated PSA levels are predictive for the need of BPH-related surgery. PSA has also been shown to be a strong predictor of future prostate growth[18], which is related to a higher risk of acute urinary retention and, subsequently, to the need for BPH-related surgery. A broader approach for the use of PSA seems justified. In a multicenter study in men suffering from LUTS, a correlation between PSA and the category of BOO was observed[19], Above a PSA-level of 4 ng/mL, mild or definite BOO was observed (in 89% of the cases) and below 2 ng/mL, the chances of a patient not suffering from BOO were one in three[19]. In previous papers, we have shown that BOO can be expected in a very particular group of patients with elevated and/or rising PSA, (multiple) negative multisided prostate biopsies and minor LUTS (mild + moderate IPSS)[20,21]. In a retrospective analysis of 82 consecutive patients, 95.9% were clearly obstructed (PdetQmax≥ 40 cm H2O)[18]. Similar results were obtained in a prospective analysis (n = 33), with a mean PdetQmax of 80.3 cm H2O[21]. A positive correlation between PSA velocity and PdetQmax was found (r = 0.5014; P = 0.006)[22]. As already mentioned, rising PSA levels can also be observed in patients with prostate cancer. Notwithstanding the presence of a tumor in these patients, the elevated PSA levels can also be influenced by BOO, implying that PSA should be included in the pre-treatment workup and the post-therapy evaluation of these patients. Therefore, PSA should be considered an additional indicator in the BOO decision tree.
We found some interesting correlations in this prospective analysis of 222 consecutive patients undergoing TURP for bothersome LUTS. A significant correlation was shown between log PSA and PdetQmax. In line with the literature[22], log PSA was also correlated with prostate volume (the resected prostate weight) (r = 0.54; P < 0.001). Interestingly, a negative correlation between IPSS and log PSA was observed (r = -0.14; P = 0.04), confirming the findings of our previous studies. This negative correlation could partly be explained by the correlation between high PSA levels and high detrusor pressures due to an increased compensation level of the detrusor for the urethral resistance, resulting in high flow rates and less symptoms.
On the other hand, no statistically significant correlation was observed between log PSA and post voiding residual volume, and between IPSS and the weight of the resected tissue. This might mean that the response of the detrusor to obstruction is more related to the symptoms than the degree of obstruction as such. Last but not least, a highly significant correlation between the weight of the resected tissue and PdetQmax (r = 0.39; P < 0.001) was observed, implying that bigger prostates are more frequently associated with obstruction and therefore are more prone to BPH-related surgery.
The role of chronic prostate inflammation in PSA elevation in asymptomatic men is still unclear. Many papers have covered this subject with various and sometimes contradictory outcomes and conclusions. In a prospective analysis of a small group of asymptomatic patients (n = 51) who underwent prostate puncture biopsies, a statistically significant correlation was found between the inflammatory process and the PSA values (P = 0.02)[23]. Similarly, in a retrospective analysis of 238 men, a correlation between PSA and inflammation was observed[24]. In an analysis of the prostate puncture biopsy of 80 asymptomatic patients, the extent of inflammation was positively correlated with total PSA levels (P < 0.001)[25]. In all these studies, histological examination was performed on prostate puncture biopsies in asymptomatic men, limiting the results obtained.
In contrast to the previously mentioned reports, multiple papers claimed the opposite and concluded that there is no correlation between PSA and inflammation. In a retrospective analysis of cancer negative prostate biopsies (n = 233), it was concluded that the degree of chronic inflammation did not correlate with PSA levels[26].
Chronic prostatitis was encountered in 68.3% of 284 patients with negative prostate puncture biopsies, while active prostatitis was observed in 8.4% of patients[27]. However, inflammation did not correlate with total PSA levels. In a prostate puncture biopsy driven evaluation (n = 49), the presence of inflammation did not correlate statistically with the PSA levels[28]. Finally, Nickel et al[29] studied a cohort of 80 patients without a history of prostatitis who underwent TURP. After histological examination on the resected tissue, prostatic inflammation was found to be extremely common. On the other hand, no correlation was observed between inflammation and PSA levels.
We performed a prospective analysis in 222 patients undergoing TURP according to the EAU guidelines[30] but without proven infection, known prostate cancer and a history of chronic prostatitis. We made a histological distinction between active and chronic inflammation, each with 4 subcategories. Additionally, since we used TURP tissue and not prostate puncture biopsy derived tissue, we were able to evaluate a substantial prostatic tissue volume. Our results showed that most patients have some degree of inflammation. Especially in the active inflammation group, a correlation between log PSA and inflammatory parameters was observed (r = 0.21; P < 0.0001). However, we did not encounter a statistically relevant correlation between symptoms (IPSS) and inflammation. The correlation between age and active inflammation (r = 0.24; P < 0.0001) is interesting to note, which was not the case for the chronic inflammation group (r = 0.09; P = 0.08). A possible explanation for this finding could be the accumulation of debris in the prostate during the lifetime, resulting in more frequent active inflammations. This finding may also be the explanation for the correlation between prostate weight (TURP) and active (r = 0.13; P = 0.011) and chronic (r = 0.1; P = 0.34) inflammation.
In conclusion, elevated and/or rising PSA levels with regard to underlying prostate cancer and prostatic inflammations are well known. In this paper, we have shown that PSA could also be indicative of BOO, not only with respect to observational data but also with regard to statistically relevant correlations between PSA and PdetQmax. Therefore, PSA levels should be taken into account and could even be used as a biomarker when a treatment strategy is determined or executed for patients suffering from clinical BPH. Additionally, the results obtained in this study indicate that inflammation can be correlated with PSA levels. This implies that elevated PSA levels should also be considered as predictive for prostate inflammation as well as for prostate cancer.
The authors wish to thank Dr. S. Deferme (PharmaXL, Belgium) for his assistance in writing and editing the text and Dr. W. M. Wami (Center for statistics, Hasselt University, Belgium) for his help in performing the statistic analyses for this study. The authors also acknowledge the ‘Kwaliteitsfonds Jessa ZH’ for the financial support.
Since its introduction, prostate-specific antigen (PSA) has played a key role in prostatic evaluation. However, it is not a cancer specific parameter but merely organ specific. Consequently, elevated PSA levels are a challenging problem for urologists in assessing or excluding potential life-threatening prostate cancer. A better understanding of other possible mechanisms causing PSA elevation is required. Therefore, the aim of this study was to investigate the relationship between PSA and the degree of prostate inflammation and to investigate whether PSA levels can be used as a biomarker for bladder outlet obstruction (BOO).
The cause of elevated PSA serum levels is not always prostate cancer. In the area of interpreting PSA elevation, the research hotspot is to have an insight in to other mechanisms that can be responsible for a PSA elevation, apart from prostate cancer.
In this study the authors showed a correlation between BOO (PdetQmax) and PSA (logarithmic). Furthermore, the authors demonstrated a weak correlation between PSA (logarithmic) and active as well as chronic prostatic inflammation.
PSA could also be indicative of BOO, not only with respect to observational data but also with regard to statistically relevant correlations between PSA and PdetQmax. Therefore, PSA levels should also be taken into account and could even be used as a biomarker when a treatment strategy is determined or executed for patients suffering from clinical benign prostate hyperplasia (BPH). Additionally, the results obtained in this study indicate that inflammation can be correlated with PSA levels. This implies that elevated PSA levels should also be considered as predictive for prostate inflammation as well as for prostate cancer.
BOO: a blockage at the base of the bladder that reduces or prevents the flow of urine into the urethra, the tube that carries urine out of the body. This condition is most common in aging men. It is often caused by BPH. As a man ages, his chance of developing these diseases increases dramatically; Prostate inflammation: inflammation of the prostate gland. There are four types of prostatitis: acute bacterial prostatitis, chronic bacterial prostatitis, chronic prostatitis without infection, asymptomatic inflammatory prostatitis; PSA (prostate-specific antigen): a protein manufactured exclusively by the prostate gland. PSA is produced for the ejaculate where it liquefies the semen and allows sperm cells to swim freely. Elevated levels of PSA in blood serum are associated with benign prostatic hyperplasia and prostate cancer.
This prospective analysis investigates the association of high PSA levels and bladder outflow obstruction and prostatic inflammation. Its results, as described in the discussion, add to the literature and the controversy that exists in these issues among other studies.
P- Reviewers: Baldi E, Cai T, Mazaris E, Zhang JJ S- Editor: Song XX L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317:909-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1782] [Cited by in F6Publishing: 1658] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10002] [Cited by in F6Publishing: 10353] [Article Influence: 739.5] [Reference Citation Analysis (0)] |
| 3. | Djavan B, Fong YK, Ravery V, Remzi M, Horninger W, Susani M, Kreuzer S, Boccon-Gibod L, Bartsch G, Marberger M. Are repeat biopsies required in men with PSA levels & lt; or =4 ng/ml? A Multiinstitutional Prospective European Study. Eur Urol. 2005;47:38-44; discussion 44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 5. | Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1747] [Cited by in F6Publishing: 1795] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 6. | Smeenge M, de la Rosette JJ, Wijkstra H. Current status of transrectal ultrasound techniques in prostate cancer. Curr Opin Urol. 2012;22:297-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Goode RR, Marshall SJ, Duff M, Chevli E, Chevli KK. Use of PCA3 in detecting prostate cancer in initial and repeat prostate biopsy patients. Prostate. 2013;73:48-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Arrighi HM, Metter EJ, Guess HA, Fozzard JL. Natural history of benign prostatic hyperplasia and risk of prostatectomy. The Baltimore Longitudinal Study of Aging. Urology. 1991;38:4-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, Lieber MM. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85-89. [PubMed] [Cited in This Article: ] |
| 10. | Kirby RS. The clinical assessment of benign prostatic hyperplasia. Cancer. 1992;70:284-290. [DOI] [Cited in This Article: ] |
| 11. | Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 12. | de la Rosette JJ, Alivizatos G, Madersbacher S, Perachino M, Thomas D, Desgrandchamps F, de Wildt M. EAU Guidelines on benign prostatic hyperplasia (BPH). Eur Urol. 2001;40:256-263; discussion 264. [PubMed] [Cited in This Article: ] |
| 13. | Griffiths D, Höfner K, van Mastrigt R, Rollema HJ, Spångberg A, Gleason D. Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction. International Continence Society Subcommittee on Standardization of Terminology of Pressure-Flow Studies. Neurourol Urodyn. 1997;16:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Rosai J. Ackerman’s surgical pathology. 10th ed. St. Louis: Mosby Year Book 2011; . [Cited in This Article: ] |
| 15. | Mochtar CA, Kiemeney LA, Laguna MP, van Riemsdijk MM, Barnett GS, Debruyne FM, de la Rosette JJ. Prognostic role of prostate-specific antigen and prostate volume for the risk of invasive therapy in patients with benign prostatic hyperplasia initially managed with alpha1-blockers and watchful waiting. Urology. 2005;65:300-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Marberger MJ, Andersen JT, Nickel JC, Malice MP, Gabriel M, Pappas F, Meehan A, Stoner E, Waldstreicher J. Prostate volume and serum prostate-specific antigen as predictors of acute urinary retention. Combined experience from three large multinational placebo-controlled trials. Eur Urol. 2000;38:563-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Roehrborn CG, McConnell JD, Saltzman B, Bergner D, Gray T, Narayan P, Cook TJ, Johnson-Levonas AO, Quezada WA, Waldstreicher J. Storage (irritative) and voiding (obstructive) symptoms as predictors of benign prostatic hyperplasia progression and related outcomes. Eur Urol. 2002;42:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Roehrborn CG, McConnell J, Bonilla J, Rosenblatt S, Hudson PB, Malek GH, Schellhammer PF, Bruskewitz R, Matsumoto AM, Harrison LH. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol. 2000;163:13-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Laniado ME, Ockrim JL, Marronaro A, Tubaro A, Carter SS. Serum prostate-specific antigen to predict the presence of bladder outlet obstruction in men with urinary symptoms. BJU Int. 2004;94:1283-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | van Renterghem K, Van Koeveringe G, Achten R, Van Kerrebroeck P. Clinical relevance of transurethral resection of the prostate in “asymptomatic” patients with an elevated prostate-specific antigen level. Eur Urol. 2007;52:819-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | van Renterghem K, Van Koeveringe G, Achten R, van Kerrebroeck P. Prospective study of the role of transurethral resection of the prostate in patients with an elevated prostate-specific antigen level, minor lower urinary tract symptoms, and proven bladder outlet obstruction. Eur Urol. 2008;54:1385-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 223] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Simardi LH, Tobias-MacHado M, Kappaz GT, Taschner Goldenstein P, Potts JM, Wroclawski ER. Influence of asymptomatic histologic prostatitis on serum prostate-specific antigen: a prospective study. Urology. 2004;64:1098-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Schatteman PH, Hoekx L, Wyndaele JJ, Jeuris W, Van Marck E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol. 2000;37:404-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Gui-Zhong L, Libo M, Guanglin H, Jianwei W. The correlation of extent and grade of inflammation with serum PSA levels in patients with IV prostatitis. Int Urol Nephrol. 2011;43:295-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Chang SG, Kim CS, Jeon SH, Kim YW, Choi BY. Is chronic inflammatory change in the prostate the major cause of rising serum prostate-specific antigen in patients with clinical suspicion of prostate cancer? Int J Urol. 2006;13:122-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Morote J, Lopez M, Encabo G, de Torres IM. Effect of inflammation and benign prostatic enlargement on total and percent free serum prostatic specific antigen. Eur Urol. 2000;37:537-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Moser PL, Brunner A, Horninger W, Bartsch G, Mikuz G. Correlation between inflammatory cells (T and B lymphocytes, macrophages) in prostate biopsies and elevated PSA levels in a PSA screening population. Urology. 2002;59:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84:976-981. [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, Wolff J, Zattoni F. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |