Published online Jul 27, 2023. doi: 10.4254/wjh.v15.i7.904
Peer-review started: March 27, 2023
First decision: April 19, 2023
Revised: May 17, 2023
Accepted: June 12, 2023
Article in press: June 12, 2023
Published online: July 27, 2023
Intrahepatic cholestasis of pregnancy (ICP) is a rare but severe complication for both the mother and the unborn child. The diagnosis is primarily based on elevated serum levels of bile acids. In a large ICP cohort, we here study in detail liver stiffness (LS) using transient elastography (TE), now widely used to non-invasively screen for liver cirrhosis within minutes.
To specifically explore LS in a large cohort of women with ICP compared to a control group with uncomplicated pregnancy.
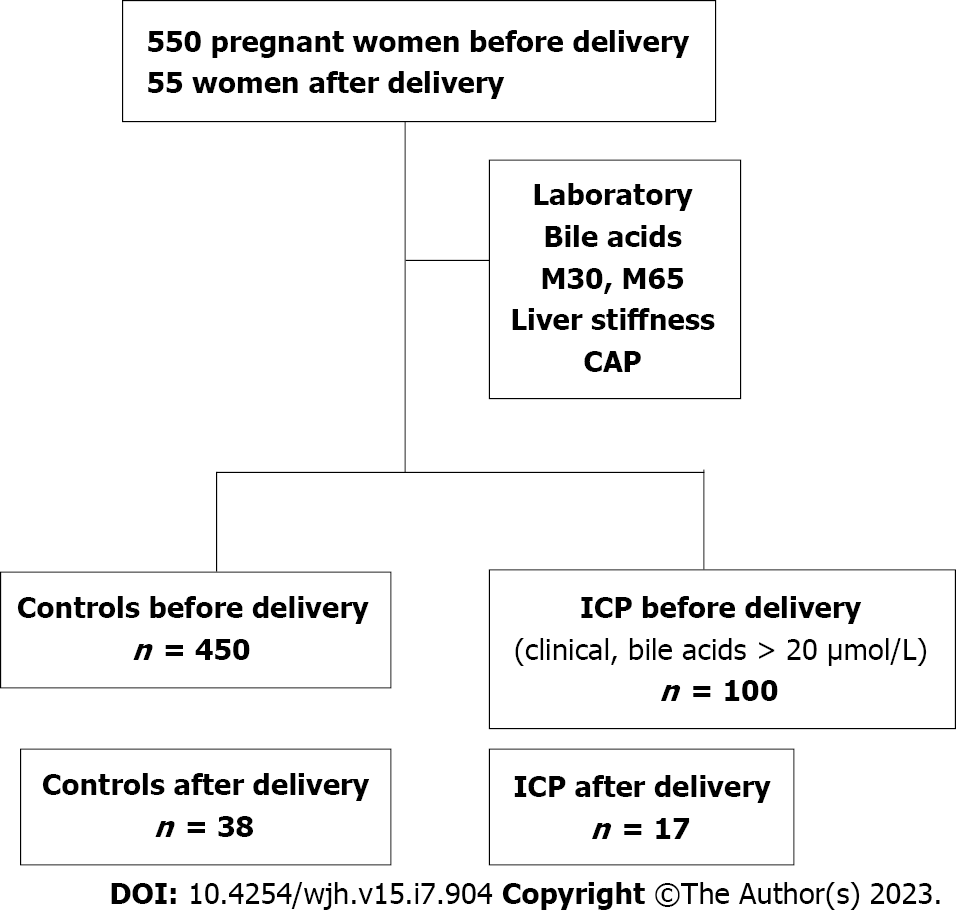
LS and hepatic steatosis marker controlled attenuation parameter (CAP) were measured in 100 pregnant women with ICP using TE (Fibroscan, Echosens, Paris, France) between 2010 and 2020. In 17 cases, LS could be measured postpartum. 450 women before and 38 women after delivery with uncomplicated pregnancy served as control group. Routine laboratory, levels of bile acids and apoptosis marker caspase-cleaved cytokeratin 18 fragment (M30) were also measured.
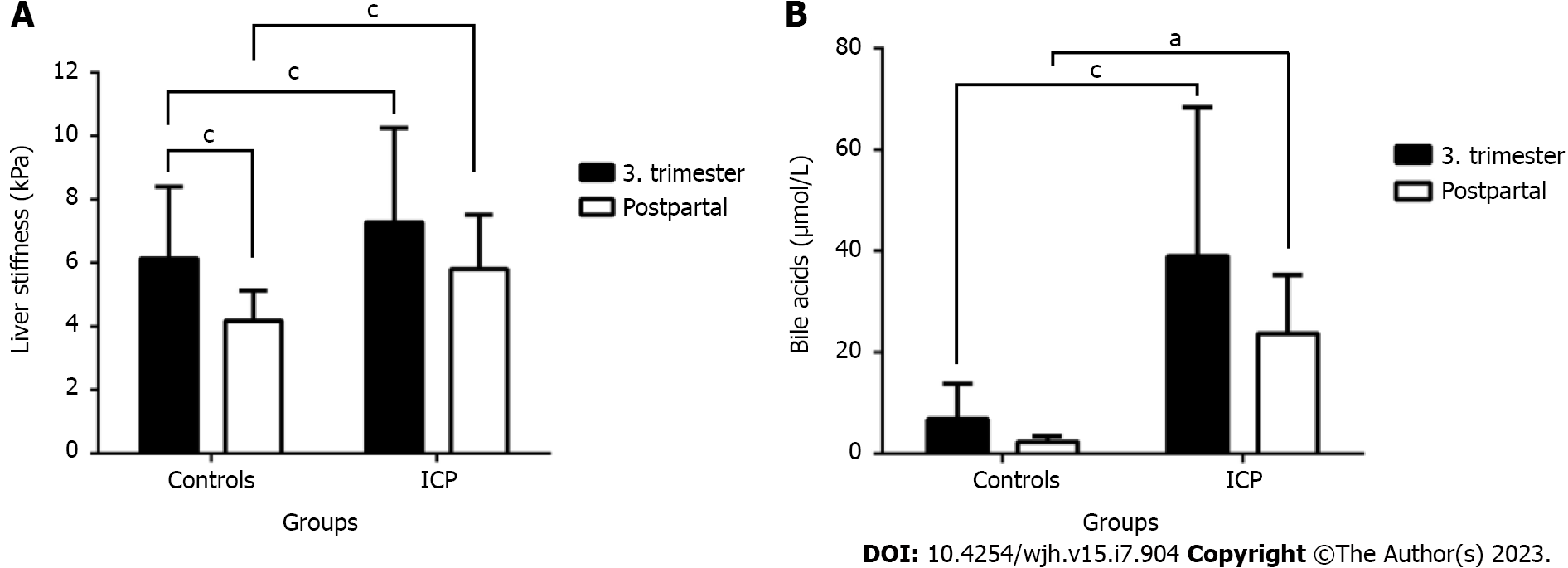
Women with ICP had significantly elevated transaminases but normal gamma-glutamyl transferase (GGT). Mean LS was significantly increased at 7.3 ± 3.0 kPa compared to the control group at 6.2 ± 2.3 kPa (P < 0.0001). Postpartum LS decreased significantly in both groups but was still higher in ICP (5.8 ± 1.7 kPa vs 4.2 ± 0.9 kPa, P < 0.0001), respectively. In ICP, LS was highly significantly correlated with levels of bile acids and M30 but not transaminases. No correlation was seen with GGT that even increased significantly after delivery in the ICP group. Bile acids were mostly correlated with the liver apoptosis marker M30, LS and levels of alanine aminotransferase, aspartate aminotransferase, and bilirubin. In multivariate analysis, LS remained the sole parameter that was independently associated with elevated bile acids.
In conclusion, LS is significantly elevated in ICP which is most likely due to toxic bile acid accumulation and hepatocyte apoptosis. In association with conventional laboratory markers, LS provides additional non-invasive information to rapidly identify women at risk for ICP.
Core Tip: Intrahepatic cholestasis of pregnancy (ICP) is a rare but severe complication in both mothers and unborn children. In a large ICP cohort, we studied liver stiffness (LS) in detail using transient elastography, which is now widely used for non-invasive screening of liver cirrhosis within minutes. LS is significantly elevated in pregnancies with ICP, most likely owing to toxic bile acid accumulation and hepatocyte apoptosis. Interestingly, no correlation was observed with γ-glutamyl transferase. In association with conventional laboratory markers, LS provides a novel non-invasive tool to rapidly identify women at risk for pregnancy complications.
- Citation: Nees J, Ammon FJ, Mueller J, Fluhr H, Mueller S. Liver stiffness in pregnant women with intrahepatic cholestasis of pregnancy: A case control study. World J Hepatol 2023; 15(7): 904-913
- URL: https://www.wjgnet.com/1948-5182/full/v15/i7/904.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i7.904
Approximately 3% of pregnant women have liver disorders that can cause severe problems for the mother and unborn child, e.g., liver failure, preterm labor, and stillbirth[1-3]. Despite intensive research on pregnancy-related liver complications in recent decades, treatment options are still insufficient, and no effective screening tests for early assessment have been established[4-6]. Intrahepatic cholestasis of pregnancy (ICP) with elevated serum bile acid levels higher than 20 μmol/L is the most common pregnancy-specific liver disease. Its etiology is complex and consists of genetic, endocrine (circulating estrogen and progesterone), and environmental factors (reduced vitamin D and selenium levels in winter). Severe forms with bile acids levels higher than > 40 μmol/L are associated with abnormal fetal echocardiography, meconium-stained amniotic fluid, spontaneous preterm labor and fetal asphyxia. Moreover, women with a total serum bile acid level of 100 μmol/L have an increased risk of stillbirth. The incidence varied between 0.05% and 27.6% for all pregnancies[7-12].
ICP typically presents in the third trimester as nocturnal pruritus of the soles and palms[13]. ICP also increases the risk of gestational diabetes and pre-eclampsia[14]. Long-term consequences of ICP include a higher risk of cancer of the liver and biliary tree, diabetes mellitus, thyroid disease, autoimmune diseases (psoriasis, inflammatory polyarthropathies, and Crohn’s disease), and cardiovascular disease[15]. Treatment with ursodeoxycholic acid has been proven to significantly improve itching, blood levels, and fetal outcomes in numerous studies; however, pregnancy termination remains the only causal therapy[2,7]. A rapid diagnosis of ICP is essential to protect mothers and children from (long-term) complications[16]. The diagnosis is normally based on elevated serum levels of bile acids. Unfortunately, these blood tests usually take several hours, even at maximum care facilities and are not available at any time. To date, no screening tests are available for liver disease during pregnancy, except serological testing for viral hepatitis in the third trimester.
Despite the scarcity of ICP data, enormous progress has been made in the molecular understanding of cholestatic liver disease in recent decades[10]. Hepatocytes and cholangiocytes cooperatively produce bile, which is a mixture of organic and inorganic compounds[17]. Cholestasis usually describes the impairment of bile flow caused by defects in hepatocytes, which form and secrete bile, and/or defects in the secretory machinery of cholangiocytes[17]. The detergent properties of bile render it highly toxic to cells and tissues[17]. In addition to drugs, inflammation, liver disease, and hormones, several gene mutations have been discovered that can cause cholestasis and ICP[8-10,18-21].
Measurement of liver stiffness (LS) using elastographic techniques has become the gold standard for the noninvasive diagnosis of liver fibrosis and cirrhosis and it often avoids invasive liver biopsies[22]. Transient elastography (TE) (FibroScan, Echosens, Paris, France), the first elastographic technique, is an ultrasound-based technique that uses a transducer probe to create an elastic shear wave[23]. Pulse-echo ultrasound is used to measure shear wave velocity, which is directly associated with LS expressed in kilopascals (kPa). TE requires only a few minutes, is highly accurate, and has a lower sampling error than biopsy, thus allowing for repetitive measurements[24]. LS values below 6 kPa are considered normal, while the generally accepted cutoff values for liver fibrosis (F3) and cirrhosis (F4) are 8 and 12.5 kPa[25]. However, LS is not only elevated by the fibrosis stage but also by other important confounding factors, including physiological conditions such as food and alcohol intake, or pathological confounders such as inflammation or pressure elevation[22]. Of note, all these confounders and artifacts will always increase LS but never decrease it, which is the most important reason, while normal LS has a very high negative predictive value in excluding liver pathologies[24].
Therefore, liver elastography is an ideal diagnostic tool to address hepatic complications during pregnancy. In the first elastography study of > 500 pregnant women without liver disease, we recently demonstrated that LS increased significantly in the third trimester and was an independent predictive factor for pre-eclampsia[26]. These findings were independently confirmed in a smaller study in Denmark[27]. Moreover, in pregnant women with cirrhosis, LS predicts hepatic decompensation after delivery[28]. The aim of the present study was to specifically explore LS in a large cohort of women with ICP compared with a control group with uncomplicated pregnancies.
The study protocol (435/2006 and S201/2015) of this observational, prospective, case-control study was approved by the Ethics Committee of the University of Heidelberg and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The study design is shown in Figure 1. Briefly, between February 2010 and March 2020, 652 women were recruited at the Department of Gynecology at the University of Heidelberg or Salem Medical Center in Heidelberg during prenatal ultrasound or who presented to the prenatal outpatient department with prenatal complications or in the ward. Postpartum examinations occurred 24 h after delivery. Inclusion criteria were age ≥ 18 years and an intact pregnancy at weeks 9 to 42 or status postpartum. The healthy control cohort was obtained from our previous study[26]. ICP was diagnosed based on typical clinical symptoms, such as pruritus, and laboratory markers, such as elevated transaminase and serum bile acid levels > 20 μmol/L. The exclusion criteria were as follows: No signed informed consent; other pregnancy complications, such as preeclampsia or HELLP syndrome; and no valid LS measurements.
Routine blood parameters were measured at the General Laboratory of University Hospital Heidelberg and Limbach Laboratory in Heidelberg. In 100 patients, serologically detected caspase-cleaved (M30) and total (M65) cytokeratin 18 levels as markers of liver apoptosis was measured as described previously using ELISA (Peviva, Bromma, Sweden)[29]. We also measured total bile acids not only in women suspected of having ICP but also in 60 women in the control group for comparative purposes.
LS and controlled attenuation parameter (CAP) were measured using TE (FibroScan, Echosens, Paris, France). M or XL probes were used according to the manufacturer’s specifications and placed in the right lobe of the liver at the intercostal position, as described previously[30]. LS and CAP values were calculated as the medians of at least 10 consecutive measurements. In parallel to liver elastography in the control group, routine abdominal ultrasound was performed to exclude liver pathologies, such as liver cirrhosis, liver congestion, or liver tumors. In addition, the degree of liver steatosis was graded (0–3) and the spleen size was determined. Cutoff values from a recent meta-analysis were used[31]. Valid LS measurements were obtained for all the women.
Statistical analyses were performed using the SPSS Statistics [version 23.0 (IBM, New York, United States), Excel 2016 (Microsoft, Redmond, United States), and GraphPad Prism 6 (GraphPad Software, San Diego, United States)]. For group comparisons, means and standard deviations were calculated, and an independent samples t-test was used. The Spearman rank-order correlation coefficient was calculated to conduct correlation analysis. Univariate and multivariate binary logistic regression analyses were used to identify the independent predictors of pregnancy complications, and receiver operating characteristic (ROC) analysis was performed.
For a better comparison, Table 1 presents only the characteristics of women (control and ICP) in the third trimester and after delivery. Almost all women with ICP (98 of 100, 98%) were in their third trimester, which is consistent with the literature[32]. In the control cohort, 228 of 450 patients (50.7%) were in the third trimester. A smaller number of women were followed up 1 d after delivery for both controls and ICP (n = 38 and n = 17). Supplementary Table 1 shows patient characteristics of the entire cohort in all trimesters. Accordingly, differences between the controls and patients with ICP remain. Women with ICP were significantly younger, and by definition, bile acids were significantly increased by a factor of approximately 6 (P < 0.0001). Women with ICP also had significantly elevated transaminase [predominantly alanine aminotransferase (ALT)] and bilirubin levels, but not elevated levels of alkaline phosphatase (AP) and gamma-glutamyl transferase (GGT). The levels of caspase 3-cleaved CK18, liver apoptosis marker M30, and uncleaved CK18 (M65), representative of liver necrosis, were significantly elevated. However, M65 showed a twofold increase in women with ICP, whereas M30 was only slightly higher in this group. Interestingly, although all liver-related parameters decreased after delivery, GGT was the only marker with postpartum elevation compared with controls.
| Parameters | Normal range1 | ICP | Control | ||
| 3. trimester, n = 98 | Postpartal, n = 17 | 3. trimester, n = 228 | Postpartal, n = 38 | ||
| Age (years) | 31.0 ± 4.2a | 28.3 ± 3.5c | 32.2 ± 5.2 | 32.9 ± 4.3 | |
| Bile acids (µmol/L) | 2-5 | 39.0 ± 29.4d | 23.7 ± 11.6a | 6.8 ± 6.9 | 2.3 ± 1.2 |
| AST (U/L) | < 35 | 116 ± 106d | 88.2 ± 80a | 26 ± 27 | 28 ± 6 |
| ALT (U/L) | < 35 | 211 ± 202d | 127 ± 106b | 18 ± 26 | 15 ± 8 |
| GGT (U/L) | < 40 | 23 ± 22 | 48 ± 40a | 30 ± 55 | 18 ± 18 |
| AP (U/L) | 35-105 | 178 ± 68 | 182 ± 73 | 150 ± 95 | 283 ± 344 |
| Bilirubin total (mg/dL) | < 1.3 | 0.65 ± 0.27d | 0.52 ± 0.56 | 0.47 ± 0.26 | 0.65 ± 0.35 |
| M30 (U/L) | < 200 | 432 ± 220a | 293 ± 75a | 339 ± 207 | 296 ± 50 |
| M65 (U/L) | < 400 | 1180 ± 571d | 777 ± 426d | 680 ± 343 | 486 ± 89 |
| Creatinine (mg/dL) | < 1.3 | 0.69 ± 0.38a | 0.88 ± 0.39a | 0.57 ± 0.12 | 0.61 ± 0.12 |
| Urea (mg/dL) | < 50 | 19.8 ± 6.8a | 25.5 ± 6.4b | 16.9 ± 4.5 | 19.4 ± 5.3 |
| Uric acid (mg/dL) | 3.5-7.2 | 5.6 ± 2.4d | 6.3 ± 2.8 | 3.9 ± 0.9 | 4.7 ± 1.0 |
| Total protein (g/L) | 66-83 | 67.6 ± 4.2b | 65.2 ± 6.8 | 64.7 ± 4.4 | 66.3 ± 9.5 |
| Albumin (g/L) | 38-59 | 35.6 ± 2.7 | 36.3 ± 12.7 | 35.7 ± 2.7 | |
| Leukocytes (1/nL) | 3.7-10.0 | 8.8 ± 2.4d | 12.0 ± 3.4a | 10.8 ± 3.0 | 15.5 ± 4.8 |
| Erythrocytes (1/pL) | 4.1-5.1 | 3.9 ± 0.4 | 3.6 ± 0.6 | 4.0 ± 0.4 | 3.7 ± 0.5 |
| Hemoglobin (g/dL) | 12-16 | 11.4 ± 1.1 | 10.3 ± 1.5 | 11.7 ± 1.3 | 10.8 ± 1.5 |
| Hematocrit (%) | 36-43 | 33 ± 3 | 30 ± 6 | ||
| MCV (/pL) | 80-96 | 86.1 ± 4.4 | 88.0 ± 3.5 | ||
| Platelets (1/nL) | 150-360 | 228 ± 72 | 241 ± 61 | 227 ± 68 | 237 ± 61 |
| Haptoglobin (g/L) | 0.3-2.0 | 0.8 ± 0.4 | 1.0 ± 0.8 | ||
| Quick (%) | 70-120 | 123.3 ± 5.1 | 119.4 ± 21.1 | 123.0 ± 13.0 | 124.5 ± 4.4 |
| INR | < 1.1 | 0.91 ± 0.04 | 0.90 ± 0.03 | 0.90 ± 0.03 | 0.90 ± 0.03 |
| Grade of steatosis in United States (0-3) | 0 | 0.42 ± 0.50a | 0.14 ± 0.38 | 0.18 ± 0.42 | 0.00 ± 0.00 |
| Spleen size (cm) | < 11 | 11.4 ± 1.9 | 11.3 ± 1.1 | 11.1 ± 1.5 | 11.1 ± 1.7 |
| Liver stiffness (kPa) | < 6 | 7.3 ± 3.0c | 5.8 ± 1.7d | 6.2 ± 2.3 | 4.2 ± 0.9 |
| CAP (dB/m) | < 290 (S3) | 206 ± 49c | 213 ± 43 | 228 ± 39 | 224 ± 46 |
As shown in Figure 2A and Table 1, LS was significantly higher in women with ICP, both before and after delivery. Moreover, in both groups, we observed an increase in LS as pregnancy progressed. In the ICP group, mean LS was significantly increased at 7.3 ± 3.0 kPa compared with the control group at 6.2 ± 2.3 kPa (P < 0.0001). An increase in LS to > 6 kPa was observed in 24.9% of healthy pregnant women and 44.2% of the ICP group, whereas an elevated LS of > 8 kPa was observed in 7.1% of the control and 15.6% of the ICP group. LS of > 12.5 kPa considered above the cutoff value for cirrhosis was measured in 3.0% of the control and ICP groups. In confirmation to our initial study[26], however, postpartum LS decreased significantly in both groups to 5.8 ± 1.7 kPa in the ICP group and 4.2 ± 0.9 kPa in the control group, respectively. Finally, hepatic steatosis, as measured by the CAP, was normal in most women. It slightly but significantly increased in the ICP group during delivery, from 206 to 213 dB/m, whereas no significant changes were observed in the controls (Table 1). In summary, although LS generally increases during pregnancy, the liver is signifi
Next, we performed Spearman’s rho correlation analysis with clinical and laboratory parameters to identify potential confounders associated with elevated LS. Table 2 shows the results of the ICP, control, and total cohorts. In the ICP cohort, only a few parameters were significantly correlated with LS, namely, serum levels of bile acids and the liver apoptosis marker M30. Bilirubin levels hardly met the level of significance, whereas leukocyte count and Quick’s test results were negatively correlated. No association between LS and bile acid levels was observed in the control group, and M30 Levels were weakly but significantly correlated with LS. In contrast, as described recently[26], LS significantly correlated with the duration of pregnancy, onset of gestational diabetes, body weight, mean arterial diastolic pressure, and AP and M65 levels in the total cohort. Interestingly, AST levels, which are usually highly associated with LS in liver diseases[33], were significantly correlated in the total cohort but not in the ICP or control groups alone. No correlation was observed between GGT and ALT levels in the ICP cohort. Finally, no association was observed between markers of hemolysis or anemia. In conclusion, in women with diagnosed ICP, bile acids are tightly associated with elevated LS and markers of liver apoptosis, but not with conventional liver function tests, except for bilirubin.
| Parameter | Spearman rho correlation with liver stiffness | ||
| ICP, n = 100, r | Control, n = 450, r | All, n = 550, r | |
| Bile acids total (μmol/L) | 0.368c | 0.085 | 0.438d |
| M30 (U/L) | 0.881b | 0.313b | 0.385c |
| Quick (%) | -0.276b | 0.210a | -0.010 |
| Leukocytes (1/nL) | -0.223a | 0.012 | -0.203b |
| Bilirubin total (mg/dL) | 0.213a | -0.091 | 0.124 |
| MAD (mmHg) | -0.379 | 0.137b | 0.154c |
| Gestational diabetes (1 or 0) | 0.376 | 0.269d | 0.301d |
| Pruritus (1 or 0) | 0.353 | -0.015 | 0.246d |
| Spleen size (cm) | 0.340 | -0.032 | 0.014 |
| Uric acid (mg/dL) | 0.308 | 0.261a | 0.411d |
| AST (U/L) | 0.146 | 0.103 | 0.327d |
| Creatinine (mg/dL) | 0.281 | 0.057 | 0.163 |
| Body weight (kg) | 0.241 | 0.301d | 0.317d |
| Platelets (1/nL) | -0.119 | 0.000 | -0.074 |
| AP (U/L) | 0.237 | 0.329c | 0.378d |
| Urea (mg/dL) | 0.234 | -0.064 | 0.041 |
| Hemoglobin (g/dL) | -0.112 | -0.022 | -0.104 |
| CAP (dB/m) | 0.097 | 0.083 | -0.006 |
| M65 (U/L) | 0.357 | 0.389c | 0.452d |
| ALT (U/L) | 0.048 | 0.047 | 0.273d |
| GGT (U/L) | -0.010 | 0.114 | 0.150 |
Figure 2B shows the levels of bile acids in both controls and women with ICP before and after delivery. Bile acid levels, which are a major criterion for the diagnosis of ICP were about six times elevated in the ICP cohort and promptly decreased after delivery. A slight but significant decrease was observed in the control group, suggesting that pregnancy causes bile acid elevation. Notably, bile acid levels were markedly elevated in the ICP cohort after delivery. Supple
In this study, we noninvasively measured LS through TE and steatosis using CAP in a large cohort of pregnant women with diagnosed ICP, primarily through elevated bile acids. Our data clearly show that LS is higher in women with ICP than those in controls. Although LS decreased rapidly after delivery, as described recently[26,27], it remained significantly higher in women with ICP despite identical follow-up observation times. Women with ICP predominantly had elevated ALT levels, followed by those with elevated AST and bilirubin levels. In addition to AP levels, no differences were observed in GGT levels. Although all parameters, including LS, improved after delivery, GGT levels increased significantly in the ICP cohort. In addition, hepatic fat content, as measured using CAP, although within the normal range, was lower in patients with ICP than in controls. Finally, the liver apoptosis marker M30 showed the highest association with bile acid levels in univariate regression analysis, whereas LS remained the strongest independent predictor of bile acid levels > 20 mol/L in multivariate regression analysis.
In confirmation of earlier reports[26-28], the present study demonstrates that noninvasive assessment of LS through elastography is feasible and well accepted in pregnant women. In contrast to reports from internal medicine departments[34], elastography can be performed in all women. Second, we showed that LS is significantly elevated in women with ICP and higher than that in controls, which is remarkable, as we and others showed that LS is generally and reversibly elevated in the third trimester[26,27]. Consequently, and comparable to the previously reported LS elevation in women with preeclampsia, LS can be considered a feasible and noninvasive tool for screening, identifying, and following women with pregnancy-related liver complications.
What are the confounding factors for LS elevation in women with ICP? In contrast to initial beliefs, LS can be elevated because of many confounding factors, including inflammation, arterial and venous pressure elevation, and physiological conditions such as meal intake[22,24]. Mechanic cholestasis has been demonstrated to reversibly increase LS[35]. Of note, however, continued elevation of LS owing to these confounders has a negative impact on the liver, and the first long-term mortality data demonstrated that LS is one of the best long-term predictors of liver-related and all-cause mortality[22]. LS measurement can also be used to identify and monitor pregnant women with preexisting liver cirrhosis and predict hepatic decompensation[28]. The first study in women with uncomplicated pregnancies showed that elevated LS was significantly correlated with AP, leukocytes, gestational age, and an increase in body weight[26]. In the present study, in women with ICP, the confounders were completely different, and LS was tightly associated with elevated serum levels of bile acids and serum markers of liver apoptosis (M30). This is particularly interesting with regard to the fact that in liver diseases, such as alcoholic liver disease or viral hepatitis, LS elevation is typically associated with transaminase levels, namely, AST but not ALT[33]. Although we show here that LS is an independent predictor of elevated bile acids, the performance of LS in predicting ICP was only moderate and lower than that in the previous smaller ICP cohort[26].
To the best of our knowledge, this is the first study to show an exceptionally strong association between the established serum liver apoptosis marker (M30) with levels and bile acid levels in women with ICP and its association with elevated LS. In the multivariate analysis, LS remained the only parameter independently associated with elevated bile acid levels. In patients with liver disease, the association between liver apoptosis and LS elevation has already been shown both at the histological level[36] and using serum markers, such as M30[29]. The tight association of bile acids with LS and M30 levels in pregnancy is new. Bile acids are synthesized in hepatocytes as essential components for bile formation[9,17]. Owing to their detergent nature, however, they are highly cytotoxic and can disrupt cellular membranes if not protected, e.g., by phospholipids[9,17,19]. Specifically, serum cholic acid becomes the primary bile acid in women with ICP, in contrast to normal pregnant and non-pregnant women, whose proportion is similar to that of chenodeoxycholic acid[37]. Typical examples are cholestatic liver diseases, such as primary biliary cirrhosis, which ultimately cause chronic bile duct inflammation, and later cirrhosis and cancer. Even simple mechanical cholestasis through obstruction of the major bile ducts by biliary stones can cause severe tissue damage.
In the last three decades, many gene mutations have been discovered that can cause cholestasis through the impairment of hepatocyte or cholangiocyte transport proteins relevant to bile formation[8-10,18-20]. These findings have resulted in a group of diseases known as progressive familial intrahepatic cholestasis. The normal GGT levels in our ICP cohort are a strong argument for a genetic cause. Hormonal changes/normalization after delivery with subsequent normalization of bile acid export through the hepatocellular apical membrane are considered important for the role of sex hormones in ICP[9]. In line with this, we observed a postpartum increase in GGT in our ICP cohort, suggesting a reinduction of GGT with the onset of bile flow.
Surprisingly, hepatic steatosis, as measured by CAP, which is now widely explored in patients with fatty liver[38], did not show any conclusive data in women with normal pregnancy or with ICP. The reason for this remains unclear because we expected that at least some women would present with steatosis, which can be a severe complication of pregnancy. We also briefly discuss some of the limitations of our study, which are mostly due to the challenging setting of performing clinical studies during late pregnancy, particularly in women with suspected complications. Serum was not available for all women to allow for the subsequent measurement of markers such as M30 and M65. In addition, we managed to measure LS sequentially before and after delivery in only a few cases in the same person. Another limitation with regard to postpartum follow-up measurements was that we only included women 24 h after delivery. This short time may explain why some parameters did not reach statistical significance.
In conclusion, we showed in a large cohort that women with ICP show significantly elevated LS compared with women with uncomplicated pregnancies. In contrast to the large body of evidence in the liver literature, elevated LS in ICP is primarily correlated with the accumulation of bile acids known to be highly toxic to hepatocytes, and liver apoptosis, as measured through M30 levels, but not with transaminases, bilirubin, or GGT. We also showed for the first time that typically low GGT levels in ICP increase after delivery. Consequently, we believe that screening for LS in pregnancy is not “another diagnostic tool” to further complicate the already intensive surveillance during pregnancy, but could provide a novel non-invasive strategy to early identify women at risk for complications.
Intrahepatic cholestasis of pregnancy (ICP) is a rare but severe hepatic complication for both mother and unborn child. Diagnosis is normally based on elevated serum levels of bile acids. Unfortunately, these blood tests usually take several hours, even at maximum care facilities. So far, there are no screening test for liver disease in pregnancy besides serological testing for viral hepatitis in the third trimester.
Measurement of liver stiffness (LS) through elastographic techniques has become the novel gold standard for the non-invasive diagnosis of liver cirrhosis often avoiding invasive liver biopsies. LS is not only highly correlated to the hepatic fibrosis stage but can also be elevated due to other important confounding factors such as inflammation, congestion or cholestasis. For these reasons, liver elastography could be an ideal diagnostic tool to address hepatic complications during pregnancy.
The aim of the present study was to specifically explore LS in a large cohort of women with ICP before and after delivery compared to a control group with uncomplicated pregnancy.
LS and the hepatic steatosis marker controlled attenuation parameter (CAP) were measured in 100 pregnant women with ICP using transient elastography (Fibroscan, Echosens, Paris, France). In 17 cases, LS could be measured after delivery. A large cohort of women with uncomplicated pregnancy served as control group. Routine laboratory, levels of bile acids and the apoptosis marker M30 were also measured.
In the third trimester, women with ICP show a significantly increased LS at 7.3 ± 3.0 kPa compared to controls (6.2 ± 2.3 kPa, P < 0.0001). LS decreases significantly 24 h after deliver and remains higher in ICP (5.8 ± 1.7 kPa vs 4.2 ± 0.9 kPa, P < 0.0001). In ICP, LS is mainly correlated with levels of bile acids and the apoptosis marker M30. No correlation was seen with GGT and GGT even increased after delivery in women with ICP.
In conclusion, LS is significantly elevated in ICP which is most likely due to toxic bile acid accumulation and hepatocyte apoptosis. In association with conventional laboratory markers, LS provides additional non-invasive information to rapidly identify women at risk for ICP.
In the future, elastography should be further validated in order to early identify women at risk for complications. Moreover, elastography studies should be combined with genetic risk assessment, as several mutations of bile transport proteins are involved in the development of ICP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver, No. 3538; European Society for Biomedical Research on Alcoholism.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Italy; Sirli RLD, Romania S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA. Liver disease in pregnancy. Lancet. 2010;375:594-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64:933-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Hay JE. Liver disease in pregnancy. Hepatology. 2008;47:1067-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Mihu D, Costin N, Mihu CM, Seicean A, Ciortea R. HELLP syndrome - a multisystemic disorder. J Gastrointestin Liver Dis. 2007;16:419-424. [PubMed] [Cited in This Article: ] |
| 5. | Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010;24:104-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Suresh I, Tr V, Hp N. Predictors of Fetal and Maternal Outcome in the Crucible of Hepatic Dysfunction During Pregnancy. Gastroenterology Res. 2017;10:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 7. | Ovadia C, Williamson C. Intrahepatic cholestasis of pregnancy: Recent advances. Clin Dermatol. 2016;34:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Sticova E, Jirsa M, Pawłowska J. New Insights in Genetic Cholestasis: From Molecular Mechanisms to Clinical Implications. Can J Gastroenterol Hepatol. 2018;2018:2313675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Pauli-Magnus C, Meier PJ, Stieger B. Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy. Semin Liver Dis. 2010;30:147-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Amirneni S, Haep N, Gad MA, Soto-Gutierrez A, Squires JE, Florentino RM. Molecular overview of progressive familial intrahepatic cholestasis. World J Gastroenterol. 2020;26:7470-7484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (4)] |
| 11. | Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15:2049-2066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 367] [Cited by in F6Publishing: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 524] [Cited by in F6Publishing: 464] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 13. | Kenyon AP, Tribe RM, Nelson-Piercy C, Girling JC, Williamson C, Seed PT, Vaughan-Jones S, Shennan AH. Pruritus in pregnancy: a study of anatomical distribution and prevalence in relation to the development of obstetric cholestasis. Obstet Med. 2010;3:25-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Wikström Shemer E, Marschall HU, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG. 2013;120:717-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Wikström Shemer EA, Stephansson O, Thuresson M, Thorsell M, Ludvigsson JF, Marschall HU. Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: A population-based cohort study. J Hepatol. 2015;63:456-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Mikolasevic I, Filipec-Kanizaj T, Jakopcic I, Majurec I, Brncic-Fischer A, Sobocan N, Hrstic I, Stimac T, Stimac D, Milic S. Liver Disease During Pregnancy: A Challenging Clinical Issue. Med Sci Monit. 2018;24:4080-4090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 446] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 18. | Carlton VE, Pawlikowska L, Bull LN. Molecular basis of intrahepatic cholestasis. Ann Med. 2004;36:606-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Jansen PL, Müller M. Genetic cholestasis: lessons from the molecular physiology of bile formation. Can J Gastroenterol. 2000;14:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Hoofnagle JH, Björnsson ES. Drug-Induced Liver Injury - Types and Phenotypes. N Engl J Med. 2019;381:264-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 21. | Palmer KR, Xiaohua L, Mol BW. Management of intrahepatic cholestasis in pregnancy. Lancet. 2019;393:853-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Mueller S. Liver Elastography: Clinical Use and Interpretation. Germany: Springer International Publishing, 2020. [Cited in This Article: ] |
| 23. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 24. | Mueller S. Introduction to Liver Stiffness: A Novel Parameter for the Diagnosis of Liver Disease. Liver Elastography: Springer, 2020: 3-9. [Cited in This Article: ] |
| 25. | Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2010;2:49-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Ammon FJ, Kohlhaas A, Elshaarawy O, Mueller J, Bruckner T, Sohn C, Fluhr G, Fluhr H, Mueller S. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. World J Gastroenterol. 2018;24:4393-4402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Stenberg Ribeiro M, Hagström H, Stål P, Ajne G. Transient liver elastography in normal pregnancy - a longitudinal cohort study. Scand J Gastroenterol. 2019;54:761-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Elshaarawy O, Abdelaziz R, Zayed N, Hany A, Hammam Z, Mueller S, Yosry A, Shousha HI. Acoustic radiation force impulse to measure liver stiffness and predict hepatic decompensation in pregnancy with cirrhosis: A cohort study. Arab J Gastroenterol. 2022;23:89-94. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 29. | Mueller S, Nahon P, Rausch V, Peccerella T, Silva I, Yagmur E, Straub BK, Lackner C, Seitz HK, Rufat P, Sutton A, Bantel H, Longerich T. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology. 2017;66:96-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:14626-14641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 99] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (2)] |
| 31. | Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, de Lédinghen V, Baumeler S, Chan WK, Perlemuter G, Cardoso AC, Aggarwal S, Sasso M, Eddowes PJ, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Cobbold JF, Naveau S, Lupsor-Platon M, Mueller S, Krag A, Irles-Depe M, Semela D, Wong GL, Wong VW, Villela-Nogueira CA, Garg H, Chazouillères O, Wiegand J, Karlas T. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 32. | Kamimura K, Abe H, Kawai H, Kamimura H, Kobayashi Y, Nomoto M, Aoyagi Y, Terai S. Advances in understanding and treating liver diseases during pregnancy: A review. World J Gastroenterol. 2015;21:5183-5190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Mueller S, Englert S, Seitz HK, Badea RI, Erhardt A, Bozaari B, Beaugrand M, Lupșor-Platon M. Inflammation-adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 2015;35:2514-2521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Boursier J. Quality criteria for liver stiffness measurement by transient elastography. Liver Elastography: Springer, 2020: 479-494. [Cited in This Article: ] |
| 35. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 36. | Mueller S, Lackner C. Histological Confounders of Liver Stiffness. Liver Elastography: Springer, 2020: 233-242. [Cited in This Article: ] |
| 37. | Heikkinen J, Mäentausta O, Ylöstalo P, Jänne O. Changes in serum bile acid concentrations during normal pregnancy, in patients with intrahepatic cholestasis of pregnancy and in pregnant women with itching. Br J Obstet Gynaecol. 1981;88:240-245. [PubMed] [Cited in This Article: ] |
| 38. | Karlas T, Mueller S. Liver Steatosis (CAP) as Modifier of Liver Stiffness. Liver Elastography: Springer, 2020: 459-467. [Cited in This Article: ] |