Published online Jul 18, 2014. doi: 10.5312/wjo.v5.i3.171
Revised: April 2, 2014
Accepted: May 16, 2014
Published online: July 18, 2014
Symptomatic chondral or osteochondral defects of the talus reduce the quality of life of many patients. Although their pathomechanism is well understood, it is well known that different aetiologic factors play a role in their origin. Additionally, it is well recognised that the talar articular cartilage strongly differs from that in the knee. Despite this fact, many recommendations for the management of talar cartilage defects are based on approaches that were developed for the knee. Conservative treatment seems to work best in paediatric and adolescent patients with osteochondritis dissecans. However, depending on the size of the lesions, surgical approaches are necessary to treat many of these defects. Bone marrow stimulation techniques may achieve good results in small lesions. Large lesions may be treated by open procedures such as osteochondral autograft transfer or allograft transplantation. Autologous chondrocyte transplantation, as a restorative procedure, is well investigated in the knee and has been applied in the talus with increasing popularity and promising results but the evidence to date is poor. The goals of the current article are to summarise the different options for treating chondral and osteochondral defects of the talus and review the available literature.
Core tip: The goals of the current article are to summarise the different options for treating chondral and osteochondral defects of the talus and review the available literature.
- Citation: Baums MH, Schultz W, Kostuj T, Klinger HM. Cartilage repair techniques of the talus: An update. World J Orthop 2014; 5(3): 171-179
- URL: https://www.wjgnet.com/2218-5836/full/v5/i3/171.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i3.171
In contrast to other joints of the lower extremity, chondral and osteochondral lesions of the talus are frequently being recognised as being caused by traumata. The impact of shear and compression forces causes a cartilage contusion and is often transmitted to the subchondral bone, thus causing subchondral microfractures. In addition to trauma other causes include endocrine or metabolic factors genetic predisposition, vascular or synovial abnormalities, localised hyperpressure, or chronic microtrauma[1-3].
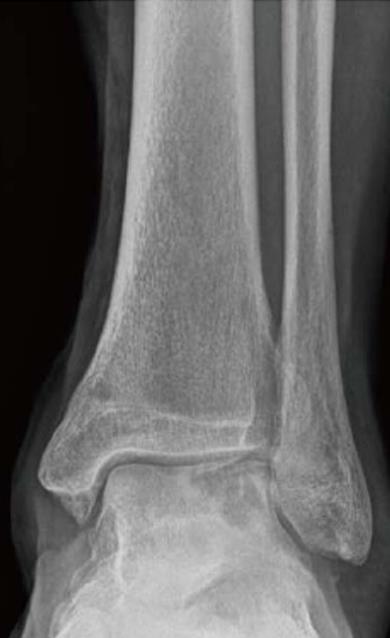
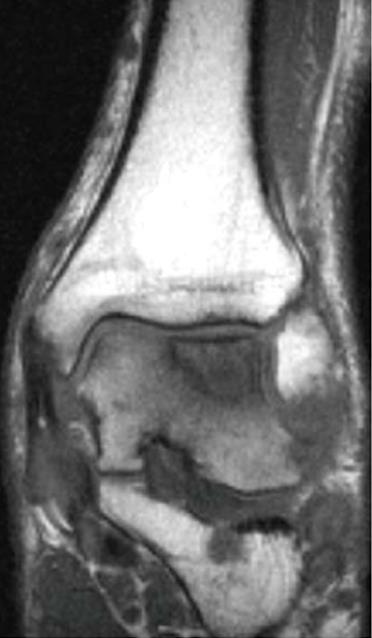
Irrespective of their aetiology, these lesions remain important problems (Figure 1), a consequence of the limited reparative potential of human cartilages. During repair, the cartilage usually produces a fibrocartilaginous tissue that has inferior mechanical properties and may deteriorate gradually[4]. For these lesions, diverse treatment options have been published in the last decades[5-10].
The goals of the current article are to summarise the different options for treating chondral and osteochondral defects of the talus and review the available literature.
Many recommendations for the management of talar cartilage defects are based on approaches for the knee. However, some well-known and important attributes clearly distinguish the cartilage of the talus from other cartilage, especially from that of the knee joint.
First, the ankle is a highly congruent joint, which is important to know when using different methods for cartilage repair, such as autologous osteochondral transplantation. Additionally, the nature of the joint will affect the development of pain in osteochondral defects of the talus[11]. Of note, the average thickness of the talar articular cartilage is approximately 0.89 mm whereas knee cartilage thickness reaches 6 mm[12,13]. Moreover, the tensile stiffness of healthy talar cartilage has only minimal topographical variability and the dynamical stiffness is higher than in the knee[14,15]. A further difference is the lower contact area and the lack of absorbability that makes the cartilage able to tolerate higher maximum loads[16]. Additionally, its metabolic activity appears to be greater than that of the knee, with a higher turnover as well as a higher level of proteoglycan synthesis[16].
Finally, the capability to maintain its mechanical properties more successfully during ageing appears to be more favourable in the talar articular cartilage compared to other joints[17].
The intended purpose of a non-operative approach is to unload the injured cartilage and thereby allow the subchondral oedema to resolve, prevent osseous necrosis, or enable healing of a minimal detached fragment. Unfortunately, the reasons for choosing this treatment are not always clearly described[18]. Additionally, the overall results of the non-operative treatment of cartilage lesions of the talus indicate only a low success rate[19,20].
Despite this fact, conservative management may be considered and favourable for some types of lesions. Non-operative treatment is appropriate in fresh cartilage injuries that are non-displaced and have a potential for healing, depending on their size and location as well as on patient parameters, such as age, socio-professional context, or smoking[1]. Asymptomatic lesions, minimally symptomatic lesions that involve cartilage alone or show an intact cartilage surface, and low-grade osteochondritis dissecans lesions in children may recover using temporarily protected weight-bearing with or without joint immobilisation[1,3,21].
Marrow stimulation techniques: Human articular cartilage has a limited reparative capability because of its avascularity, among other reasons. Although the basic purpose of the surgical treatment is to re-vascularise the bony defect, many cartilage defects of the talus can be treated arthroscopically using bone marrow stimulation methods involving drilling or microfracture.
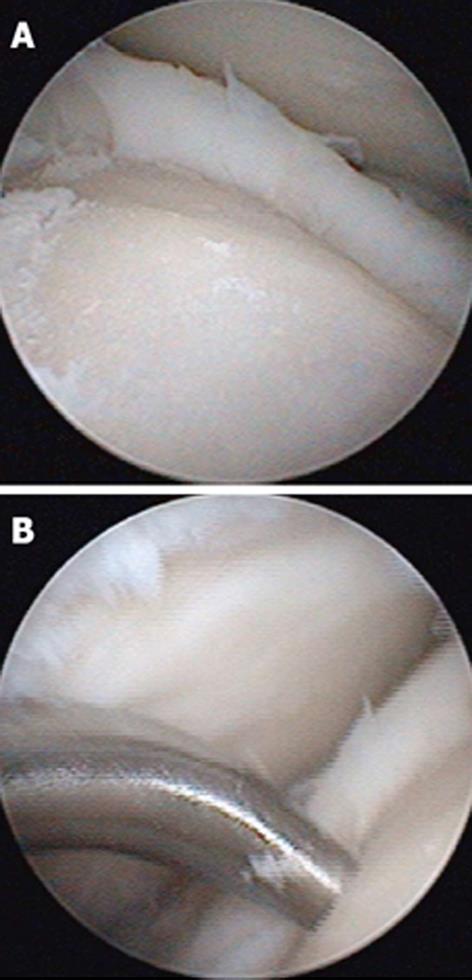
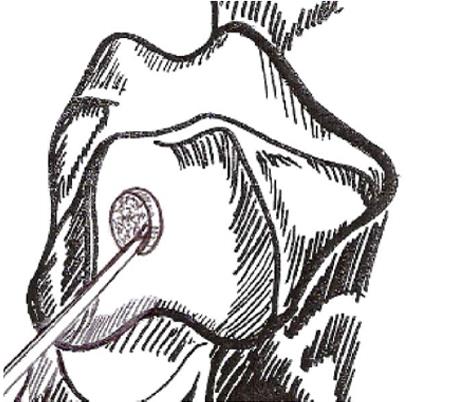
These techniques attempt to promote the development of a fibrocartilageous formation over the defect, which may suffice for small lesions. The principle is to breach the subchondral plate at multiple intervals to allow the subsequent inflow of serum factors as well as to stimulate chondroprogenitor cells of the marrow into the base of the defect site[22] (Figure 2A and B). The release of fatty drops from the created fracture apertures provides a clinical indicator that the depth of the microfracture is adequate. To remove the calcified layer and to obtain stable edges of vital cartilage, it is recommended that the procedure be supplemented by excision and curettage[23,24] (Figure 3).
Of note, a recent study of 2nd look arthroscopy at 12 mo postoperatively revealed incomplete healing of osteochondral lesions treated using these techniques in 40% of the patients[25]. Interestingly, good clinical results were achieved, which agrees with most series demonstrating pain relief and optimisation of function[26-28]. O’Driscoll[29] summarised that this technique may be best for the treatment of small (< 6 mm), shear-type lesions with minimal subchondral involvement.
Increased age has been considered to be an independent risk factor for a poor outcome, but has not been confirmed by recent studies[27,30]. In contrast, a higher body mass index, a history of trauma, and the presence of degenerative changes will certainly worsen the outcome[5,27]. Moreover, the defect’s size is a predictor of clinical outcome: a defect dimension larger than 150 mm2 appears to result in a significantly higher failure rate[5,31].
Autologous osteochondral transplantation: The uncertain value of bone marrow stimulation techniques for defects larger than 150 mm2 has encouraged the search for alternative resurfacing procedures, such as autologous osteochondral transplantation. This technique was developed principally to treat focal cartilage defects of the knee[32].
This procedure involves autologous grafting using one or more cylindrical components consisting of cartilage and its underlying bone. The components were harvested from a less weight-bearing part of the femur of the ipsilateral knee. Hangody et al[8] introduced this mosaicplasty to treat large cartilage defects using a one-step procedure. This can be performed using an open approach or, in special cases, arthroscopically. The size of the defect determines whether more than one osteochondral plug is needed: the plugs may vary in size and are placed in a side-by-side configuration into the prepared defect site. Distinctive cystic lesions could be treated using the osteochondral autograft transfer system (OATS)[3]. Several authors reported favourable results based on short- to mid-term follow-up[8,33-35]. Good results may be expected for a moderate talar dome defect of approximately 2 cm2 in size and more than 5 mm in depth[36]. Others recommend this treatment for lesions that are 4 cm2 or smaller[3].
In contrast to bone marrow stimulation the aim of osteochondral transplantation techniques is to resurface the defect with a viable hyaline cartilage. Therefore, this procedure attempts to reproduce the mechanical, structural, and biomechanical characteristics of the primary hyaline talar cartilage[18].
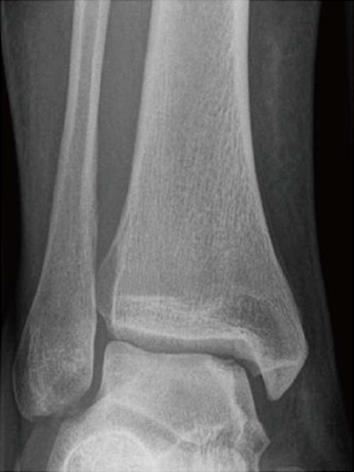
Despite these advantages, some disadvantages must be considered when planning osteochondral autografts. Only a circumscribed surface can be treated anatomically due to the limited number of suitable donor sites, which is primarily due to differences in the surface curvature between the graft and the host tissue[4] (Figure 4). Additionally, restoring lesions of the talar shoulder can be difficult[17]. Any type of surface incongruity or irregularity caused by differences in thicknesses of the grafts or differences between the size of the graft and the size of the defect should be carefully avoided. These surface differences often result in an uneven surface or the development of “dead spaces” between each graft that is filled only with a fibrous regrind. Therefore, circular lesions could often be resurfaced better than elliptical defects[17].
Based on the location of the lesion and depending on the approach needed a malleolar osteotomy is necessary. In some patients the use of an osteotomy may worsen the clinical outcome and affect the potential benefit of cartilage resurfacing[37], but this does not appear to cause widespread concern[38]. Several techniques were described for performing the osteotomy[39]. However, the surgeons have to be aware of potentially related problems. First, it is essential to be conscious of a proper level to avoid violating the articular surface as well as to gain optimal visibility of the defect[40]. Second, one must focus on a precise reduction and sufficient fixation to avoid a fibrous non-union or malunion[3]. For example, Lamb et al[41] described a chevron-type medial malleolar osteotomy that appears safe and reduces the risk of non-union. At a median follow-up of 34.5 mo 94% of the patients were non-symptomatic. The median time to radiographic healing was six weeks.
Donor-site knee morbidity could pose problems for patients, but it is not discussed in any of the published series[17]. Therefore, some authors suggest harvesting the osteochondral plugs from the talus itself to avoid donor-site knee pain, stiffness, or even arthritic changes[42]. Two series specifically addressed donor-site morbidity[43,44]. In a retrospective study of 11 patients, Reddy et al[44] showed that the number of grafts obtained had no effect on clinical outcome. Paul et al[43] found that a high body-mass index influenced the outcome score negatively.
Osteochondral allograft transplantation: The use of fresh osteochondral allografts is a different technique especially designed to reconstruct massive osteochondral defects that have substantial loss or cystic degeneration of subchondral bone[40] (Figure 5). Indications for choosing this method for reconstruction are similar to those for osteochondral autologous transplantation, but without limitations based on size[36]. In patients with severe tibiotalar arthritis, the use of bipolar osteochondral allografts has been described[45].
In osteochondral allografts, a cadaver graft, consisting of both articular cartilage and its underlying bone, is transplanted into the defect site. An advantage of this technique is that the transplanted allograft can be tailored to match the shape of the defect precisely, which is particularly necessary due to the above-mentioned high congruity of the ankle joint. Therefore, even severe defects that involve the talar shoulder can be treated successfully[46]. Regardless, a malleolar osteotomy is required in some cases. A viable articular cartilage is provided and graft harvesting from a healthy knee joint is not needed; these are other advantages of this method.
Nevertheless, the success of such allografts is related to the percentage of chondrocytes that remain viable after graft procurement[47]. The storage of a fresh human allograft for more than fourteen days was revealed to substantially decrease the viability, cell density, metabolic activity of the chondrocytes, and lead to an approximately 30% decrease in viable chondrocytes after 28 d[47,48]. Despite these drawbacks, the biomechanical characteristics appear not to be affected by storage for this time interval[39]. However, many tissue banks need almost one month for screening to minimise the risk of disease transmission via the graft[36]. To date, the authors are not aware of any viral transmission via such allografts; however, the screening period is necessary and patients have to be informed of this hypothetical risk.
An immunologic reaction that adversely affects the chondrocytes, the limited availability of grafts, and the acceptance of costs may be further disadvantages[47]. Several authors have investigated the treatment of large osteochondral defects of the talus using osteochondral allograft transplantation in case series[7,46,49-52]. The overall clinical results were promising, especially considering the size of the defects. However, in certain of these studies, only a few patients were reported to be symptom-free[51]: some patients needed further surgical treatment, or the procedure failed[46,49,51].
In summary, the evidence for the use of osteochondral allograft transplantation has to be interpreted carefully. Most series included a small number of patients, studied patients retrospectively, had only a short- or mid-term follow-up, or presented no description of the underlying size of the defect[7,46,49,50,52,53]. Additionally, in several of these investigations, patients were lost to follow-up or were excluded because of graft failure[46,50,52].
Autologous chondrocyte transplantation/ implantation: Brittberg et al[54] implemented the technique of autologous chondrocyte transplantation in 1987. The first results were published in 1994 after treating chondral defects of the knee with this technique. Since then, it has become a promising tool for the repair of cartilage defects. Several long-term trials have provided strong evidence of the efficacy of this procedure, primarily studying its application in the knee[55-57]. Young patients suffering from a single focal cartilage defect with only a short duration of symptoms should expect good results[58]. However, to our best knowledge, equivalent data do not exist regarding the treatment of the talus. Additionally, a clearly recommendation regarding the defect size in which this procedure works best cannot be given: reported defect sized vary between 2 cm2 and 12 cm2[59].
Autologous chondrocyte transplantation (ACT) is a cell-based, two-stage procedure that involves the transplantation of viable and cultured chondrocytes into a defect. In the first step, cartilaginous material is harvested from the knee or the ankle itself[10,40]. In some cases, the cartilage was harvested from a detached osteochondral fragment without any reported adverse effect on the chondrocytes’ viability[60]. Usually, the second-stage of the procedure is performed after three to four weeks of cell culturing.
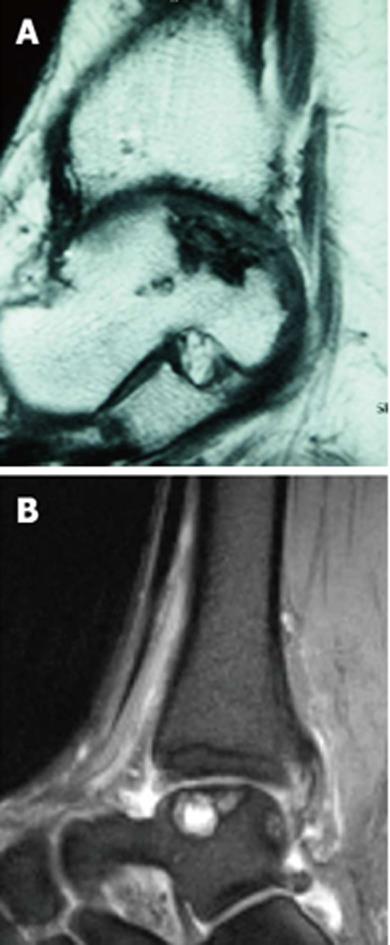
The aim of ACT is to promote the development of a regrind that meets the requirements of human hyaline cartilage or, at best, will facilitate a hyaline-like repair tissue. The ideal indication for an ACT is a full-thickness cartilage defect with an intact subchondral plate with stable edges of the surrounding cartilage[59]. The conditions for its application do not differ from that of the above-mentioned techniques: all pathologic cartilage should be carefully debrided to achieve vertical and stable edges surrounding the defect[10,61]. In case of an osseous deficiency (Figures 6 and 7), concomitant bone-grafting is suggested to provide a sufficient bony base[61]. Indications and contraindications are summarised in Table 1.
| Indication | Contraindication |
| symptomatic full-thickness chondral/osteochondral lesions | Osteoarthritis/rheumatoid arthritis |
| focal lesion > 1.5 cm2 in size | so-called kissing lesions |
| lesion with necrotic bone/fibrous tissue base | ligamentous instability (can be corrected in conjunction with the ACT procedure) |
| failed previous traditional surgery | axial malalignment |
| (i.e., drilling or microfracture) | (should be previously corrected) |
| patients younger than 45 yr of age | children/teenagers |
| patients older than 45 yr of age |
A method using a periosteum-covered ACT is called the first generation of this technique. A periosteal flap is harvested, i.e., from the distal part of the tibia, and then placed over the defect with the cambium layer facing toward the aforementioned prepared bed[40,61]. Then, the cultured cell suspension is injected beneath the sutured flap. However, this technically demanding procedure induced complications, such as delamination, uneven distribution of cells within the defect, cell leakage, or periosteal hypertrophy[38].
Due to these complications, a second generation of ACT, using matrix-associated techniques, was developed. In matrix-induced autologous chondrocyte implantation/transplantation (MACI/MACT), cells are embedded into a bioabsorbable, porcine type-I/III collagen membrane[62]. In the second stage of the procedure this membrane is placed over talar cartilage defect. Advantages of MACI/MACT are the avoidance of periosteal graft harvesting and a more even cell distribution potentially delivering more viable cells to the defect[17].
Furthermore, a third-generation of ACT, a three-dimensional, biomaterial-free MACT with chondrospheres, is available[63]. To apply it entirely arthroscopically and therefore reduce morbidity is a further advantage. However, to date, it is unclear whether the chondrospheres will remain securely in the defect because they are placed without coverage.
Analysing of the literature reveals various trials of ACT of the talus[4,40,43,63,64]. Although, many of the reports publicised promising results, the available evidence is of poor quality. A recent meta-analysis showed that many publications address ACT of the talus[65]. However, only 16 of 54 studies could be included in this systematic review. Due to the use of several products for ACT, several “generations” of ACT, the low case numbers, inhomogeneous indications, and the use of different outcome parameters, it was not possible to draw any conclusion about what type of ACT is superior[65]. Additionally, there were no controlled studies available. Therefore, a safe and significant superiority of other techniques of cartilage repair could not be estimated until now.
Further methods to optimise techniques for cartilage repair have been introduced, but most of them are in the early stages of development or are only described in isolated case series. In summary, there is insufficient evidence to support recommending their use. However, they are mentioned below for completeness.
Mesenchymal stem cells may be able to differentiate into articular cartilage and may be used as an adjunct to microfracture treatment[6]. However, to date, the only relevant investigations were either animal or uncontrolled trials[66,67].
Additionally, platelet-rich plasma (PRP) may function as a scaffold for cultured cells and provide a reservoir of growth-stimulating factors[9,68].
Finally, viscosupplementation therapy using of hyaluronic acid has great popularity despite the lack of convincing outcomes[3]. In a recent study, after arthroscopic debridement and microfracture in osteochondral defects of the talus, hyaluronic acid was added postoperatively. Functional and pain scores were significantly improved compared to the group treated with microfracture alone[53].
In summary, no technique appears to be superior to the others, and treatment of chondral/osteochondral lesions of the talus remains controversial. Patients should be analysed rigorously. Before selecting an appropriate procedure, the socio-professional context and the patient’s compliance, as well as the characteristics of the patients job-related or sports activities, have to be considered.
Based on the evidence available as well as our own experience we agree with others that, depending on the lesion’s size, arthroscopic treatment using marrow stimulation and debridement may be a reasonable strategy to treat these lesions effectively[3,18,38]. Therefore, this approach can be recommended as first-line treatment.
For larger lesions, autologous osteochondral transplantation can be utilised as primary treatment with good success as well. Moreover, it can be recommended as second-line treatment in cases in which the bone marrow stimulation technique fails.
Patients with large-volume or cystic lesions who cannot be treated with the standard autograft procedures due to evidence of poor quality results, should be chosen for osteochondral allograft transplantation carefully.
Finally, autologous chondrocyte transplantation techniques should be individualised and applied to cautiously selected patients in whom the above-mentioned first-line treatment methods have failed. Table 2 gives an overview about the different treatment options.
| Procedure | Concept | Indication | Potential Advantage | Worth knowing | Evidence |
| Conservative | Unload injured cartilage | Low-grade OD in children | Healing without surgical risk | Results in literature low but recommended first-line treatment in low-grade lesions | Poor |
| Marrow stimulation techniques | Recruits mesenchymal stem cells from bone marrow Stimulates differentiation of repair tissue | Lesions < 150 mm2 with none/minimal subchondral involvement | Can be administered arthroscopically Can be done repeatedly | Fibrocartilaginous repair tissue Results deteriorate over time | Fair |
| Autologous osteochondral transplantation | Resurfaces defect with viable hyaline cartilage + underlying bone | Osteochondral defects (2-4 cm2) | Reproduces mechanical, structural, biomechanical characteristics of primary cartilage One-stage procedure | Donor site morbidity Potential need for osteotomy | Fair |
| Osteochondral allograft transplantation | Resurfaces defect with viable hyaline cartilage + underlying bone | Large-volume/ cystic lesions | No limitations based on size of defect One-stage procedure | Potential decrease in viable chondrocytes due to disease screening | Poor |
| Autologous chondrocyte transplantation (ACT) | Cultured chondrocyte-like cells will stimulate a hyaline-like repair tissue | Second-line treatment in large defects (> 2 cm2) | Nearly perfect fit with defect (no ”dead spaces”) | Adverse effects of 1st generation MACT with better cell distribution Osseous defect has to be grafted before ACT | Poor |
| Further treatment options (hyaluronic acid, PRP, mesenchymal stem cells) | Not clear May function as an biological adjunct | Not clear May be added to repair techniques | Not clear May improve final outcome | Mode of function not completely understood | Insufficient |
P- Reviewer: Niyibizi C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Laffenêtre O. Osteochondral lesions of the talus: Current concept. Orthop Traumatol Surg Res. 2010;96:554-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1979;131-136. [PubMed] [Cited in This Article: ] |
| 3. | O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Giannini S, Buda R, Grigolo B, Vannini F. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001;22:513-517. [PubMed] [Cited in This Article: ] |
| 5. | Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-1937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22:385-391. [PubMed] [Cited in This Article: ] |
| 8. | Hangody L, Kish G, Kárpáti Z, Szerb I, Eberhardt R. Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique--a preliminary report. Foot Ankle Int. 1997;18:628-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 161] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Munirah S, Samsudin OC, Chen HC, Salmah SH, Aminuddin BS, Ruszymah BH. Articular cartilage restoration in load-bearing osteochondral defects by implantation of autologous chondrocyte-fibrin constructs: an experimental study in sheep. J Bone Joint Surg Br. 2007;89:1099-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8:291-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc. 2010;18:570-580. [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Al-Ali D, Graichen H, Faber S, Englmeier KH, Reiser M, Eckstein F. Quantitative cartilage imaging of the human hind foot: precision and inter-subject variability. J Orthop Res. 2002;20:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J Biomech. 1991;24:761-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 211] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Swann AC, Seedhom BB. The stiffness of normal articular cartilage and the predominant acting stress levels: implications for the aetiology of osteoarthrosis. Br J Rheumatol. 1993;32:16-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 99] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Aurich M, Venbrocks RA, Fuhrmann RA. [Autologous chondrocyte transplantation in the ankle joint. Rational or irrational?]. Orthopade. 2008;37:188, 190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Mitchell ME, Giza E, Sullivan MR. Cartilage transplantation techniques for talar cartilage lesions. J Am Acad Orthop Surg. 2009;17:407-414. [PubMed] [Cited in This Article: ] |
| 18. | Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 372] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 19. | Shearer C, Loomer R, Clement D. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int. 2002;23:651-654. [PubMed] [Cited in This Article: ] |
| 20. | Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21:119-126. [PubMed] [Cited in This Article: ] |
| 21. | Zengerink M, Szerb I, Hangody L, Dopirak RM, Ferkel RD, van Dijk CN. Current concepts: treatment of osteochondral ankle defects. Foot Ankle Clin. 2006;11:331-359, vi. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532-553. [PubMed] [Cited in This Article: ] |
| 23. | Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34:1824-1831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Takao M, Uchio Y, Kakimaru H, Kumahashi N, Ochi M. Arthroscopic drilling with debridement of remaining cartilage for osteochondral lesions of the talar dome in unstable ankles. Am J Sports Med. 2004;32:332-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Lee KB, Bai LB, Yoon TR, Jung ST, Seon JK. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37 Suppl 1:63S-70S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26:583-589. [PubMed] [Cited in This Article: ] |
| 27. | Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:656-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22:1085-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795-1812. [PubMed] [Cited in This Article: ] |
| 30. | Choi WJ, Kim BS, Lee JW. Osteochondral lesion of the talus: could age be an indication for arthroscopic treatment? Am J Sports Med. 2012;40:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37:1974-1980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 32. | Hangody L, Kárpáti Z. [New possibilities in the management of severe circumscribed cartilage damage in the knee]. Magy Traumatol Ortop Kezseb Plasztikai Seb. 1994;37:237-243. [PubMed] [Cited in This Article: ] |
| 33. | Al-Shaikh RA, Chou LB, Mann JA, Dreeben SM, Prieskorn D. Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int. 2002;23:381-389. [PubMed] [Cited in This Article: ] |
| 34. | Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84:237-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Hangody L, Kish G, Módis L, Szerb I, Gáspár L, Diószegi Z, Kendik Z. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22:552-558. [PubMed] [Cited in This Article: ] |
| 36. | Kadakia AR, Espinosa N. Why allograft reconstruction for osteochondral lesion of the talus? The osteochondral autograft transfer system seemed to work quite well. Foot Ankle Clin. 2013;18:89-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Henle P, Niemeyer P. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006;34:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95:1045-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 39. | Verghese N, Morgan A, Perera A. Osteochondral lesions of the talus: defining the surgical approach. Foot Ankle Clin. 2013;18:49-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am. 2006;88:303-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Lamb J, Murawski CD, Deyer TW, Kennedy JG. Chevron-type medial malleolar osteotomy: a functional, radiographic and quantitative T2-mapping MRI analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21:1283-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int. 2002;23:693-698. [PubMed] [Cited in This Article: ] |
| 43. | Paul J, Sagstetter A, Kriner M, Imhoff AB, Spang J, Hinterwimmer S. Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J Bone Joint Surg Am. 2009;91:1683-1688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 44. | Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80-85. [PubMed] [Cited in This Article: ] |
| 45. | Bugbee WD, Khanna G, Cavallo M, McCauley JC, Görtz S, Brage ME. Bipolar fresh osteochondral allografting of the tibiotalar joint. J Bone Joint Surg Am. 2013;95:426-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Adams SB, Viens NA, Easley ME, Stinnett SS, Nunley JA. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93:648-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306. [PubMed] [Cited in This Article: ] |
| 48. | Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111-2120. [PubMed] [Cited in This Article: ] |
| 49. | Berlet GC, Hyer CF, Philbin TM, Hartman JF, Wright ML. Does fresh osteochondral allograft transplantation of talar osteochondral defects improve function? Clin Orthop Relat Res. 2011;469:2356-2366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93:1634-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94:1105-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 52. | Raikin SM. Stage VI: massive osteochondral defects of the talus. Foot Ankle Clin. 2004;9:737-744, vi. [PubMed] [Cited in This Article: ] |
| 53. | Doral MN, Bilge O, Batmaz G, Donmez G, Turhan E, Demirel M, Atay OA, Uzumcugil A, Atesok K, Kaya D. Treatment of osteochondral lesions of the talus with microfracture technique and postoperative hyaluronan injection. Knee Surg Sports Traumatol Arthrosc. 2012;20:1398-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895. [PubMed] [Cited in This Article: ] |
| 55. | Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-2112. [PubMed] [Cited in This Article: ] |
| 56. | Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 561] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 57. | Vasiliadis HS, Wasiak J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2010;CD003323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88:61-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 59. | Vanlauwe J, Almqvist F, Bellemans J, Huskin JP, Verdonk R, Victor J. Repair of symptomatic cartilage lesions of the knee: the place of autologous chondrocyte implantation. Acta Orthop Belg. 2007;73:145-158. [PubMed] [Cited in This Article: ] |
| 60. | Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13:601-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. The surgical technique of autologous chondrocyte transplantation of the talus with use of a periosteal graft. Surgical technique. J Bone Joint Surg Am. 2007;89 Suppl 2 Pt.2:170-182. [PubMed] [Cited in This Article: ] |
| 62. | Jones CW, Willers C, Keogh A, Smolinski D, Fick D, Yates PJ, Kirk TB, Zheng MH. Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J Orthop Res. 2008;26:292-303. [PubMed] [Cited in This Article: ] |
| 63. | Thermann H, Driessen A, Becher C. [Autologous chondrocyte transplantation in the treatment of articular cartilage lesions of the talus]. Orthopade. 2008;37:232-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Aurich M, Bedi HS, Smith PJ, Rolauffs B, Mückley T, Clayton J, Blackney M. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 65. | Niemeyer P, Salzmann G, Schmal H, Mayr H, Südkamp NP. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc. 2012;20:1696-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 66. | Han SH, Kim YH, Park MS, Kim IA, Shin JW, Yang WI, Jee KS, Park KD, Ryu GH, Lee JW. Histological and biomechanical properties of regenerated articular cartilage using chondrogenic bone marrow stromal cells with a PLGA scaffold in vivo. J Biomed Mater Res A. 2008;87:850-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Kennedy JG, Murawski CD. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: surgical technique. Cartilage. 2011;2:327-336. [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Brehm W, Aklin B, Yamashita T, Rieser F, Trüb T, Jakob RP, Mainil-Varlet P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthritis Cartilage. 2006;14:1214-1226. [PubMed] [Cited in This Article: ] |