Published online Apr 10, 2016. doi: 10.5306/wjco.v7.i2.149
Peer-review started: June 16, 2015
First decision: September 22, 2015
Revised: September 24, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: April 10, 2016
Fortunately, the landscape of the systemic treatment for grade 1 and 2 pancreatic neuroendocrine tumors has changed in the last decade with at least four different alternatives approved in the field. Chemotherapy, somatostatin analogues, sunitinib and everolimus remind valid options according to the most referenced international guidelines. However, and although this is something done in the routine practice, there is a lack of evidence for the use of any of these strategies after failure to the others. Moreover, further sequential alternatives in third or fourth line have never been tested prospectively. The need for a better understanding of the rationale to sequence different systemic options is even greater in non-pancreatic neuroendocrine tumors since available therapies are scarce. Sequential strategies in other solid tumors have led to a clear improvement in overall survival. This is also believed to occur in neuroendocrine tumors but no clear data on it has been delivered yet. We postulate that the different mode of action of the systemic options available for the treatment of neuroendocrine tumors may avoid the complete resistance of one option after the other and that sequential use of these agents will be translated into a longer overall survival of patients. Prospective and randomized trials that seek for the activity of drugs after failure to another systemic alternatives are highly needed in this field of neuroendocrine tumors.
Core tip: There is a need to improve the rationale we use when approaching to a sequential systemic treatment strategy in disseminated neuroendocrine tumors. Up to now, we do not have level 1 evidence to use any systemic alternative after failure to a prior one. Widely heterogeneous populations have been recruited in larger phase III pivotal trials in neuroendocrine tumors. Therefore, it is difficult to find final conclusions from the registration trials. In this article we aim to summarize the available evidence behind the use of different alternatives after failure to standard somatostatin analogs.
- Citation: Grande E. Sequential treatment in disseminated well- and intermediate-differentiated pancreatic neuroendocrine tumors: Common sense or low rationale? World J Clin Oncol 2016; 7(2): 149-154
- URL: https://www.wjgnet.com/2218-4333/full/v7/i2/149.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i2.149
The sequencing of different systemic options is something that is routinely done in metastatic solid tumors where other alternatives are available. Moreover, the use of successive lines of treatment have led to an improvement in the overall survival of all major tumors like breast cancer[1], colorectal cancer[2], non-small cell lung cancer[3] or renal cell carcinoma[4] among others. In addition, the enlargement of survival is accompanied by the maintenance of patient’s quality of life[5-8]. Both goals, survival and quality of life, are the two main objectives that we are looking for in our patients with disseminated solid tumors.
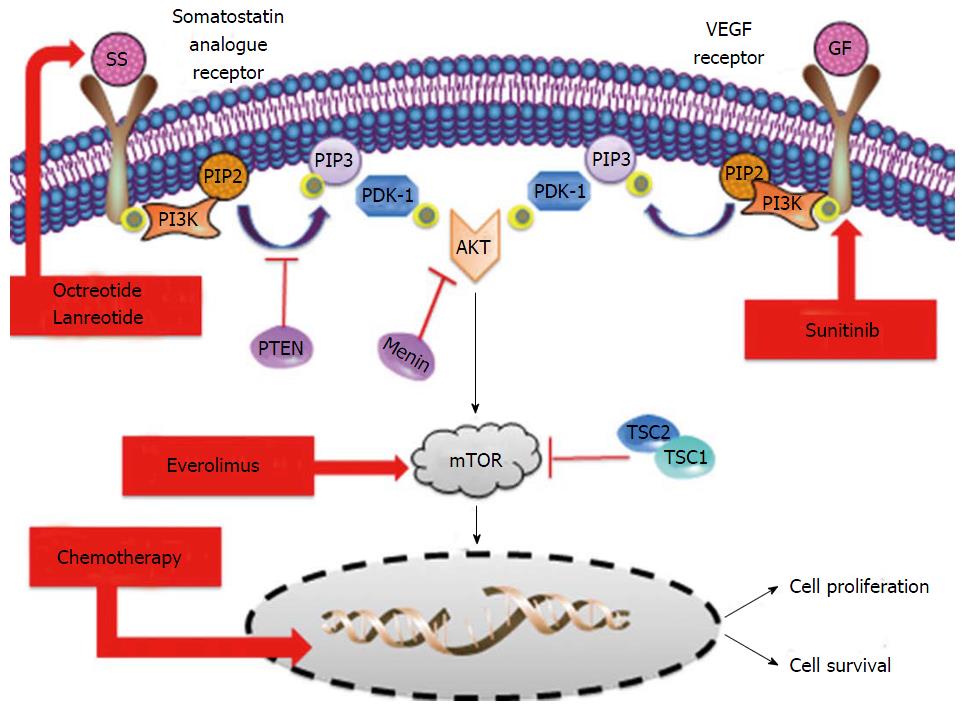
The systemic management of pancreatic neuroendocrine tumors (pNETs) has dramatically changed in the last few years. Everolimus and sunitinib are novel targeted agents that have been approved recently based on a significant improvement in progression free survival against placebo[9,10]. Caplin and cols. Recently showed that somatostatin analogues, particularly lanreotide, may have also a role in tumor growth control and not only as a symptom-relievers in pNETs[11]. Streptozocin-based schemes of chemotherapy have been widely used worldwide for the last three decades to treat metastatic pNETs on the basis of high tumor and symptom responses found in prospective and retrospective studies[12-14]. Temozolomide is an alkylating agent that has shown impressive response rates according to objectives RECIST criteria but in non-randomized trials and retrospective series in pNETs[15,16]. Based on the induced-rate of tumor shrinkage, temozolomide is also considered an option for the systemic treatment of pNETs in the most referenced international guidelines[17-19] (Figure 1).
The deep exomic sequences analysis of tumor samples from pNETs patients showed that there are at least two genetic key drivers that are leading the development of these tumors[20]. The phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (mTOR) pathway is directly or indirectly affected by mutations in patients with pNETs[20]. Indirect mutations related with the mTOR pathway are related with the biallelic inactivation of the MEN1 tumor supressor gene. The activity of the protein encoded by MEN1 gene, the menin, is involved in the regulation of AKT access to the cell membrane. Therefore, a loss of function of the menin activity translates into a highest availability of AKT in tumor cell membrane that activates the mTOR signalling pathway[21].
The second most frequently mutated genes in patients with pNETs are affecting to domain-associated protein gene (DAXX) and ATR-X gene (ATRX). A total of 43% of the patients are harbouring mutations in either DAXX or ATRX genes. Both genes are encoding proteins involved in chromatin remodelling process and are also related to the activation of alternative lengthening of telomeres[22].
From the histology analysis and phenotype features perspective, these tumors are also characterized by high presence of newly tumor-formed blood vessels and somatostatin receptors on the surface of tumor cells. Overexpression of vascular endothelial growth factor receptors not only in the endothelial cells or in the stroma but also in the surface of the neuroendocrine cells has been extensively observed[23,24]. The presence of somatostatin receptors over the surface of pNETs tumor cells is over 90%[25,26]. Somatostatin receptors are playing a crucial role not only in the production and release of active peptides by the tumor cells but also in the growth and tumor cell survival process.
The complexity of the underlying biology behind the development of neuroendocrine tumors makes that it is difficult to believe that by targeting one of these involved mechanisms we can control the growing of the tumor for a prolonged period of time in the majority of the patients. At the same time, this complexity means that it is also very likely that one agent targeting one of these pathways could easily revert the resistance to the action of another agent against other mechanism. Overlapping resistance is not frequent among different approaches in the mode of action.
A wide range of agents with distinct mode of actions is used in the systemic treatment of pNETs. In renal cell carcinomas (RCC) it is established that mTOR inhibitors can overcome the resistance to tyrosine kinase inhibitors[27]. Moreover, there is a clear strategy for sequencing treatments in RCC; tyrosine kinase inhibitors should go first and after progression mTOR inhibitors are leading to a higher overall survival[28]. This clear superiority of sunitinib vs everolimus has not been established in neuroendocrine tumors so far.
In the RADIANT-3 trial, everolimus significantly prolonged median progression-free survival compared with placebo in 410 patients with pNETs (11.0 mo vs 4.6 mo; HR = 0.35; 95%CI: 0.27-0.45; P < 0.001)[9]. In an ulterior retrospective analysis of the RADIANT-3 trial, it was shown that everolimus improved median progression free survival by 7.8 mo in patients who previously received chemotherapy (11.0 mo vs 3.2 mo; HR = 0.34; 95%CI: 0.25-0.48; P < 0.0001) and by 6.0 mo (11.4 mo vs 5.4 mo; HR = 0.42; 95%CI: 0.29-0.60; P < 0.0001) in patients who were chemonaive. It is important also to highlight that patients recruited for the RADIANT-3 trial were stratified according to if they received or not prior treatment with chemotherapy. Therefore, no significant differences are found in baseline characteristics of the two subgroups of patients[29]. Same findings were found in the retrospective subgroup analysis of those patients who were recruited for the RADIANT-3 trial and received prior treatment with somatostatin analogues (0.40: 95%CI: 0.28-0.57) vs those who did not receive prior treatment with either lanreotide or octreotide (0.36: 95%CI: 0.25-0.51)[9].
Similar findings were observed at the SUN-1111, the pivotal trial for sunitinib in advanced pNETs patients. When we take a look in deep to the forest plot analysis of the clinical outcome of patients treated at the SUN-1111 trial we observe that those patients who were treated before with somatostatin analogues had very similar HR for progression free survival (0.43: 95%CI: 0.21-0.89) than those patients recruited who were naïve for somatostatin analogues treatment (0.41: 95%CI: 0.22-0.75). In addition, there was also a highly significant benefit in terms of progression free survival in favour to those patients who did not receive or only received one systemic prior treatment and were randomized to sunitinib vs placebo (0.33: 95%CI: 0.19-0.59). Although not statistically significant there was observed also a trend to a higher benefit for sunitinib vs placebo in those patients who received more than two prior lines of systemic treatments (0.61: 95%CI: 0.27-1.37)[10].
To our knowledge, there are not prospective data to support the clinical activity of sunitinib followed by everolimus or the other sequence around in advanced pNETs patients. In an evidence-based sense, there is no available evidence to support the use of somatostatin analogues or chemotherapy after failure to novel targeted agents. The fact that novel targeted agents have shown activity after failure to somatostatin analogues and/or chemotherapy does not necessarily means that novel drugs should be used later in our sequential strategy of management of these patients. Actually, everolimus and sunitinib could be sequenced before the use of other systemic alternatives.
The Spanish Task Force Group for Neuroendocrine tumors (GETNE) has recently published the results of the PAZONET trial. We recruited 44 patients with advanced NETs that were treated with the multitargeted tyrosine kinase inhibitor pazopanib as a single agent (n = 14) or in combination with somatostatin analogues (n = 30) according to the investigators decision. Eleven (25%) patients had previously received everolimus, 16 (36.4%) previously received a tyrosine kinase inhibitor, and 8 patients previously had failed to both mTOR and antiangiogenic systemic treatments. The median progression free survival that we achieved in the intention to treat population was 9.5 mo (95%CI: 4.8-14.1), however the median progression free survival in the subgroup of patients with one prior multitargeted inhibitor o a prior mTOR treatment were 12.4 mo (95%CI: 11.3-13.5) and 6.8 mo (95%CI: 0.0-15.3) respectively. The time to treatment failure was much lower in those patients who received both different alternatives before since median progression free survival was only 4.0 mo (95%CI: 1.3-6.8). Interestingly, those patients who received pazopanib plus somatostatin analogues did it better in terms of progression free survival that those who received pazopanib alone (11.7 mo, 95%CI: 9.7-13.7 vs 4.2 mo, 95%CI: 3.3-5.1; P = 0.043)[30].
Bevacizumab has been widely studied either alone[31] or in combination with octreotide[32,33], temsirolimus[34], everolimus[35,36], chemotherapy[37-39] or sorafenib[40] in patients with disseminated NETs. Although there were some of these patients previously treated with chemotherapy or other targeted agents, no data regarding clinical outcome for these subgroup populations has been released yet.
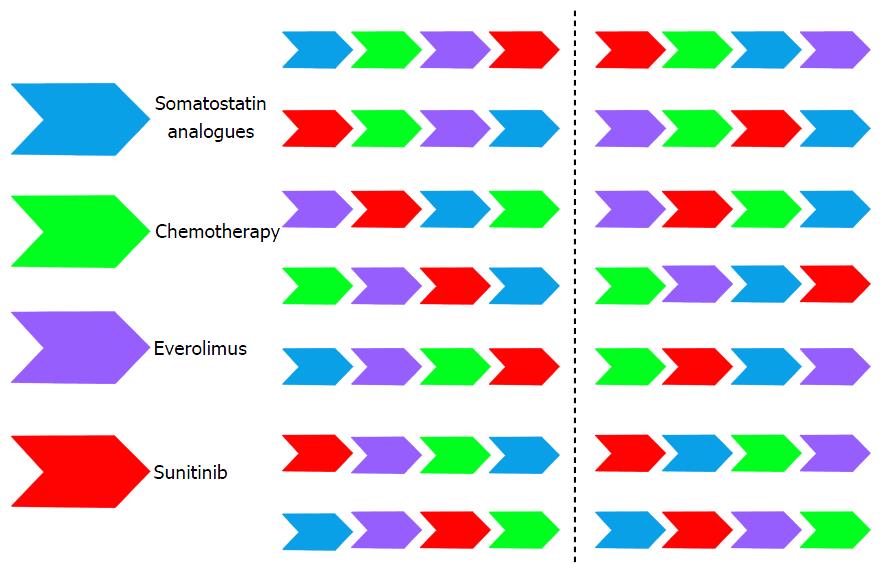
There are four different strategies currently approved or widely accepted for the treatment of advanced pNETs: Somatostatin analogues (lanreotide and octreotide), chemotherapy schemes (streptozocin- and temozolamide-based), everolimus and sunitinib. In addition, there are colleagues that are using interferon as well for pNETs patients and in those sites with availabi–lity the use of peptide receptor radionuclide therapy is also common. Therefore, we can count on at least six different systemic approaches for the routine management of disseminated pNETs in addition of local techniques. How we can best order these alternatives is crucial to achieve the longest survival with the higher preservation of patient’s quality of life.
There is a wide range of different sequential alternatives that can be followed in our patients with advanced pNETs (Figure 2). It is not reliable to conduct one trial in each of these situations. To our knowledge, the only randomized sequential trial in pNETs that is now undergoing is the European Neuroendocrine Tumors Society/Spanish Task Force Group for Neuroendocrine Tumors (GETNE) trial called SEQTOR (NCT02246127). The SEQTOR trial seeks to compare the efficacy and safety of everolimus followed by chemotherapy with streptozocin-fluorouracil (STZ-5FU) upon progression or the reverse sequence in advanced progressive pNETs.
The GETNE group is also leading the PALBONET trial that consists on a phase II trial in which the cyclin-dependent kinase-4 (CDK4)/CDK6 inhibitor palbociclib is assessed as single agents in disseminated pNETs patients after progression to at least one prior novel targeted agent.
Nowadays, and in order to be pragmatic, we should consider classical clinical parameters like if the tumor is functioning or not, presence of symptoms because of tumor bulk, rapidness of tumor growth, Ki67 proliferation index, histology grade of differentiation, comorbidities, etc., to customize our treatment. After progression to our first approach we need to consider once again all these parameters and customize the treatment one more time in a common sense based more than in evidence based sense. There is no single sequential strategy that has proved to be superior to others as of yet. Moreover, there is no single treatment that has shown to be superior to the others. This remains the challenge of neuroendocrine tumors in which “art” is still the standard of care line after line of treatment. There is an urgent need for increasing our systemic armamentarium against these tumors and for ordering our sequential strategies. More evidence is needed across different lines of treatment for pNETs. In addition, the concomitant use of the available options could be synergistic but we do not know if the total time to tumor growth is superior to the administration of the different strategies alone one after the other. Probably, the greatest advances and efforts that are being performed in the field of molecular biology of these tumors may translate sooner than later into a better management of our patients in the clinic.
P- Reviewer: Di Lorenzo G, Qiao QD, Sakata N S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Dear RF, McGeechan K, Jenkins MC, Barratt A, Tattersall MH, Wilcken N. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2013;12:CD008792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Asmis T, Berry S, Cosby R, Chan K, Coburn N, Rother M. Strategies of sequential therapies in unresectable metastatic colorectal cancer: a meta-analysis. Curr Oncol. 2014;21:318-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Grossi F, Aita M, Follador A, Defferrari C, Brianti A, Sinaccio G, Belvedere O. Sequential, alternating, and maintenance/consolidation chemotherapy in advanced non-small cell lung cancer: a review of the literature. Oncologist. 2007;12:451-464. [PubMed] [Cited in This Article: ] |
| 4. | Albiges L, Choueiri T, Escudier B, Galsky M, George D, Hofmann F, Lam T, Motzer R, Mulders P, Porta C. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2015;67:100-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, Kyriakides S, Costa A, Cufer T, Albain KS. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Verhaar S, Vissers PA, Maas H, van de Poll-Franse LV, van Erning FN, Mols F. Treatment-related differences in health related quality of life and disease specific symptoms among colon cancer survivors: results from the population-based PROFILES registry. Eur J Cancer. 2015;51:1263-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Sancho A, Carrera S, Arietaleanizbeascoa M, Arce V, Gallastegui NM, Giné March A, Sanz-Guinea A, Eskisabel A, Rodriguez AL, Martín RA. Supervised physical exercise to improve the quality of life of cancer patients: the EFICANCER randomised controlled trial. BMC Cancer. 2015;15:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Calvo E, Grünwald V, Bellmunt J. Controversies in renal cell carcinoma: treatment choice after progression on vascular endothelial growth factor-targeted therapy. Eur J Cancer. 2014;50:1321-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2039] [Cited by in F6Publishing: 1969] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 10. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1805] [Cited by in F6Publishing: 1693] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 11. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1142] [Cited by in F6Publishing: 1122] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 12. | Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189-1194. [PubMed] [Cited in This Article: ] |
| 13. | Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523. [PubMed] [Cited in This Article: ] |
| 14. | Reidy-Lagunes DL. Systemic therapy for advanced pancreatic neuroendocrine tumors: an update. J Natl Compr Canc Netw. 2012;10:777-783. [PubMed] [Cited in This Article: ] |
| 15. | Kulke MH, Hornick JL, Frauenhoffer C, Hooshmand S, Ryan DP, Enzinger PC, Meyerhardt JA, Clark JW, Stuart K, Fuchs CS. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 297] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Ekeblad S, Sundin A, Janson ET, Welin S, Granberg D, Kindmark H, Dunder K, Kozlovacki G, Orlefors H, Sigurd M. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986-2991. [PubMed] [Cited in This Article: ] |
| 17. | Öberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124-vii130. [PubMed] [Cited in This Article: ] |
| 18. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 329] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 19. | Kulke MH, Shah MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF, Fanta P, Giordano T. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78-108. [PubMed] [Cited in This Article: ] |
| 20. | Calvo E, Escudier B, Motzer RJ, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Ravaud A. Everolimus in metastatic renal cell carcinoma: Subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer. 2012;48:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1227] [Cited by in F6Publishing: 1235] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 22. | Lairmore TC, Chen H. Role of menin in neuroendocrine tumorigenesis. Adv Exp Med Biol. 2009;668:87-95. [PubMed] [Cited in This Article: ] |
| 23. | Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, Hunger F, Pasquinelli S, Speel EJ, Perren A. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146:453-460.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 24. | De Dosso S, Grande E, Barriuso J, Castellano D, Tabernero J, Capdevila J. The targeted therapy revolution in neuroendocrine tumors: in search of biomarkers for patient selection and response evaluation. Cancer Metastasis Rev. 2013;32:465-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Teulé A, Casanovas O. Relevance of angiogenesis in neuroendocrine tumors. Target Oncol. 2012;7:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Okuwaki K, Kida M, Mikami T, Yamauchi H, Imaizumi H, Miyazawa S, Iwai T, Takezawa M, Saegusa M, Watanabe M. Clinicopathologic characteristics of pancreatic neuroendocrine tumors and relation of somatostatin receptor type 2A to outcomes. Cancer. 2013;119:4094-4102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Alonso-Gordoa T, Díez JJ, Durán M, Grande E. Advances in thyroid cancer treatment: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:22-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, Srimuninnimit V, Pittman K, Sabbatini R, Rha SY. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:2765-2772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 303] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 29. | Lombard-Bohas C, Yao JC, Hobday T, Van Cutsem E, Wolin EM, Panneerselvam A, Stergiopoulos S, Shah MH, Capdevila J, Pommier R. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial. Pancreas. 2015;44:181-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 30. | Grande E, Capdevila J, Castellano D, Teulé A, Durán I, Fuster J, Sevilla I, Escudero P, Sastre J, García-Donas J. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE)†. Ann Oncol. 2015;26:1987-1993. [PubMed] [Cited in This Article: ] |
| 31. | Hobday TJ, Yin J, Pettinger A, Strosberg JR, Reidy DL, Chen XH, Erlichman C. Multicenter prospective phase II trial of bevacizumab (bev) for progressive pancreatic neuroendocrine tumor (PNET). J Clin Oncol. 2015;33 suppl:abstr 4096. [Cited in This Article: ] |
| 32. | Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, Hess K, Ng C, Abbruzzese JL, Ajani JA. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 33. | Yao JC, Guthrie K, Moran C, Strosberg JR, Kulke MH, Chan JA, LoConte NK, McWilliams RR, Wolin EM, Mattar BI, McDonough S, Chen HX, Blanke CD, Hochster HS. SWOG S0518: Phase III prospective randomized comparison of depot octreotide plus interferon alpha-2b versus depot octreotide plus bevacizumab (NSC #704865) in advanced, poor prognosis carcinoid patients (NCT00569127). Presented at the 2015 ASCO annual meeting: Abstract 4004. . [Cited in This Article: ] |
| 34. | Hobday TJ, Qin R, Reidy-Lagunes D, Moore MJ, Strosberg J, Kaubisch A, Shah M, Kindler HL, Lenz HJ, Chen H. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J Clin Oncol. 2015;33:1551-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Yao JC, Phan AT, Hess K, Fogelman D, Jacobs C, Dagohoy C, Leary C, Xie K, Ng CS. Perfusion computed tomography as functional biomarker in randomized run-in study of bevacizumab and everolimus in well-differentiated neuroendocrine tumors. Pancreas. 2015;44:190-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Kulke MH, Niedzwiecki D, Foster NR, Fruth B, Kunz PL, Kennecke HF, Wolin EM, Venook AP. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). J Clin Oncol. 2015;33 suppl:abstr 4005. [Cited in This Article: ] |
| 37. | Ducreux M, Dahan L, Smith D, O’Toole D, Lepère C, Dromain C, Vilgrain V, Baudin E, Lombard-Bohas C, Scoazec JY. Bevacizumab combined with 5-FU/streptozocin in patients with progressive metastatic well-differentiated pancreatic endocrine tumours (BETTER trial)--a phase II non-randomised trial. Eur J Cancer. 2014;50:3098-3106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Berruti A, Fazio N, Ferrero A, Brizzi MP, Volante M, Nobili E, Tozzi L, Bodei L, Torta M, D’Avolio A. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: the XELBEVOCT study. BMC Cancer. 2014;14:184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 39. | Kasuya K, Nagakawa Y, Suzuki M, Suzuki Y, Kyo B, Suzuki S, Matsudo T, Itoi T, Tsuchida A, Aoki T. Combination therapy of gemcitabine or oral S-1 with the anti-VEGF monoclonal antibody bevacizumab for pancreatic neuroendocrine carcinoma. Exp Ther Med. 2012;3:599-602. [PubMed] [Cited in This Article: ] |
| 40. | Castellano D, Capdevila J, Sastre J, Alonso V, Llanos M, García-Carbonero R, Manzano Mozo JL, Sevilla I, Durán I, Salazar R. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumour: a phase II study of Spanish Neuroendocrine Tumour Group (GETNE0801). Eur J Cancer. 2013;49:3780-3787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |