Published online Jul 18, 2023. doi: 10.5312/wjo.v14.i7.547
First decision: March 1, 2023
Revised: March 9, 2023
Accepted: June 12, 2023
Article in press: June 12, 2023
Published online: July 18, 2023
Presepsin is an emerging biomarker in the diagnosis of sepsis. In the field of orthopaedics, it could be useful in diagnosing and managing periprosthetic joint infections.
To define the normal postoperative presepsin plasmatic curve, in patients undergoing primary cementless total hip arthroplasty (THA).
Patients undergoing primary cementless THA at our Institute were recruited. Inclusion criteria were: Primary osteoarthritis of the hip; urinary catheter time of permanence < 24 h; peripheral venous cannulation time of permanence < 24 h; no postoperative homologous blood transfusion administration and hospital stay ≤ 8 d. Exclusion criteria were: The presence of other articular prosthetic replacement or bone fixation devices; chronic inflammatory diseases; chronic kidney diseases; history of recurrent infections or malignant neoplasms; previous surgery in the preceding 12 mo; diabetes mellitus; immunosuppressive drug or corticosteroid assumption. All the patients received the same antibiotic prophylaxis. All the THA were performed by the same surgical and anaesthesia team; total operative time was defined as the time taken from skin incision to completion of skin closure. At enrollment, anthropometric data, smocking status, osteoarthritis stage according to Kellgren and Lawrence, Harris Hip Score, drugs assumption and comorbidities were recorded. All the patients underwent serial blood tests, including complete blood count, presepsin (PS) and C-reactive protein 24 h before arthroplasty and at 24, 48, 72 and 96 h postoperatively and at 3, 6 and 12-mo follow-up.
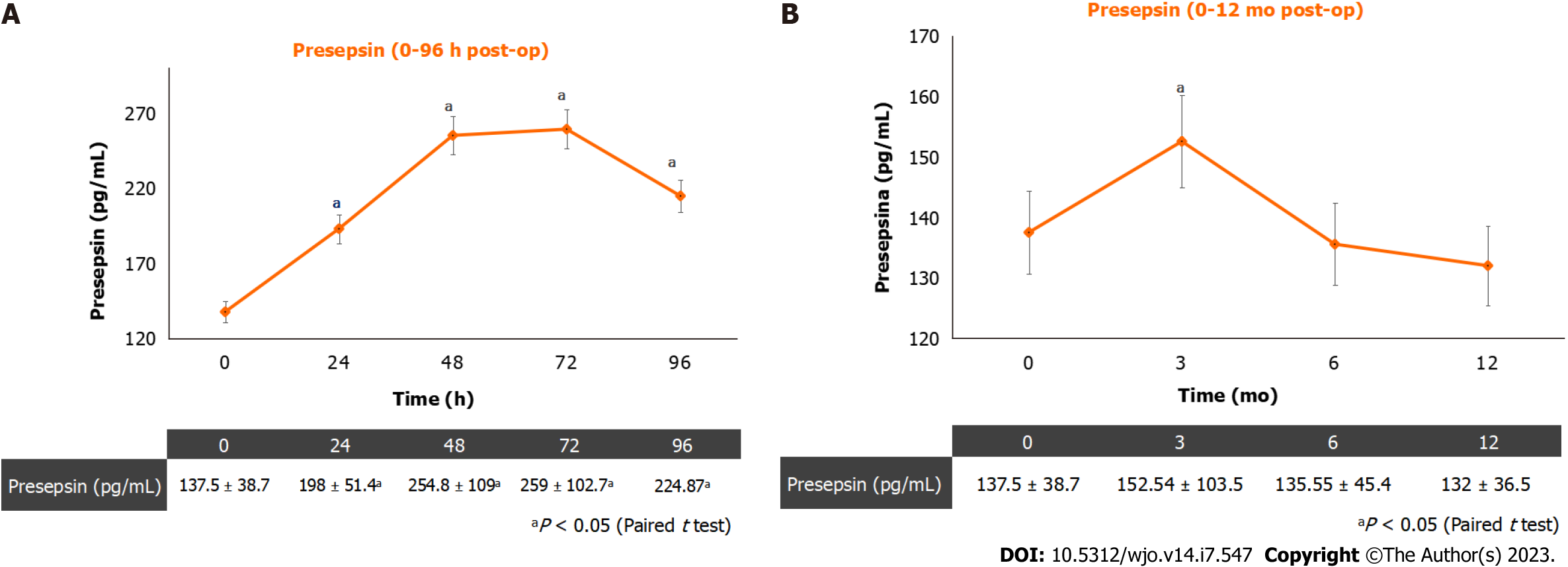
A total of 96 patients (51 female; 45 male; mean age = 65.74 ± 5.58) were recruited. The mean PS values were: 137.54 pg/mL at baseline, 192.08 pg/mL at 24 h post-op; 254.85 pg/mL at 48 h post-op; 259 pg/mL at 72 h post-op; 248.6 pg/mL at 96-h post-op; 140.52 pg/mL at 3-mo follow-up; 135.55 pg/mL at 6-mo follow-up and 130.11 pg/mL at 12-mo follow-up. In two patients (2.08%) a soft-tissue infection was observed; in these patients, higher levels (> 350 pg/mL) were recorded at 3-mo follow-up.
The dosage of plasmatic PS concentration is highly recommended in patients undergoing THA before surgery to exclude the presence of an unknown infection. The PS plasmatic concentration should be also assessed at 72 h post-operatively, evaluate the maximum postoperative PS value, and at 96 h post-operatively when a decrease of presepsin should be found. The lack of a presepsin decrease at 96 h post-operatively could be a predictive factor of infection.
Core Tip: The dosage of plasmatic presepsin (PS) concentration is highly recommended in patients undergoing total hip arthroplasty before surgery to exclude the presence of an unknown infection. The PS plasmatic concentration should be also assessed at 72 h post-operatively, to evaluate the maximum postoperative PS value, and at 96 h post-operatively when a decrease of presepsin should be found. The lack of a presepsin decrease at 96 h post-operatively should be a predictive factor of infection.
- Citation: Bizzoca D, Piazzolla A, Moretti L, Vicenti G, Moretti B, Solarino G. Physiologic postoperative presepsin kinetics following primary cementless total hip arthroplasty: A prospective observational study. World J Orthop 2023; 14(7): 547-553
- URL: https://www.wjgnet.com/2218-5836/full/v14/i7/547.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i7.547
Periprosthetic joint infections (PJIs) are a relevant cause of prosthetic surgery revision, accounting for 15% of failed total hip arthroplasties (THA)[1-3].
PJIs are fearsome complications in orthopaedics, as they might significantly affect the patient’s quality of life. Therefore, in the last two decades, an increasing interest towards the prevention of these complications has developed[1-3]. In the meantime, several research groups have investigated the potential role of presepsin (PS) in this PJI prevention and diagnosis[1-3].
PS, from a molecular point of view, is the N-terminal fragment of the soluble cluster of differentiation 14-SubType[14]. It is released into circulation after the activation of defence mechanisms, mainly bacterial phagocytosis[4].
Yaegashi et al[5], in 2004, hypothesized for the first time that PS could be useful as a biomarker to predict clinical prognosis in septic conditions. In the following years, different studies have highlighted the potential of PS in several infectious diseases, including severe community-acquired pneumonia, severe acute pancreatitis, infections in patients with haematological malignancies, implantable cardioverter-defibrillator (ICD) pocket infections, neonatal sepsis, pacemaker and PJIs and surgical site infections (SSIs)[6-22].
Furthermore, PS has been also studied in the risk stratification in patients undergoing cardiac surgery and as a biomarker in the perioperative management of patients undergoing total knee arthroplasty (TKA) or THA[23-29].
In a previous preliminary study, our research group defined the normal perioperative plasmatic levels of presepsin at 96 h postoperatively in 50 patients undergoing primary cementless THA and primary cemented TKA[25].
The present study aims at depicting the normal postoperative PS plasmatic curve, in patients undergoing primary cementless THA, at 12-mo follow-up.
Patients undergoing primary cementless THA at our Institute were recruited. Ethical clearance was obtained from our centre’s Clinical Research Ethics Committee, as per the 1964 Declaration of Helsinki.
All the patients gave written informed consent before enrolment in the study. Inclusion criteria were: Primary osteoarthritis of the hip; urinary catheter time of permanence < 24 h; peripheral venous cannulation time of permanence < 24 h; no postoperative homologous blood transfusion administration and hospital stay ≤ 8 d.
Exclusion criteria were: The presence of other articular prosthetic replacement or bone fixation devices; chronic inflammatory diseases; chronic kidney diseases; history of recurrent infections or malignant neoplasms; previous surgery in the preceding 12 mo; diabetes mellitus; immunosuppressive drug or corticosteroid assumption.
All the patients received the same antibiotic prophylaxis with cephazolin 2 g. All the THA were performed by the same surgical and anaesthesia team; total operative time was defined as the time taken from skin incision to completion of skin closure. At enrolment anthropometric data, smocking status, osteoarthritis stage according to Kellgren and Lawrence, Harris Hip Score, drugs assumption and comorbidities were recorded.
All the patients underwent serial blood tests, including complete blood count, PS and C-reactive protein (CRP) 24 h before arthroplasty and at 24, 48, 72 and 96 h postoperatively and at 3, 6 and 12-mo follow-up. The complication rate was recorded during the 12-mo follow-up.
Statistical analysis was performed with SPSS v25.0 (SPSS Inc, Chicago, IL, United States). The Shapiro-Wilk Test was conducted. Pearson correlation test was performed to assess any relationship between non-modifiable risk factors and preoperative PS values at baseline.
Compared to the baseline, the paired t-student test was performed to assess PS and CRP modifications at 3, 6 and 12-mo follow-ups. P values below 0.05 were considered significant.
A total of 96 patients (51 females; 45 males; mean age = 65.74 ± 5.58) were recruited in the present study. The main data of the study are summarized in Table 1.
| Characteristic | Values |
| Patients (n) | 96 |
| Gender, male, n (%) | 45 (46.875) |
| Age, mean ± SD | 65.74 ± 5.58 |
| Range | 54-77 |
| BMI (kg/m2), mean ± SD | 26.34 ± 7.22 |
| Total operative time (min), mean ± SD | 86.54 ± 43.5 |
| Hospital staying (d), mean ± SD | 5.45 ± 1.76 |
Table 2 shows the correlation between non-modifiable risk factors and preoperative PS values at baseline; a significant correlation was observed between patients’ age and preoperative PS levels. Notably, no correlation was found between preoperative creatinine and preoperative PS levels, since patients with chronic kidney disease were excluded from the present study.
| Presepsin | ||
| R | P value | |
| Age | 0.74 | 0.018a |
| BMI (kg/m2) | -0.259 | 0.734 |
| Gender | 0.19 | 0.44 |
| KLS | 0.287 | 0.16 |
| HHS | -0.17 | 0.645 |
| Creatinine | 0.056 | 0.852 |
Figure 1 shows the mean postoperative PS levels (Figure 1A) and the mean PS levels at 12-mo follow-ups (Figure 1B). The PS plasmatic concentration showed an increasing trend until 72 h post-op and started decreasing 96 h after surgery. During the 12-mo follow-up, the plasmatic PS concentration showed a significant increase at the three-month follow-up, when three patients out of 96 (3.125%) reported a soft-tissues infection. Higher levels (> 350 pg/mL) were recorded in these three patients at a 3-mo follow-up. No PJIs were diagnosed during the 12-mo follow-up.
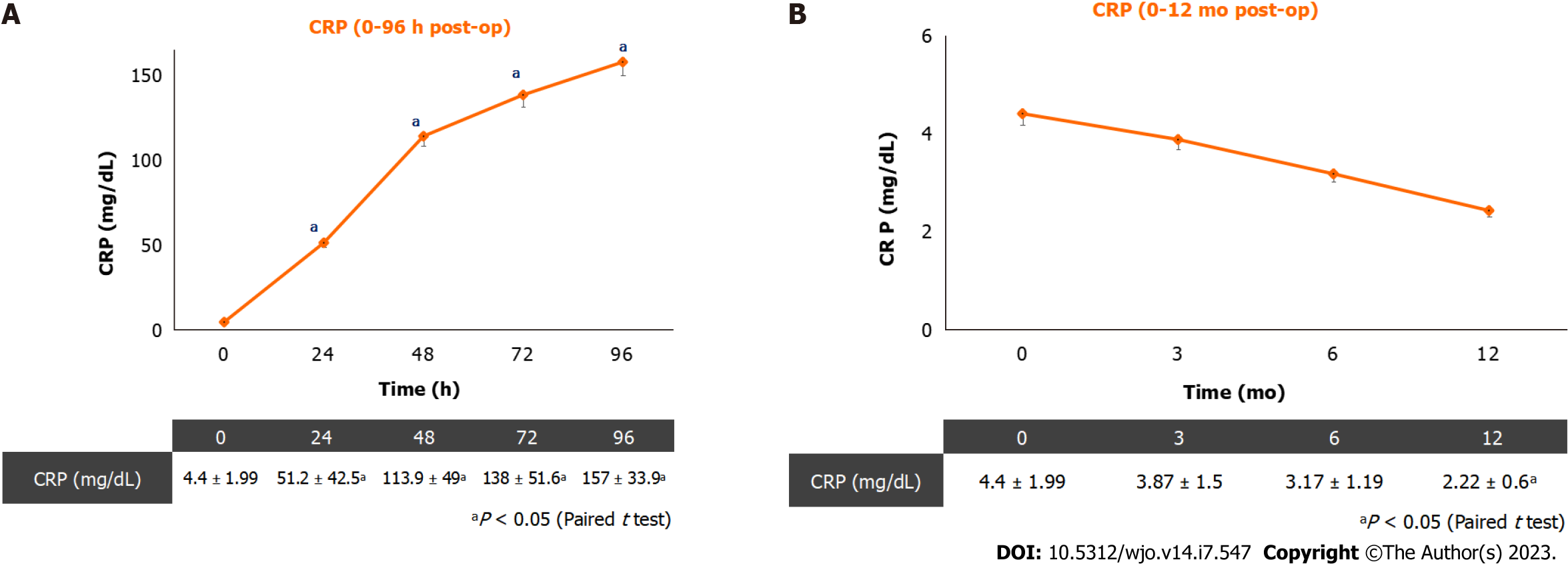
Figure 2 shows plasmatic CRP kinetics after surgery (Figure 2A) and at 12-mo follow-up (Figure 2B). The plasmatic CRP curve still showed an increasing trend at 96 h after surgery. During the 12-mo follow-up, plasmatic CRP showed a reducing trend and it was not influenced by the presence of soft-tissue infections.
PJIs are an emerging complication in prosthetic surgery, accounting for a relevant percentage of revision surgeries[27,28].
The pursuit of a biomarker able to improve the diagnosis and management of PJI, together with clinical findings and imaging modalities, is currently playing a central role in orthopaedics and traumatology[1].
Presepsin is an emerging biomarker studied in a wide range of infective conditions, including severe acute pancreatitis, neonatal sepsis, severe community-acquired pneumonia, pacemaker and ICD pocket infections, infections in patients with haematological malignancies, SSIs and PJIs. The present paper aims at depicting the normal postoperative plasmatic PS trend, in patients undergoing primary cementless THA, at 12-mo follow-up, to further depict a presepsin cut-off level for hip PJIs.
Our data showed PS has an increasing trend until 72 h post-op after THA surgery and starts decreasing 96 h after surgery. Furthermore, during the 12-mo follow-up, the plasmatic PS concentration showed a significant increase at the three-month follow-up, when three patients reported a soft-tissues infection. Hence, in the present study, plasmatic PS values are more sensitive to soft-tissues inflection, than CRP. Although the study sample is not so big and no cases of PJI have been observed in this trial, based on the study findings, the dosage of plasmatic PS concentration is recommended in patients undergoing THA before surgery, to exclude a concomitant unknown infection. However, future studies with a bigger sample size are needed to better define the limits of presepsin physiologic interval. The PS plasmatic concentration should be also assessed at 72 h after surgery, to assess the highest postoperative PS value, and at 96 h after surgery, when a decrease in plasmatic PS concentration is awaited. The lack of a plasmatic PS decrease, at 96 h after surgery could be a predictive factor of SSI or PJI.
These findings are consistent with the data reported by our research group in a preliminary report on perioperative presepsin levels in patients undergoing THA and TKA[25].
PS has been also studied in the assessment of PJIs by Marazzi et al[23], in a prospective multicentre study recruiting 100 patients who underwent revision surgery for aseptic loosening or PJI. These authors reported PS plasmatic levels were higher in the PJI group compared to the aseptic loosening group[23].
Koakutsu et al[30] have recently investigated the potential of PS in SSIs in a prospective observational study recruiting 118 patients who underwent elective major spine surgical procedures. These authors depicted an SSI in 3 patients out of 118; in these patients, higher levels (i.e., > 300 pg/mL) were depicted in the first postoperative week[30].
Imagama et al[31] recently evaluated synovial fluid and serum PS, together with procalcitonin (PCT) serum levels, in 28 patients affected by osteoarthritis (OA), compared with 18 patients suffering from septic arthritis (SA). These authors observed that synovial fluid, plasmatic PS and plasmatic PCT were significantly higher in the SA group compared with the OA group[31]. Hence, Imagama et al[31] concluded that synovial fluid PS could be a useful biomarker to differentiate SA from OA.
On the other hand, Busch et al[32], in a prospective cohort study on 53 patients with aseptic painful total shoulder, knee and hip arthroplasty and 27 patients affected by PJI, reported synovial fluid PS was not significantly higher in the PJI group, compared with the aseptic group. Synovial fluid presepsin specificity was 51% and sensitivity was 29%, with a cut-off value above 0.06 ng/mL[32].
Considering the above-mentioned findings, further studies with larger samples are awaited to better define the potential of serum and synovial PS concentration in the diagnosis of PJI.
The present study has some limitations. First of all, the sample size is not so large and no cases of PJI were recorded in the recruited patients during follow-up, hence the findings of the present study should be validated in a study with a larger sample size. Moreover, no control group was included in the present study and presepsin accuracy was not compared to other emerging biomarkers.
The dosage of plasmatic PS concentration is highly recommended in patients undergoing THA, in the preoperative phase, to rule out any unknown infection.
The PS plasmatic concentration should be also assessed at 72 h after surgery, to assess the higher postoperative PS value, and at 96 h after surgery, when a PS decrease should be found. The lack of a presepsin decrease at 96 h after surgery might be a predictive factor of infection.
Periprosthetic joint infections (PJIs) are a relevant cause of prosthetic surgery revision, accounting for 15% of failed total hip arthroplasties (THA).
Presepsin (PS) is released into the circulation following bacterial phagocytosis and the activation of other innate defence mechanisms. In a previous preliminary study, our research group defined the normal perioperative plasmatic levels of presepsin at 96 h postoperatively in 50 patients undergoing primary cementless THA and primary cemented total knee arthroplasties.
This paper aims at depicting e normal postoperative PS plasmatic curve, in patients undergoing primary cementless THA, at 12-mo follow-up.
Patients undergoing primary THA were prospectively recruited. All the procedures were performed by the same anaesthesia and surgical équipe. The recruited patients underwent serial blood tests, including complete blood count, PS and C-reactive protein 24 h before arthroplasty and at 24, 48, 72 and 96 h postoperatively and at 3, 6 and 12-mo follow-up.
Ninety-six patients (51 female; mean age = 65.74 years old) were included in the present study. The mean PS values were: 137.54 pg/mL before surgery, 192.08 pg/mL at 24 h post-op; 254.85 pg/mL at 48 h post-op; 259 pg/mL at 72 h post-op; 248.6 pg/mL at 96-h post-op; 140.52 pg/mL at 3-mo follow-up; 135.55 pg/mL at 6-mo follow-up and 130.11 pg/mL at 12-mo follow-up.
The assessment of plasmatic PS concentration is highly recommended in patients undergoing THA in the preoperative phase, to rule out any unknown infection. The PS plasmatic concentration should be also assessed at 72 h after surgery, to quantify the higher postoperative PS value, and at 96 h after surgery, when a decrease in PS should be found. The lack of a presepsin decrease at 96 h after surgery might predict a local infection.
Presepsin is an emerging biomarker in the diagnosis of PJIs. However, further studies with bigger samples are awaited to better define the role of serum and synovial PS in the diagnosis of PJI.
Provenance and peer review: Invited article; Externally peer-reviewed.
Peer-review model: Single-blind
Peer review started: December 23, 2022
Speciality type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hooper GJ, New Zealand; Maruccia F, Italy; Yan ZQ, China S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
| 1. | Solarino G, Bizzoca D, Moretti L, Vicenti G, Piazzolla A, Moretti B. What's New in the Diagnosis of Periprosthetic Joint Infections: Focus on Synovial Fluid Biomarkers. Trop Med Infect Dis. 2022;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Solarino G, Abate A, Vicenti G, Spinarelli A, Piazzolla A, Moretti B. Reducing periprosthetic joint infection: what really counts? Joints. 2015;3:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Bizzoca D, Moretti L, Gnoni A, Moretti FL, Scacco S, Banfi G, Piazzolla A, Solarino G, Moretti B. The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections. J Funct Morphol Kinesiol. 2022;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Yaegashi Y, Shirakawa K, Sato N, Suzuki Y, Kojika M, Imai S, Takahashi G, Miyata M, Furusako S, Endo S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother. 2005;11:234-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Wu J, Hu L, Zhang G, Wu F, He T. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0133057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, Borggrefe M, Hoffmann U. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care. 2014;18:507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Vodnik T, Kaljevic G, Tadic T, Majkic-Singh N. Presepsin (sCD14-ST) in preoperative diagnosis of abdominal sepsis. Clin Chem Lab Med. 2013;51:2053-2062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Kweon OJ, Choi JH, Park SK, Park AJ. Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. J Crit Care. 2014;29:965-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Sargentini V, Ceccarelli G, D'Alessandro M, Collepardo D, Morelli A, D'Egidio A, Mariotti S, Nicoletti AM, Evangelista B, D'Ettorre G, Angeloni A, Venditti M, Bachetoni A. Presepsin as a potential marker for bacterial infection relapse in critical care patients. A preliminary study. Clin Chem Lab Med. 2015;53:567-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Su MH, Shou ST. Prognostic value of presepsin for diagnosis and severity assessment of sepsis. Linchang Jianyan Zazhi. 2014;32:106-111. [Cited in This Article: ] |
| 12. | Yu J, Shao Q, Wang Q, Zhang XH, Huang K. Combined determination of presepsin, procalcitonin and C reactive protein for early diagnosis and prognostic assessment of severe trauma patients with sepsis. Linchang Jianyan Zazhi. 2014;32:200-203. [Cited in This Article: ] |
| 13. | Liu B, Chen YX, Yin Q, Zhao YZ, Li CS. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care. 2013;17:R244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, Morello F, Lupia E, Moiraghi C, Mengozzi G, Battista S. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17:R168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Klouche K, Cristol JP, Devin J, Gilles V, Kuster N, Larcher R, Amigues L, Corne P, Jonquet O, Dupuy AM. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann Intensive Care. 2016;6:59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Ozdemir AA, Elgormus Y. Diagnostic Value of Presepsin in Detection of Early-Onset Neonatal Sepsis. Am J Perinatol. 2017;34:550-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Montaldo P, Rosso R, Santantonio A, Chello G, Giliberti P. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res. 2017;81:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Lin J, Li Z, Zheng Y, Zhang Y, Shao C, Liu G, Li J. Elevated Presepsin Levels are Associated with Severity and Prognosis of Severe Acute Pancreatitis. Clin Lab. 2016;62:1699-1708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 19. | Ebihara Y, Kobayashi K, Ishida A, Maeda T, Takahashi N, Taji Y, Asou N, Ikebuchi K. Diagnostic performance of procalcitonin, presepsin, and C-reactive protein in patients with hematological malignancies. J Clin Lab Anal. 2017;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Leoni D, Rello J. Severe community-acquired pneumonia: optimal management. Curr Opin Infect Dis. 2017;30:240-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Bomberg H, Klingele M, Wagenpfeil S, Spanuth E, Volk T, Sessler DI, Schäfers HJ, Groesdonk HV. Presepsin (sCD14-ST) Is a Novel Marker for Risk Stratification in Cardiac Surgery Patients. Anesthesiology. 2017;126:631-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Lennerz C, Vrazic H, Haller B, Braun S, Petzold T, Ott I, Lennerz A, Michel J, Blažek P, Deisenhofer I, Whittaker P, Kolb C. Biomarker-based diagnosis of pacemaker and implantable cardioverter defibrillator pocket infections: A prospective, multicentre, case-control evaluation. PLoS One. 2017;12:e0172384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Marazzi MG, Randelli F, Brioschi M, Drago L, Romanò CL, Banfi G, Massaccesi L, Crapanzano C, Morelli F, Corsi Romanelli MM, Galliera E. Presepsin: A potential biomarker of PJI? A comparative analysis with known and new infection biomarkers. Int J Immunopathol Pharmacol. 2018;31:394632017749356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Vicenti G, Solarino G, Bizzoca D, Caringella N, Nappi VS, Moretti L, Belluati A, Moretti B. The limits and potentials of presepsin in orthopaedic surgery: state of the art and future directions. J Biol Regul Homeost Agents. 2020;34:259-262. Congress of the Italian Orthopaedic Research Society. [PubMed] [Cited in This Article: ] |
| 25. | Vicenti G, Pesce V, Bizzoca D, Nappi V, Palmiotto F, Carrozzo M, Moretti B. Perioperative plasmatic presepsin levels in patients undergoing total hip or knee replacement: a preliminary study. J Biol Regul Homeost Agents. 2017;31:1081-1086. [PubMed] [Cited in This Article: ] |
| 26. | Vicenti G, Bizzoca D, Cotugno D, Carrozzo M, Riefoli F, Rifino F, Belviso V, Elia R, Solarino G, Moretti B. The use of a gentamicin-coated titanium nail, combined with RIA system, in the management of non-unions of open tibial fractures: A single centre prospective study. Injury. 2020;51 Suppl 3:S86-S91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | McNally M, Sousa R, Wouthuyzen-Bakker M, Chen AF, Soriano A, Vogely HC, Clauss M, Higuera CA, Trebše R. The EBJIS definition of periprosthetic joint infection. Bone Joint J. 2021;103-B:18-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 231] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 28. | Sigmund IK, Luger M, Windhager R, McNally MA. Diagnosing periprosthetic joint infections : a comparison of infection definitions: EBJIS 2021, ICM 2018, and IDSA 2013. Bone Joint Res. 2022;11:608-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 29. | Peng B, Li H, Peng XX. Functional metabolomics: from biomarker discovery to metabolome reprogramming. Protein Cell. 2015;6:628-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 30. | Koakutsu T, Sato T, Aizawa T, Itoi E, Kushimoto S. Postoperative Changes in Presepsin Level and Values Predictive of Surgical Site Infection After Spinal Surgery: A Single-Center, Prospective Observational Study. Spine (Phila Pa 1976). 2018;43:578-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Imagama T, Tokushige A, Seki K, Seki T, Nakashima D, Ogasa H, Sakai T, Taguchi T. Early diagnosis of septic arthritis using synovial fluid presepsin: A preliminary study. J Infect Chemother. 2019;25:170-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Busch A, Jäger M, Engler H, Wasssenaar D, Bielefeld C, Wegner A. Diagnostic Accuracy of Synovial Neopterin, TNF-α and Presepsin in Periprosthetic Joint Infection: A Prospective Study. Z Orthop Unfall. 2022;160:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |