Published online May 18, 2017. doi: 10.5312/wjo.v8.i5.400
Peer-review started: October 28, 2016
First decision: December 1, 2016
Revised: January 5, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 18, 2017
To undertook a systematic review to determine factors that increase a patient’s risk of developing lower limb periprosthetic joint infections (PJI).
This systematic review included full-text studies that reviewed risk factors of developing either a hip or knee PJI following a primary arthroplasty published from January 1998 to November 2016. A variety of keywords were used to identify studies through international databases referencing hip arthroplasty, knee arthroplasty, infection, and risk factors. Studies were only included if they included greater than 20 patients in their study cohort, and there was clear documentation of the statistical parameter used; specifically P-value, hazard ratio, relative risk, or/and odds ratio (OR). Furthermore a quality assessment criteria for the individual studies was undertaken to evaluate the presence of record and reporting bias.
Twenty-seven original studies reviewing risk factors relating to primary total hip and knee arthroplasty infections were included. Four studies (14.8%) reviewed PJI of the hip, 3 (11.21%) of the knee, and 20 (74.1%) reviewed both joints. Nineteen studies (70.4%) were retrospective and 8 (29.6%) prospective. Record bias was identified in the majority of studies (66.7%). The definition of PJI varied amongst the studies but there was a general consensus to define infection by previously validated methods. The most significant risks were the use of preoperative high dose steroids (OR = 21.0, 95%CI: 3.5-127.2, P < 0.001), a BMI above 50 (OR = 18.3, P < 0.001), tobacco use (OR = 12.76, 95%CI: 2.47-66.16, P = 0.017), body mass index below 20 (OR = 6.00, 95%CI: 1.2-30.9, P = 0.033), diabetes (OR = 5.47, 95%CI: 1.77-16.97, P = 0.003), and coronary artery disease (OR = 5.10, 95%CI: 1.3-19.8, P = 0.017).
We have highlighted the need for the provider to optimise modifiable risk factors, and develop strategies to limit the impact of non-modifiable factors.
Core tip: This systematic review determines the most statistically significant factors that increase a patient’s risk of developing lower limb periprosthetic joint infections. Reviewing all relevant papers until November 2016 through international databases, we have included 27 original studies. The results include multiple factors relating to the patient and the Institute, as well as post-operative predictors and causes of infection. This ultimately reiterates the importance of optimising the patients pre-operatively by addressing modifiable risk factors (such as their immunosuppression, nutrition, diabetes, and smoking), and develops strategies to limit the impact of non-modifiable factors.
- Citation: George DA, Drago L, Scarponi S, Gallazzi E, Haddad FS, Romano CL. Predicting lower limb periprosthetic joint infections: A review of risk factors and their classification. World J Orthop 2017; 8(5): 400-411
- URL: https://www.wjgnet.com/2218-5836/full/v8/i5/400.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i5.400
Chronic periprosthetic joint infections (PJI) have received increasing interest in the medical literature as the profession has acknowledged the real-life implications to the patient and the health service[1,2]. The treatment of PJI is costly to the health service with strain upon limited resources as multiple operations and trials of antibiotic therapy may be attempted. But the cost to the patient is greatest, with loss or reduced joint function, deterioration in their physical and psychological health, and loss in trust with the profession.
Prevention is key. Despite improved outcomes following the various treatment modalities for treating established infections today, the patient has to endure the consequences of the infection[3]. Prior to the initial surgery it is imperative the patient is medically optimised and any reversible risk factors be corrected. Such risk factors are well known such as diabetes[4], systemic infections[5], and immunocompromise[6].
However, risk factors vary and are dependent upon the patient cohort, and often findings from isolated studies are not transferable. Therefore, we undertook a systematic review of the literature to determine overall predictive factors that increase a patient’s risk of developing a lower limb PJI, and determine which risk factors are most predictive of infection.
In this review, we categorised risk factors in order to better understand the relative role of the host, of the healthcare provider, and of post-surgical conditions, the latter acting more as prognostic factors since the surgical procedure has already taken place. To this aim, we have subdivided known risk factors for PJI in three groups: (1) those relating to the host (host-related risk factors); (2) those that are related to the treatment provider and to the surgical environment (provider-related risk factors); and (3) those that arise from clinical interventions, increasing the patient’s inherent risk (post-surgical risk factors). We have then compared the absolute number of risk factors in each main category, scored them according to their relative weight and divided in “modifiable” and “non-modifiable” risk factors.
This systematic review included full-text studies that reviewed risk factors of developing either a hip or knee PJI following a primary arthroplasty published from January 1998 to November 2016. These were identified through international databases, such as EMBASE, PubMed/MEDLINE, MEDLINE Daily Update, MEDLINE In-Process, Google Scholar, SCOPUS, CINAHL, Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews.
A variety of keywords were used either alone or in combinations to identify the studies. This included references to hip infections (total hip replacement; THR; periprosthetic hip infection, hip arthroplasty infection), knee infections (total knee replacement; TKR; periprosthetic knee infection, knee arthroplasty infection), general joint infections (PJI, PPI), and “risk factors”. We did not use specific keywords to search for individual risk factors, such as diabetes, etc.
Studies were only included if the risk factors were calculated by involving greater than 20 patients in their study cohort, and there was clear documentation of the statistical parameter used, and were only included if the P-value was quoted and one or more of the following; hazard ratio (HR), relative risk (RR), or/and odds ratio. Studies were excluded if they referred to recurrent infection following a revision procedure, hip or knee fracture, and a risk factor was excluded if the P-value was greater than 0.05. Results from combined studies, as seen in meta-analysis, were also excluded.
Two investigators, DAG and CLR, independently searched and reviewed the literature and determined if the study should be included based on their title and abstract. Once the two lists were compared, if the same material was presented in more than one study, only the most recent one was included.
The quality assessment criteria for the inclusion of the individual studies was adapted from George et al[7]. to reflect the information we expect to be present in each study. Therefore we evaluated the presence of (1) record bias reflecting the source of data, and whether the analysis was retrospective or prospective; and (2) reporting bias; each study’s definition of PJI (the measured outcome).
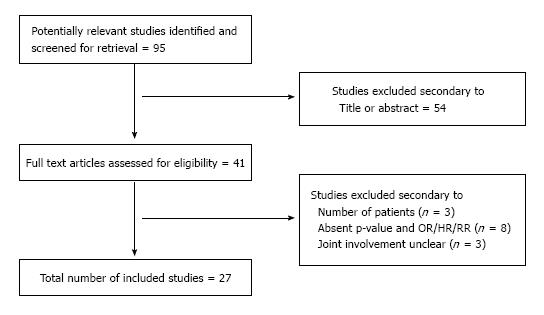
Figure 1 demonstrates the overall selection process according to the Prisma model[8]. DAG, CLR, SS and EG compared the overall findings and any discrepancies were solved by reclassification as mutually agreed.
In all, 27 original studies reviewing risk factors relating to primary total hip and knee arthroplasty infections were included. The number of risk factors identified ranged from 1 to 18. Four studies (14.8%) reviewed PJI on the hip, 3 (11.21%) on the knee, and 20 (74.1%) reviewed both joints. The statistical methods used to determine significance are also shown in Table 1[4,5,9-33].
| Ref. | Year | Patients (n) | Statistical method used | Site | Follow-up (mo) | ||||
| Infected (cases) | Non-infected (controls) | Total | Min | Max | Mean | ||||
| Berbari et al[9] | 1998 | 462 | 462 | 924 | OR, CI, P | Both | - | - | - |
| Lai et al[10] | 2007 | 51 | - | - | OR, CI, P | Both | - | 84 | - |
| Parvizi et al[11] | 2007 | 78 | 156 | 234 | OR, CI, P | Both | - | - | - |
| Pulido et al[12] | 2008 | 63 | 9182 | 9245 | HR, CI, P | Both | 12 | 72 | 43 |
| Malinzak et al[13] | 2009 | 43 | 8451 | 8494 | OR, P | Both | 24 | 192 | 74.4 |
| Ong et al[14] | 2009 | 887 | 39042 | 39929 | OR, P | Hip | - | 108 | - |
| Berbari et al[5] | 2010 | 339 | 339 | 678 | OR, CI, P | Both | - | - | - |
| Peel et al[15] | 2011 | 63 | 126 | 189 | OR, CI, P | Both | - | - | - |
| Bozic et al[16] | 2012 | - | - | 40919 | HR, CI, P | Hip | 12 | - | - |
| Jämsen et al[17] | 2012 | 52 | 7129 | 7181 | HR, CI, P | Both | 0 | 12 | 12 |
| Bozic et al[4] | 2012 | - | - | 83011 | OR, CI, P | Knee | 12 | - | - |
| Dale et al[18] | 2012 | 2778 | 429390 | 432168 | RR, CI, P | Hip | 0 | 60 | 60 |
| Greenky et al[19] | 2012 | 389 | 15333 | 15722 | OR, CI, P | Both | 36 | 108 | 62.4 |
| Namba et al[20] | 2013 | 404 | 55812 | 56216 | HR, CI, P | Knee | - | - | - |
| Somayaji et al[21] | 2013 | 5 | 254 | 259 | OR, CI, P | Both | 12 | 124 | 24 |
| Coelho-Prabhu et al[22] | 2013 | 339 | 339 | 678 | OR, CI, P | Both | 2 | 24 | - |
| Maoz et al[23] | 2014 | 47 | 3625 | 3672 | OR, CI, P | Hip | 12 | 48 | 24 |
| Gómez-Lesmes et al[24] | 2014 | 32 | 1299 | 1331 | OR, CI, P | Knee | - | 3 | - |
| Yi et al[25] | 2014 | 126 | 375 | 501 | OR, CI, P | Both | 3 | - | - |
| Wu et al[26] | 2014 | 45 | 252 | 297 | OR, CI, P | Both | 12 | 144 | 28 |
| Sousa et al[27] | 2014 | 43 | 2454 | 2497 | OR, CI, P | Both | 1 | 12 | 12 |
| Jiang et al[28] | 2014 | - | - | 306946 | HR, P | Hip | 6 | - | - |
| 2014 | - | - | 573840 | HR, P | Knee | 6 | - | - | |
| Duchman et al[29] | 2015 | 8062+ | 70129+ | 78191 | OR, CI, P | Both | - | - | - |
| Chrastil et al[30] | 2015 | - | - | 13272 | HR, CI, P | Both | 24 | 120 | - |
| Crowe et al[31] | 2015 | 26 | 3393 | 3419 | OR, CI, P | Both | - | 12 | - |
| Debreuve-Theresette et al[32] | 2015 | 45 | 90 | OR, CI, P | Both | - | - | - | |
| Bohl et al[33] | 2015 | - | - | 49603 | RR, CI, P | Both | - | 1 | - |
The quality of the included studies is demonstrated in Table 2. Nineteen studies (70.4%) were retrospective and 8 (29.6%) prospective. Record bias was identified in the majority of studies (66.7%). The definition of PJI varied amongst the studies but there was a general consensus to define infection by previously validated methods.
| Ref. | Design | Record bias | Reporting bias (outcome measure); definition of infection |
| Berbari et al[9] | Retrospective | No | 2 or more cultural examination positive for the same microorganism; sinus tract; purulence around the prosthesis/joint |
| Lai et al[10] | Retrospective | No | 2 or more cultural examination positive for the same microorganism; clinical diagnosis |
| Parvizi et al[11] | Prospective | No | Criteria based upon 3 of 5 features1 |
| Pulido et al[12] | Retrospective | Yes | Criteria based upon 3 of 5 features1 |
| Malinzak et al[13] | Retrospective | No | Unknown |
| Ong et al[14] | Retrospective | Yes | Diagnostic code in Medicare database |
| Berbari et al[5] | Prospective | Yes | 2 or more cultural examination positive for the same microorganism; acute inflammation on histopathological examination; sinus tract; purulence around the prosthesis/joint |
| Peel et al[15] | Prospective | Yes | Criteria based upon 3 of 5 features1 |
| Bozic et al[16] | Retrospective | Yes | Diagnostic code in Medicare database |
| Jämsen et al[17] | Prospective | Yes | CDC definition of surgical site infection3 |
| 1Bozic et al[4] | Retrospective | Yes | Diagnostic code in Medicare database |
| Dale et al[18] | Retrospective | Yes | Clinical as reported by the surgeon after surgery |
| Greenky et al[19] | Retrospective | No | Criteria based upon 3 of 5 features1 |
| Namba et al[20] | Retrospective | Yes | CDC definition of surgical site infection3 |
| Somayaji et al[21] | Retrospective | No | Criteria based upon 3 of 5 features1 |
| Coelho-Prabhu et al[22] | Retrospective | Yes | 2 or more cultural examination positive for the same microorganism; sinus tract; purulence around the prosthesis/joint |
| Maoz et al[23] | Retrospective | Yes | CDC definition of surgical site infection3 |
| Gómez-Lesmes et al[24] | Prospective | Yes | Criteria based upon 3 of 5 features1 |
| Yi et al[25] | Retrospective | No | Criteria based upon 3 of 5 features1 |
| Wu et al[26] | Retrospective | Yes | MSIS definition2 |
| Sousa et al[27] | Retrospective | No | Criteria based upon 3 of 5 features1 |
| Jiang et al[28] | Prospective | Yes | Diagnostic code in Medicare database |
| Duchman et al[29] | Prospective | Yes | Criteria based upon 3 of 5 features1 |
| Chrastil et al[30] | Retrospective | Yes | Diagnostic code in Medicare database |
| Crowe et al[31] | Retrospective | Yes | CDC definition of surgical site infection3 |
| Debreuve-Theresette et al[32] | Retrospective | No | CDC definition of surgical site infection3 |
| Bohl et al[33] | Prospective | Yes | American College of Surgeons National Surgical Quality Improvement Program definition |
This included the presence of 2 or more cultural positive results for the same microorganism (plus other features on infection) in 4 studies (14.8%), the CDC definition in 5 studies (18.5%), the Medicare code for infection in 5 studies (18.5%), and 9 studies (33.3%) based their definition on patients meeting 3 of the following 5 features; (1) abnormal serology (ESR > 30 mm/h; CRP > 1 mg/dL); (2) strong clinical and radiographic suspicion for infection; (3) positive joint aspiration culture for infection; (4) evidence of purulence during the subsequent surgical intervention; and (5) positive intraoperative culture.
One study used the MSIS criteria, which includes: (1) a sinus tract; (2) positive culture results from 2 or more tissue or fluid samples; and (3) 4 of the following 6 criteria are present: (I) elevated CRP/ESR; (II) elevated synovial WCC; (III) high synovial PMN leukocyte percentage; (IV) presence of purulence in the joint; (V) positive culture result from one sample from the affected joint; and (VI) PMN leukocyte count of more than 5 per high-powered field in 5 high-powered fields on histologic analysis at 400 × magnification[34].
Risk factors relating to the host have been shown in Table 3, and are the most abundant group of risk factors identified. The majority of the risk factors are systemic referring to patient co-morbidities that are negatively associated with patient outcome following a primary THR or TKR, such as presence of diabetes mellitus[4,9,17,20,26], immunocompromised[5,15,21], concomitttent systemic infection[5,10,27,31], cardiology[4,16,21] and gastroenterology disorders[22,28], high ASA (American Society of Anesthesiologists) grade[12,15,20] and malnutrition[13,17,21,23,25,26,33].
| Ref. | Statistical parameter | Site | |||||
| HR | OR | RR | 95%CI | P value | |||
| General | |||||||
| Age: 65-75 yr (compared to 45-65) | [26] | 3.36 | 1.30-8.69 | 0.013 | Hip/knee | ||
| Comorbidities (total number) | [10] | 1.35 | 1.10-1.66 | 0.005 | Hip/knee | ||
| Charlson index + 5 (compared to 0) | [14] | 2.57 | 1.96-3.37 | < 0.001 | Hip | ||
| Place of residence (rural) | [26] | 2.63 | 1.13-6.10 | 0.025 | Hip/knee | ||
| Hispanic race (compared to White) | [20] | 0.69 | 0.49-0.98 | 0.038 | Knee | ||
| Alcohol abuse | [26] | 2.95 | 1.06-8.23 | 0.039 | Hip/knee | ||
| Tobacco use | [29] | 1.47 | 1.21-1.78 | 0.001 | Hip/knee | ||
| [31] | 3.4 | 1.23-9.44 | 0.029 | Hip/knee | |||
| [32] | 3.91 | 3.4 | 1.19-12.84 | 0.032 | Hip/knee | ||
| Tobacco use (S aureus colonization) | [23] | 12.76 | 2.47-66.16 | 0.017 | Hip | ||
| Gender | |||||||
| Female | [14] | 0.83 | 0.009 | Hip | |||
| Male | [18] | 1.9 | 1.80-2.10 | < 0.001 | Hip | ||
| [20] | 1.89 | 1.54-2.32 | < 0.001 | Knee | |||
| [31] | 3.55 | 1.60-7.84 | 0.002 | ||||
| Endocrine disorders | |||||||
| Diabetes mellitus | [4] | 1.19 | 1.06-1.34 | 0.0025 | Knee | ||
| [26] | 5.47 | 1.77-16.97 | 0.003 | Hip/knee | |||
| [22] | 1.46 | 1.27-1.68 | 0.0007 | Hip | |||
| [9] | 4 | 1.13-14.18 | 0.032 | Hip/knee | |||
| [20] | 1.28 | 1.03-1.60 | 0.025 | Knee | |||
| [17] | 2.31 | 1.12-4.72 | < 0.001 | Hip/knee | |||
| [15] | 1.4 | 0.90-2.10 | 0.06 | Hip/knee | |||
| [5] | 1.8 | 1.20-2.80 | 0.006 | Hip/knee | |||
| [13] | 3.1 | 0.02 | Hip/knee | ||||
| [44] | 2.21 | 1.34-3.64 | 0.001 | Knee | |||
| Pre-op BM > 6.9 mmol/L | [17] | 2.25 | 0.60-8.50 | 0.073 | Hip/knee | ||
| Pre-operative hyperglycemia | [30] | 1.44 | 1.09-1.89 | 0.008 | Hip/knee | ||
| Psychiatric disorders | |||||||
| Depression | [4] | 1.28 | 1.08-1.51 | 0.0035 | Knee | ||
| [16] | 1.6 | 1.32-1.93 | 0.0039 | Hip | |||
| Psychosis | [16] | 1.74 | 1.38-2.20 | 0.0044 | Hip | ||
| [4] | 1.26 | 1.02-1.57 | 0.0331 | Knee | |||
| Haematological disorders | |||||||
| Preoperative anaemia | [16] | 1.36 | 1.15-1.62 | 0.0005 | Hip | ||
| [19] | 1.95 | 1.41-2.69 | < 0.001 | Hip/knee | |||
| [4] | 1.26 | 1.09-1.45 | 0.0014 | Knee | |||
| Coagulopathy | [16] | 1.58 | 1.24-2.01 | 0.0002 | Hip | ||
| Malignancy | |||||||
| Metastatic malignancy | [4] | 1.59 | 1.03-2.47 | 0.0369 | Knee | ||
| Tumour 5 yr before implant | [5] | 3.1 | 1.30-7.20 | < 0.01 | Hip/knee | ||
| Cardiovascular disorders | |||||||
| Congestive heart failure | [4] | 1.28 | 1.13-1.46 | < 0.0001 | Knee | ||
| [16] | 1.57 | 1.33-1.84 | 0.0409 | Hip | |||
| Cardiac arrhythmia | [16] | 1.48 | 1.30-1.70 | 0.0012 | Hip | ||
| Coronary artery disease | [21] | 5.10 | 1.30-19.8 | 0.017 | Hip/knee | ||
| Valvular disease | [4] | 1.15 | 1.01-1.31 | 0.039 | Knee | ||
| Peripheral vascular disease | [4] | 1.13 | 1.01-1.27 | 0.0381 | Knee | ||
| [16] | 1.44 | 1.24-1.68 | 0.0032 | Hip | |||
| Gastroenterology disorders | |||||||
| Liver cirrhosis | [28] | 5.4 | < 0.001 | Hip | |||
| [28] | 3.4 | < 0.001 | Knee | ||||
| Hepatitis B virus (amongst males) | [44] | 4.32 | 1.85-10.09 | < 0.001 | Knee | ||
| OGD with biopsy | [22] | 2.8 | 1.10-7.10 | 0.03 | Hip/knee | ||
| Respiratory disorders | |||||||
| Chronic pulmonary disease | [4] | 1.22 | 1.10-1.36 | < 0.0001 | Knee | ||
| [31] | 4.34 | 1.28-14.70 | 0.041 | Both | |||
| Pulmonary circulation disorders | [4] | 1.42 | 1.06-1.91 | 0.0205 | Knee | ||
| Renal disorders | |||||||
| Renal disease | [4] | 1.38 | 1.11-1.71 | 0.0038 | Knee | ||
| Renal function (mL/min) | [15] | 1 | 0.90-1.00 | 0.05 | Hip | ||
| Rheumatoid arthritis | |||||||
| Rheumatoid arthritis | [15] | 3.3 | 0.80-13.90 | 0.09 | Hip/knee | ||
| [4] | 1.18 | 1.02-1.37 | 0.0277 | Knee | |||
| [16] | 1.71 | 1.42-2.06 | < 0.0001 | Hip | |||
| ASA grade | |||||||
| ASA score | [15] | 2.2 | 1.30-4.00 | 0.006 | Hip/knee | ||
| Mean score | [11] | 2.07 | 1.08-1.97 | 0.03 | Hip/knee | ||
| 3 (compared to 1 or 2) | [20] | 1.65 | 1.33-2.00 | < 0.001 | Knee | ||
| > 4 | [12] | 1.95 | 1.00-3.70 | 0.04 | Hip/knee | ||
| Body mass index | |||||||
| Obesity | [4] | 1.22 | 1.03-1.44 | 0.0219 | Knee | ||
| [16] | 1.73 | 1.35-2.22 | < 0.0001 | Hip | |||
| BMI (kg/m2) | [15] | 1.1 | 1.00-1.10 | 0.05 | Hip | ||
| [12] | 3.23 | 1.60-6.50 | 0.001 | Hip/knee | |||
| < 20 | [21] | 6 | 1.20-30.9 | 0.033 | Hip/knee | ||
| 25-30 | [5] | 0.4 | 0.30-0.70 | < 0.001 | Hip/knee | ||
| ≥ 28 (compared to 18.5-28) | [26] | 2.77 | 1.20-6.40 | 0.017 | Hip/knee | ||
| 31-39 | [5] | 0.5 | 0.30-0.70 | < 0.001 | Hip/knee | ||
| 35 (compared to < 35) | [20] | 1.47 | 1.17-1.85 | 0.001 | Knee | ||
| [32] | 1.84 | 1.11-3.05 | 0.007 | Both | |||
| > 40 | [23] | 4.13 | 1.30-12.88 | 0.01 | Hip | ||
| [13] | 3.3 | 0.045 | Knee | ||||
| [17] | 6.41 | 1.67-24.59 | < 0.001 | Hip/knee | |||
| > 50 | [13] | 18.3 | < 0.001 | Hip/knee | |||
| Malnutrition | [25] | 2.3 | 1.50-3.50 | < 0.001 | Hip/knee | ||
| Serum albumin < 3.5 g/dL | [33] | 2 | 1.50-2.80 | < 0.001 | Hip/knee | ||
| Immunocompromise | |||||||
| Immunocompromise | [5] | 2.2 | 1.60-3.00 | < 0.001 | Hip/knee | ||
| Inflammatory disease | [18] | 1.4 | 1.10-1.70 | 0.001 | Hip | ||
| Prednisone dose exceeds 15 mg/d | [21] | 21 | 3.50-127.2 | < 0.001 | Hip/knee | ||
| Systemic steroid therapy | [15] | 3.3 | 0.80-13.90 | 0.09 | Hip/knee | ||
| Infection | |||||||
| Distant organ infection | [5] | 2.2 | 1.50-3.25 | < 0.001 | Hip/knee | ||
| Nasal S. Aureus Infection | [31] | 3.95 | 1.80-8.71 | < 0.001 | Hip/knee | ||
| Nasal MRSA Infection | [31] | 8.24 | 3.23-21.02 | < 0.001 | Hip/knee | ||
| Asymptomatic bacteriuria | [27] | 3.23 | 1.67-6.27 | 0.001 | Hip/knee | ||
| Genitourinary infection | [10] | 2.8 | 1.01-7.77 | 0.048 | Hip/knee | ||
| Operative indication | |||||||
| Hip fracture | [18] | 2.1 | 1.90-2.40 | < 0.001 | Hip | ||
| Post-traumatic osteoarthritis | [20] | 3.23 | 1.68-6.23 | < 0.001 | Knee | ||
| Prior operation on the index joint | [5] | 1.9 | 1.30-2.60 | < 0.001 | Hip/knee | ||
| Per additional surgery | [32] | 2.88 | 1.45-5.80 | 0.018 | Hip/knee | ||
| Avascular necrosis | [18] | 1.7 | 1.40-2.10 | < 0.001 | Hip | ||
Patient demographics also have been shown to have an impact upon risk of PJI, including age[16], rural residence[16], race[20], male gender[14,18,20,31], and alcohol[26] or tobacco use[23,29,31,32]. Previous operations to the joint (excluding revisions arthroplasty as this was excluded from analysis) increased the risk of PJI[5,32].
Risk factors relating to the provider are shown in Table 4. Prolonged operative duration of greater than 115 minutes in hip arthroplasty is a strong predictor of infection[5,14,23], as is non-same day surgery[23]. During knee arthroplasty, exposure to the joint requiring quadriceps release significantly increases the risks of infection[20].
| Ref. | Statistical parameter | Site | |||||
| HR | OR | RR | 95%CI | P value | |||
| Antibiotic use | |||||||
| Antibiotic surgical prophylaxis | [5] | 0.5 | 0.30-0.80 | 0.003 | Hip/knee | ||
| Antibiotic irrigation | [20] | 0.67 | 0.48-0.92 | 0.014 | Knee | ||
| Surgical technique | |||||||
| Exposure requiring quadriceps release | [20] | 4.76 | 1.18-19.21 | 0.029 | Knee | ||
| Use of wound drain tube | [15] | 0.09 | 0.01-0.80 | 0.03 | Knee | ||
| Side of surgery | |||||||
| Simultaneous bilateral surgery | [12] | 5.85 | 2.50-13.90 | < 0.0001 | Hip/knee | ||
| [20] | 0.51 | 0.31-0.83 | 0.007 | Knee | |||
| Single side (compared to bilateral) | [13] | 3.1 | 0.0024 | Hip/knee | |||
| [13] | 4 | 0.009 | Knee | ||||
| Cement | |||||||
| Antibiotic-laden cement | [20] | 1.53 | 1.18-1.98 | < 0.001 | Knee | ||
| Non-antibiotic cement | [8] | 1.5 | 1.30-1.80 | < 0.001 | Hip | ||
| Hybrid (compared to uncemented) | [8] | 1.6 | 1.40-1.80 | < 0.001 | Hip | ||
| Operative duration | |||||||
| Length of operation (> 115 min) | [23] | 3.38 | 1.23-9.28 | 0.018 | Hip | ||
| (> 210 min) | [14] | 1.78 | 1.40-2.26 | < 0.0001 | Hip | ||
| (≥ 240 min) | [5] | 2.7 | 1.50- 5.00 | 0.002 | Hip/knee | ||
| Hospital factors | |||||||
| Hospital volume < 100 (vs > 200/yr) | [20] | 0.33 | 0.12-0.90 | 0.03 | Knee | ||
| Medicare buy-in | [14] | 1.34 | 0.005 | Hip | |||
Protective measures include the use of antibiotic surgical prophylaxis systemically[5] and locally as irrigation[20], but antibiotic impregnated cement may or may not be protective[18,20]. In addition, bilateral procedures during the same operation have been shown by some studies to increase the risk[12], whilst in others decrease it[20].
Post-operatively patients may present with a superficial infection to the joint with a warm, cellulitic, and sometimes discharging wound, which is a high predictor of an underlying PJI[5,11,9,15]. Table 5 demonstrates other factors that have a high correlation with a PJI, including receiving a blood transfusion[11,12,15] (especially if the blood has been stored for greater than 14 d[24]), post-operative urinary tract infection (UTI)[5,12], and onset of cardiac arrhythmias[12].
| Ref. | Statistical parameter | Site | ||||
| HR | OR | 95%CI | P value | |||
| Anaesthetic factors | ||||||
| Intensive care length of stay (d) | [15] | 0.5 | 0.20-1.00 | 0.06 | Knee | |
| Haematological | ||||||
| Blood transfusion | [12] | 2.11 | 1.10-3.90 | 0.02 | Hip/knee | |
| [15] | 2.1 | 1.00-4.20 | 0.04 | Hip/knee | ||
| [11] | 1.63 | 1.14-2.33 | 0.007 | Hip/knee | ||
| Transfusion if RBCs stored > 14 d | [24] | 5.9 | 2.60-13.20 | < 0.001 | Knee | |
| Perioperative blood loss (via drain tube) | [15] | 1 | 1.00-1.01 | 0.008 | Hip | |
| Cardiac | ||||||
| Postoperative atrial fibrillation | [12] | 6.22 | 1.40-28.5 | 0.02 | Hip/knee | |
| Postoperative myocardial infarction | [12] | 20.4 | 2.10-199.9 | 0.009 | Hip/knee | |
| Hospital factors | ||||||
| Longer hospital stay | [12] | 1.09 | 1.00-1.10 | 0.0003 | Hip/knee | |
| Non same-day surgery | [23] | 4.16 | 1.44-12.02 | 0.008 | Hip | |
| Wound complications | ||||||
| All wound complications | [11] | 27 | 11.00-91.6 | 0.0002 | Hip/knee | |
| Wound discharge | [5] | 18.7 | 7.40-47.2 | < 0.001 | Hip/knee | |
| [15] | 6.3 | 1.30-30.7 | 0.02 | Knee | ||
| [15] | 5.4 | 2.00-15.0 | 0.001 | Hip | ||
| [15] | 5.7 | 2.40-13.3 | <0.001 | Hip/knee | ||
| [11] | 32.2 | 8.7-119.17 | < 0.0001 | Hip/knee | ||
| Haematoma | [5] | 3.5 | 1.30-9.50 | 0.01 | Hip/knee | |
| Surgical site infection | [1] | 35.9 | 8.30-154.6 | < 0.01 | Hip/knee | |
| Superficial incisional SSI | [15] | 3.7 | 1.10-11.9 | 0.03 | Knee | |
| [15] | 5 | 1.60-15.9 | 0.007 | Hip | ||
| [15] | 4.3 | 1.90 - 9.90 | 0.001 | Hip/knee | ||
| NNIS risk index 2 | [9] | 3.9 | 2.00-7.50 | < 0.01 | Hip/knee | |
| Urinary | ||||||
| Postoperative urinary infection | [12] | 5.45 | 1.00-8.70 | 0.04 | Hip/Knee | |
| [5] | 2.7 | 1.04-7.10 | 0.04 | Hip/Knee | ||
Several risk factors were shown to have greater significance than others, and a vast majority of the risk factors were directly related to the patient (host-factors). The most significant risks were the use of preoperative high dose steroids (OR = 21.0, 95%CI: 3.5-127.2, P < 0.001)[21], a BMI above 50 (OR = 18.3, P < 0.001)[13], tobacco use (OR = 12.76, 95%CI: 2.47-66.16, P = 0.017)[23], BMI below 20 (OR = 6.00, 95%CI: 1.2-30.9, P = 0.033)[21], diabetes (OR = 5.47, 95%CI: 1.77-16.97, P = 0.003)[26], and coronary artery disease (OR = 5.10, 95%CI: 1.3-19.8, P = 0.017)[21].
We further categorised the resultant risk factors into whether or not they were modifiable, reflecting the opportunity of the surgeon to optimise their patient pre-operatively and to reduce the risk of developing a PJI (Table 6).
| Risk factor | Minimum increase | Maximum increase | Statistical parameter | Ref. | |
| Host-related risk factors | |||||
| Modifiable | Systemic steroids | 3.3 | 21 | OR | [15,21] |
| Tobacco use | 3.4 | 12.76 | OR | [23,32] | |
| Nasal MRSA infection | - | 8.24 | OR | [31] | |
| BMI < 20 | - | 6 | OR | [21] | |
| Coronary artery disease | - | 5.1 | OR | [21] | |
| COPD | 1.22 | 4.34 | OR | [4,31] | |
| BMI > 40 | - | 4.13 | OR | [23] | |
| Pre-operative BM | - | 2.25 | [17] | ||
| Non-modifiable | Diabetes | 1.4 | 5.47 | OR | [15,26] |
| Liver cirrhosis | - | 5.4 | HR | [28] | |
| Male | 1.89 | 3.55 | HR,OR | [20,31] | |
| Age | - | 3.36 | OR | [26] | |
| Rheumatoid arthritis | 1.18 | 3.3 | OR | [4,15] | |
| Malignancy | - | 3.1 | OR | [5] | |
| Provider-related risk factors | |||||
| Modifiable | Quadriceps release (TKR) | - | 4.76 | HR | [20] |
| Non-same day procedure | - | 4.16 | OR | [23] | |
| Prolonged operation | 1.78 | 3.38 | HR | [14,23] | |
| Non-modifiable | Prolonged storage of blood | 2.6 | 13.2 | OR | [24] |
It is extremely difficult to predict if a patient will develop a post-operative infection following lower limb arthroplasty. Multiple prospective and retrospective studies have reviewed the risks associated with their patient cohort developing such infections. This paper was undertaken to combine these risks and determine if there was a consensus to which factors puts a patient at highest risk, and categorise them if they related directly to the host (patient), provider (the surgical team and their Institute), or occurred during the post-operative period.
Little is known about the interaction between, or synergistic effect, of specific patient risk factors[35], as it is likely they have a multiplicity effect, rather than additive risk, as shown by Tomás[6]. In their cohort if a patient had two (or more) significant factors the probability of infection development was 14-times higher, whereas having three (or more) factors the probability was increased 16-times.
Several themes have emerged following this systematic review of the literature, specifically the patient’s immunological and systematic responses to infection, other sources of infection, antibiotic use, and provider factors.
The most frequently quoted risk factor was diabetes mellitus[4,9,17,20,22,26], which had one of the highest odds ratios[26]. Almost all the other highest odds ratio, or hazard ratio, also belonged to medical conditions ultimately impairing a patients immunity, as demonstrated from high dose pre-operative steroids[21], malnutrition (reflective of high alcohol intake[26], BMI below 20[21] and above 50[13]), and tobacco use[23]. Malignancy[4,5], rheumatoid arthritis[4,15,16], and liver cirrhosis[28] can also impair a patient’s immunity.
Immunosuppression has long been known to increase a patient’s risk of systemic infection, and has widely been documented in arthroplasty patients. Ragni et al[36] demonstrated this in human immunodeficiency virus-positive hemophiliacs with CD4 counts of 200 mm3 or less undergoing orthopaedic surgery. Post-operative infection occurred in 10 (15.1%) of 66 patients[36]. Local steroid injection causing focal immunosuppression about the joint has also been shown to increase the risk, compared to those that have not received any joint injections in hip arthroplasty cases[37].
In rheumatoid patients treated with immunosuppressive drugs (including biologic agents) undergoing all orthopeadic procedures, a statistically significant higher risk of infection was seen in this patient cohort compared to a degenerative/post-traumatic group (OR = 2.58, 95%CI: 1.91-3.48, P < 0.001)[38]. Furthermore this risk was significantly increased in patients taking multiple disease-modifying antirheumatic drugs (DMARDs) (P = 0.036) or tumor necrosis factor α (TNFα) inhibitors (P = 0.032), especially if the last dose of TNFα inhibitor was given < 1 administration interval before surgery[38].
While not directed specifically to immunosuppression, other co-morbidities have a role in reducing the patients systemic response to infection. Cardiac dysfunction[4,16,21], renal failure[4,15], anaemia[4,9,16] and coagulopathy[16] have all been shown to increase the risk of infection. This may be directed through specific cellular pathways[39], but may demonstrate the insult the surgical procedures has in causing a secondary inflammatory insults, worsening multiple organ dysfunction[40,41].
Deranges in renal function, with progressively higher poor glomerular filtration rate (GFR) in either the acute or chronic stages, reduces the ability to remove unwanted and hazardous chemicals from the blood, and places the patient at a higher risk. Lieberman et al[42] demonstrated a high rates of infection in patients on chronic renal dialysis (19%), however in a separate patient series no significant increase in infection risk was seen[43].
We believe that if a patient is known to have systemic infection, or a localised infection but distant to the operative joint, the risk of haematological spread of infection to the implant is highly likely. We have demonstrated a statistically significant increased risk of PJI in patients with a pre-operative confirmation of a genitourinary infection[10,27], nasal S. Aureus and MRSA infections[31], or other distant organ infections[6], such as hepatitis B[44].
Conditions that further increase this risk are those that may make the patient more susceptible for the introduction of a new pathogen, such as chronic pulmonary disease[4,31] with known high rates of pneumonia, peripheral vascular disease[4,16] with high risk of skin ulceration and introduction of skin contaminates, and recent oesophagogastroduodenoscopy (EGD) with biopsy[22], risking the introduction of gut flora to the blood system.
Furthermore, perioperative blood transfusion increases the risk of PJI in both hip and knee arthroplasty[11,12,15], and allogeneic blood transfusion has been shown to instigate a detrimental immunomodulation reaction, and decreases T-cell-mediated immunity, and may enhance the acute inflammatory response[45,46]. Stored blood can cause a significant increase in inflammatory cytokine release from the stored neutrophils, and superoxide release results in delaying neutrophil apoptosis and risks cytotoxicity[47,48].
This has been confirmed in a recent systematic meta-analysis of 6 studies demonstrating the association between allogeneic blood transfusion and an increased risk for a SSI after total hip and knee arthroplasty. Data was included from over 20000 patients, and the blood transfusion group had a significantly higher frequency of infection (pooled OR = 1.71, 95%CI: 1.23-2.40, P = 0.002) compared to the non-exposed group[49].
The use of antibiotic-impregnated cement was shown by Dale et al[18] to protect against revisions due to infection, whereas Namba et al[20] identified an increased risk. Such conflicting outcomes are common in the literature regarding the use of antibiotic-impregnated cement in primary procedures. A prospective randomized study with 2948 cemented total knee arthroplasties failed to see an improvement of PJI rates by using bone cement loaded with erythromycin and colistin compared to controls[50], whereas the Norwegian Arthroplasty Register has demonstrated a synergistic effect of systemic and cement antibiotics[51]. However there is a general consensus that antibiotic-impregnated cement has a greater role in revision cases[52], and is recommended as standard practice in these high-risk cases[53].
Systemic antibiotics given at anaesthetic induction are generally the standard of care, and continued post-operatively for a further two doses in the United Kingdom, and for two days in Italy (authors experience). The choice of antibiotic varies in each Institute to reflect the prominent pathogen and patient cohort. Multiple studies have demonstrated the benefits of antibiotics given during the procedure to reduce the risk of post-operative infection[51,54].
Concerning the relative impact of the hospitals yearly volume of procedures, we found only one retrospective review of joint registry data, that suggests that the fewer total knee arthroplasties undertaken per year will result in a lower rate of infection[20]. This particular finding needs, in our opinion, further validation, since it contradicts other reports demonstrating better outcomes from greater volumes of surgery and greater experience of the surgeons, as exemplified by the Hospital for Special Surgery, New York[55], while other studies have shown no difference between the two[56].
Furthermore, the use of a drain post-operatively has been shown by Peel et al[15] to reduce the risk of PJI following knee arthroplasty, however multiple meta-analyses and prospective, randomised, controlled trials have demonstrated no significant difference in post-operative infections between the wounds treated with a drain and those without[57,58].
When the risk factors were further categorised into modifiable or not, the vast majority of factors were non-modifiable. Many risk factors increased a patient’s risk by less than 5 times (OR < 5), and very few increased the risk by more than 10 times.
However, the presence of non-modifiable risk factors still requires attention, and may be more important than modifiable ones. Alternate methods should be adopted to reduce the patient’s burden and may include a combination of implant modifications (such as silver or disposable microbiological coatings)[59,60], antibiotic impregnated cement or bone graft[61,62], or other novel therapies[63] to provide a personalized and more effective prophylaxis.
It is the responsibility of the operating team to act upon these, and modify or optimise the patient prior to surgery. For example, intensive insulin therapy, maintaining tight blood glucose concentrations between 80 and 110 mg/dL, has been shown to decrease infection-related complications and mortality[64]. Normal renal function should be sought, nutrition improved, cardiac investigations and interventions should be offered, local and systemic infections appropriately treated, as should chronic anaemia, and patients should be informed to withhold DMARDs and stop tobacco smoking and alcohol use preoperatively.
Indeed, determining individual patients risks is an important step in personalized informed consent. Surgeons may quote published rates or their own, but the risk is individual and should reflect all the aforementioned factors, which may have consequences in the medico-legal evaluation in case of damage evaluation after PJI.
Previous attempts to combine such measures in a scoring system have been attempted by The Mayo Clinic[65] who based the data on their cohort of patients at baseline and at one month. Bozic et al[35] developed a risk calculator using data from 11 years worth of Medicare claims. A similar tool has been developed in the Chinese population[26].
The main disadvantage of such tools is the calculations relate to a specific set of patients, and may not reflect the general public risks, as they have not been externally validated. In addition the data is unlikely to appreciate advances in perioperative care over the time period, and may not capture patients with late onset PJI if follow-up is short.
A wide variety of studies were included in this systematic review, which gives an overview of risk factors for hip and knee PJI but the quality of each study is generally poor. As previously discussed, only 8 studies (29.6%) were prospective, and one third of studies demonstrated record bias. Reporting bias was also seen amongst the studies, as a variety of diagnostic criteria were used. This is common amongst studies reviewing PJI as there is no gold standard measure to determine presence of infection, nor an agreement to the medical, or surgical management, for these patients[53].
Our search criteria only highlighted studies with “risk factor” in the title, and therefore we did not search for studies looking at individual risk factors. Therefore studies, some of high quality, may not have met our inclusion criteria. Furthermore, we were unable to undertake a meta-analysis due to the heterogeneity of the data.
In conclusion, as demonstrated, current data is conflicting as the influence of the risk factors vary widely, and we believe more emphasis is required regarding the multiplicity effects of risk factors. We need larger studies and novel tools to investigate single and combined risk factors, and to identify key areas of improvement and modification for these patients.
The literature has demonstrated significant variation in the number and type of risk factors that places a patient at higher risk of developing a PJI, which is heavily weighted towards the patient. However the provider has a role in addressing the modifiable risk factors pre-operatively to optimise their patient, and develop new strategies to limit the impact of non-modifiable factors.
Several studies have previously shown the impact of various risk factors on the probability of developing an infection after joint replacement. The heterogeneity of the available data notwithstanding, in this systematic review a detailed analysis of the respective weight of known risk factors, classified as host-, provider- or post-surgical-related, is performed; moreover, a further distinction in modifiable or not-modifiable risk factors is proposed.
A classification and ranking of known risk factors may open new frontiers in prevention and control of peri-prosthetic infections. Furthermore, it can be helpful to improve the information to the patient prior to surgery, to drive personalised prophylaxis and to better evaluate the cost-to-benefit ratio of new technologies, like antibacterial coatings, designed to reduce bacterial adhesion on implanted biomaterials.
This systematic review sheds new lights on the relative impact of various risk factors that increase a patient’s risk of developing lower limb periprosthetic joint infections (PJI). This ultimately reiterates the importance of optimising the patients pre-operatively by addressing modifiable risk factors (such as their immunosuppression, nutrition, diabetes, and smoking), and develops strategies to limit the impact of non-modifiable factors.
The data obtained in this systematic review may form the basis for the development of specific software, like the “PJI Risk App”, an application for smartphones, specifically designed to calculate the risk of developing a peri-prosthetic infection in a given patient. This in turn may be useful for surgeons and their patients to understand the specific risk of undergoing joint replacement and eventually to better tailor antibiotic prophylaxis.
In this manuscript authors reviewed provider risk factors of chronic PJI. This study is interesting and the objective very clear.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cui Q, Friedrich M, Lourtet-Hascoett J, Waddell BS S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Donaldson AD, Jalaludin BB, Chan RC. Patient perceptions of osteomyelitis, septic arthritis and prosthetic joint infection: the psychological influence of methicillin-resistant Staphylococcus aureus. Intern Med J. 2007;37:536-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61-65.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1101] [Cited by in F6Publishing: 1130] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 3. | George DA, Gil E, Morris-Jones S. Prevention Of Periprosthetic Joint Infections: Minimizing The Risks (2016). Periprosthetic Joint Infections: Changing Paradigms. Switzerland: Springer International Publishing 2016; Chapter 3. [Cited in This Article: ] |
| 4. | Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470:130-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | Berbari EF, Osmon DR, Carr A, Hanssen AD, Baddour LM, Greene D, Kupp LI, Baughan LW, Harmsen WS, Mandrekar JN. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis. 2010;50:8-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Tomás T. [Patient - related risk factors for infected total arthroplasty]. Acta Chir Orthop Traumatol Cech. 2008;75:451-456. [PubMed] [Cited in This Article: ] |
| 7. | George DA, Logoluso N, Castellini G, Gianola S, Scarponi S, Haddad FS, Drago L, Romano CL. Does cemented or cementless single-stage exchange arthroplasty of chronic periprosthetic hip infections provide similar infection rates to a two-stage? A systematic review. BMC Infect Dis. 2016;16:553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47017] [Cited by in F6Publishing: 43393] [Article Influence: 2892.9] [Reference Citation Analysis (0)] |
| 9. | Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247-1254. [PubMed] [Cited in This Article: ] |
| 10. | Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22:651-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 926] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 13. | Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24:105-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 15. | Peel TN, Dowsey MM, Daffy JR, Stanley PA, Choong PF, Buising KL. Risk factors for prosthetic hip and knee infections according to arthroplasty site. J Hosp Infect. 2011;79:129-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, Vail TP, Berry DJ. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94:794-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 17. | Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94:e101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 18. | Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, Pedersen AB, Kärrholm J, Garellick G, Pulkkinen P. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012;83:449-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Greenky M, Gandhi K, Pulido L, Restrepo C, Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthop Relat Res. 2012;470:2695-2701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95:775-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 21. | Somayaji R, Barnabe C, Martin L. Risk factors for infection following total joint arthroplasty in rheumatoid arthritis. Open Rheumatol J. 2013;7:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Coelho-Prabhu N, Oxentenko AS, Osmon DR, Baron TH, Hanssen AD, Wilson WR, Steckelberg JM, Baddour LM, Harmsen WS, Mandrekar J. Increased risk of prosthetic joint infection associated with esophago-gastro-duodenoscopy with biopsy. Acta Orthop. 2013;84:82-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Maoz G, Phillips M, Bosco J, Slover J, Stachel A, Inneh I, Iorio R. The Otto Aufranc Award: Modifiable versus nonmodifiable risk factors for infection after hip arthroplasty. Clin Orthop Relat Res. 2015;473:453-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Gómez-Lesmes SP, Tornero E, Martínez-Pastor JC, Pereira A, Marcos M, Soriano A. Length of storage of transfused red blood cells and risk of prosthetic joint infection after primary knee arthroplasty. J Arthroplasty. 2014;29:2016-2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Yi PH, Frank RM, Vann E, Sonn KA, Moric M, Della Valle CJ. Is potential malnutrition associated with septic failure and acute infection after revision total joint arthroplasty? Clin Orthop Relat Res. 2015;473:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Wu C, Qu X, Liu F, Li H, Mao Y, Zhu Z. Risk factors for periprosthetic joint infection after total hip arthroplasty and total knee arthroplasty in Chinese patients. PLoS One. 2014;9:e95300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Sousa R, Muñoz-Mahamud E, Quayle J, Dias da Costa L, Casals C, Scott P, Leite P, Vilanova P, Garcia S, Ramos MH. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis. 2014;59:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Jiang SL, Schairer WW, Bozic KJ. Increased rates of periprosthetic joint infection in patients with cirrhosis undergoing total joint arthroplasty. Clin Orthop Relat Res. 2014;472:2483-2491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Duchman KR, Gao Y, Pugely AJ, Martin CT, Noiseux NO, Callaghan JJ. The Effect of Smoking on Short-Term Complications Following Total Hip and Knee Arthroplasty. J Bone Joint Surg Am. 2015;97:1049-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 30. | Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is Hemoglobin A1c or Perioperative Hyperglycemia Predictive of Periprosthetic Joint Infection or Death Following Primary Total Joint Arthroplasty? J Arthroplasty. 2015;30:1197-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Crowe B, Payne A, Evangelista PJ, Stachel A, Phillips MS, Slover JD, Inneh IA, Iorio R, Bosco JA. Risk Factors for Infection Following Total Knee Arthroplasty: A Series of 3836 Cases from One Institution. J Arthroplasty. 2015;30:2275-2278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Debreuve-Theresette A, Diallo S, Siboni R, Ohl X, Dehoux E, Bajolet O. Infections in Total Hip and Total Knee Arthroplasty: Development of a Score To Assess Endogenous Risk of Surgical Site Infections. Surg Infect (Larchmt). 2015;16:794-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Bohl DD, Shen MR, Kayupov E, Della Valle CJ. Hypoalbuminemia Independently Predicts Surgical Site Infection, Pneumonia, Length of Stay, and Readmission After Total Joint Arthroplasty. J Arthroplasty. 2016;31:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 34. | Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992-2994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1212] [Cited by in F6Publishing: 1324] [Article Influence: 101.8] [Reference Citation Analysis (1)] |
| 35. | Bozic KJ, Ong K, Lau E, Berry DJ, Vail TP, Kurtz SM, Rubash HE. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471:574-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopaedic surgery in human immunodeficiency virus-infected hemophiliacs with CD4 counts & lt; or = 200/mm3. J Arthroplasty. 1995;10:716-721. [PubMed] [Cited in This Article: ] |
| 37. | Kaspar S, de V de Beer J. Infection in hip arthroplasty after previous injection of steroid. J Bone Joint Surg Br. 2005;87:454-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res (Hoboken). 2013;65:2032-2040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769-776. [PubMed] [Cited in This Article: ] |
| 40. | Waydhas C, Nast-Kolb D, Jochum M, Trupka A, Lenk S, Fritz H, Duswald KH, Schweiberer L. Inflammatory mediators, infection, sepsis, and multiple organ failure after severe trauma. Arch Surg. 1992;127:460-467. [PubMed] [Cited in This Article: ] |
| 41. | Waydhas C, Nast-Kolb D, Trupka A, Zettl R, Kick M, Wiesholler J, Schweiberer L, Jochum M. Posttraumatic inflammatory response, secondary operations, and late multiple organ failure. J Trauma. 1996;40:624-630; discussion 630-631. [PubMed] [Cited in This Article: ] |
| 42. | Lieberman JR, Fuchs MD, Haas SB, Garvin KL, Goldstock L, Gupta R, Pellicci PM, Salvati EA. Hip arthroplasty in patients with chronic renal failure. J Arthroplasty. 1995;10:191-195. [PubMed] [Cited in This Article: ] |
| 43. | Debarge R, Pibarot V, Guyen O, Vaz G, Carret JP, Bejui-Hugues J. [Total hip arthroplasty in patients with chronic renal failure transplant or dialysis]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:222-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Kuo SJ, Huang PH, Chang CC, Kuo FC, Wu CT, Hsu HC, Lin CC. Hepatitis B Virus Infection Is a Risk Factor for Periprosthetic Joint Infection Among Males After Total Knee Arthroplasty: A Taiwanese Nationwide Population-Based Study. Medicine (Baltimore). 2016;95:e3806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Innerhofer P, Luz G, Spötl L, Hobisch-Hagen P, Schobersberger W, Fischer M, Nussbaumer W, Lochs A, Irschick E. Immunologic changes after transfusion of autologous or allogeneic buffy coat-poor versus white cell-reduced blood to patients undergoing arthroplasty. I. Proliferative T-cell responses and the balance of helper and suppressor T cells. Transfusion. 1999;39:1089-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Avall A, Hyllner M, Bengtson JP, Carlsson L, Bengtsson A. Postoperative inflammatory response after autologous and allogeneic blood transfusion. Anesthesiology. 1997;87:511-516. [PubMed] [Cited in This Article: ] |
| 47. | Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13:29-33. [PubMed] [Cited in This Article: ] |
| 48. | Biffl WL, Moore EE, Zallen G, Johnson JL, Gabriel J, Offner PJ, Silliman CC. Neutrophils are primed for cytotoxicity and resist apoptosis in injured patients at risk for multiple organ failure. Surgery. 1999;126:198-202. [PubMed] [Cited in This Article: ] |
| 49. | Kim JL, Park JH, Han SB, Cho IY, Jang KM. Allogeneic Blood Transfusion Is a Significant Risk Factor for Surgical-Site Infection Following Total Hip and Knee Arthroplasty: A Meta-Analysis. J Arthroplasty. 2017;32:320-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 50. | Hinarejos P, Guirro P, Leal J, Montserrat F, Pelfort X, Sorli ML, Horcajada JP, Puig L. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am. 2013;95:769-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590-595. [PubMed] [Cited in This Article: ] |
| 52. | Chiu FY, Lin CF. Antibiotic-impregnated cement in revision total knee arthroplasty. A prospective cohort study of one hundred and eighty-three knees. J Bone Joint Surg Am. 2009;91:628-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Parvizi J, Gehrke T. Proceedings Of The International Consensus Meeting On Periprosthetic Joint Infection. Philadelphia Consensus. EFORT. 2013;. [Cited in This Article: ] |
| 54. | Meehan J, Jamali AA, Nguyen H. Prophylactic antibiotics in hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91:2480-2490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 55. | Hospital-Acquired Infection (HAI) Rates in New York State Hospitals. New York State Department of Health. Available from: Http://Www.Health.State.Ny.Us/Statistics/Facilities/Hospital. [Cited in This Article: ] |
| 56. | Kreder HJ, Grosso P, Williams JI, Jaglal S, Axcell T, Wal EK, Stephen DJ. Provider volume and other predictors of outcome after total knee arthroplasty: a population study in Ontario. Can J Surg. 2003;46:15-22. [PubMed] [Cited in This Article: ] |
| 57. | Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2004;86-A:1146-1152. [PubMed] [Cited in This Article: ] |
| 58. | Walmsley PJ, Kelly MB, Hill RM, Brenkel I. A prospective, randomised, controlled trial of the use of drains in total hip arthroplasty. J Bone Joint Surg Br. 2005;87:1397-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Fiedler J, Kolitsch A, Kleffner B, Henke D, Stenger S, Brenner RE. Copper and silver ion implantation of aluminium oxide-blasted titanium surfaces: proliferative response of osteoblasts and antibacterial effects. Int J Artif Organs. 2011;34:882-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Drago L, Boot W, Dimas K, Malizos K, Hänsch GM, Stuyck J, Gawlitta D, Romanò CL. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res. 2014;472:3311-3323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 61. | Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 62. | Winkler H, Stoiber A, Kaudela K, Winter F, Menschik F. One stage uncemented revision of infected total hip replacement using cancellous allograft bone impregnated with antibiotics. J Bone Joint Surg Br. 2008;90:1580-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | George DA, Gant V, Haddad FS. The management of periprosthetic infections in the future: a review of new forms of treatment. Bone Joint J. 2015;97-B:1162-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Butler SO, Btaiche IF, Alaniz C. Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy. 2005;25:963-976. [PubMed] [Cited in This Article: ] |
| 65. | Berbari EF, Osmon DR, Lahr B, Eckel-Passow JE, Tsaras G, Hanssen AD, Mabry T, Steckelberg J, Thompson R. The Mayo prosthetic joint infection risk score: implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol. 2012;33:774-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |