Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1317
Peer-review started: March 30, 2023
First decision: June 17, 2023
Revised: July 3, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: August 15, 2023
Colitis-associated colorectal cancer (CAC) is defined as a specific cluster of colorectal cancers that develop as a result of prolonged colitis in patients with inflammatory bowel disease (IBD). Patients with IBD, including ulcerative colitis and Crohn’s disease, are known to have an increased risk of developing CAC. Although the incidence of CAC has significantly decreased over the past few decades, individuals with CAC have increased mortality compared to individuals with sporadic colorectal cancer, and the incidence of CAC increases with duration. Chronic inflammation is generally recognized as a major contributor to the pathogenesis of CAC. CAC has been shown to progress from colitis to dysplasia and finally to carcinoma. Accumulating evidence suggests that multiple immune-mediated pathways, DNA damage pathways, and pathogens are involved in the pathogenesis of CAC. Over the past decade, there has been an increasing effort to develop clinical approaches that could help improve outcomes for CAC patients. Colonoscopic surveillance plays an important role in reducing the risk of advanced and interval cancers. It is generally recommended that CAC patients undergo endoscopic removal or colectomy. This review summarizes the current understanding of CAC, particularly its epidemiology, mechanisms, and management. It focuses on the mechanisms that contribute to the development of CAC, covering advances in genomics, immunology, and the microbiome; presents evidence for management strategies, including endoscopy and colectomy; and discusses new strategies to interfere with the process and development of CAC. These scientific findings will pave the way for the management of CAC in the near future.
Core Tip: Colitis-associated colorectal cancer (CAC) is defined as a specific cluster of colorectal cancers that develop as a result of prolonged colitis in patients with inflammatory bowel disease (IBD). Patients with IBD are known to have an increased risk of developing CAC. Accumulating evidence suggests that multiple immune-mediated pathways, DNA damage pathways, and pathogens are involved in the pathogenesis of CAC. This review summarizes the current understanding of CAC, particularly its epidemiology, mechanisms, and management. These scientific findings will pave the way for the management of CAC in the near future.
- Citation: Dan WY, Zhou GZ, Peng LH, Pan F. Update and latest advances in mechanisms and management of colitis-associated colorectal cancer. World J Gastrointest Oncol 2023; 15(8): 1317-1331
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1317.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1317
Colitis-associated colorectal cancer (CAC) is defined as a specific type of colorectal cancer (CRC) that develops in patients with inflammatory bowel disease (IBD) as a result of prolonged colitis, especially in ulcerative colitis (UC). The un
The mechanisms underpinning CAC pathogenesis remain elusive, but accumulated evidence suggests that genetics and epigenetics, immunity and inflammation, and microbiota are all involved in CAC pathogenesis. Given this evidence, new strategies are being applied to the treatment of CAC. Therefore, we provide a comprehensive and critical review of recent advances in CAC, focusing on its epidemiology, pathogenesis, and management, including surveillance, surgery, chemoprevention, and new strategies for CAC treatment.
It is widely recognized that long-standing colitis increases the risk of CAC, but estimates of the risk vary widely in the available studies. An approximately 2-fold increase in the risk of developing CAC[1,4-7] and a 1.7-fold increase in the risk of death have been reported[8]. Based on a population-based cohort study, there is 1 additional CRC diagnosis per 1058 UC patients and 1 additional CRC death per 3041 UC patients per 5 years[2]. The prevalence of CAC fluctuates with multiple factors, such as time, region, disease classification, extent, and duration. A previous meta-analysis reported that the prevalence of UC-associated CRC was 3.7%[9], while recent meta-analyses indicated a lower prevalence of UC-associated CRC in Asia at 0.85%[10], which could be explained by optimized surveillance and better chemoprevention.
The cumulative incidence of CAC varies across studies. A large Swedish population-based cohort study reported an overall cumulative incidence of CAC of 1.0%, 1.5%, and 2.7% after 10, 20, and 30 years of disease, respectively[1]. The cumulative risk of UC-associated CRC appeared to be higher in Asia and was reported to be 0.02%, 4.81%, and 13.91% in Asian patients[10] as well as 1.15%, 3.56%, and 14.36% in Chinese[11] patients at 10, 20, and 30 years of disease diagnosis. In addition, a meta-analysis of population-based cohort studies reported the cumulative risks of CAC as 1% after 10 years, 2% after 20 years, and 5% after over 20 years of disease duration[6].
In addition to disease duration, disease classification and extent are also considered to be important parameters influencing an individual’s risk of CAC. Compared with the general population, patients with UC and CD are associated with 1.47- to 2.70-fold and 1.51- to 2.10-fold increased risks of CRC, respectively[1,4-7]. Compared to those with proctitis UC, patients with extensive UC and left-sided UC are at higher risk of CRC[12]. Specially, certain populations with autoimmune diseases are known to have a higher risk of developing anal cancer than average, such as UC patients with an incidence rate of 276167 py and CD patients with an incidence rate of 614830 py[13].
It is noteworthy that the incidence of UC-associated CRC has steadily declined over the last six decades. The cumulative incidence declined from 33.1 per 1000 patients in studies published in the 1950s to 9.1 per 1000 patients in studies published in the last decade, while the incidence rates declined from 4.29 per 1000 py to 1.21 per 1000 py, respectively[14]. The prognosis for CAC has dramatically improved. Compared to the general population, cancer risk in IBD patients decreased from a 5-fold increase in the 1960s to a 2-fold increase in the 2000–2004 follow-up period[1].
The worldwide cancer incidence rates of UC-associated CRC show considerable geographical variation. The overall incidence rate varies from 5/1000 person-years duration (pyd) in the USA, 4/1000 pyd in the UK, and 2/1000 pyd in Scandinavia[9]. Geography also plays an underlying role in CAC prognosis. The time of malignant transformation started after 10-20 years of CAC duration in Asian patients, whereas it significantly increased to more than 30 years in North American patients[12].
Recent data have shown an association between family history and CAC. Patients with a family history of CRC in a first-degree relative have an almost 8-fold increase in the risk of CAC, while those without a family history have a 4-fold increase in risk[15]. Additional risk factors include hyperlipidemia, obesity, and alcohol consumption for early-onset colorectal cancer[16].
Wijnands et al[17] summarized the data that describe the prognostic factors for advanced colorectal neoplasia in IBD, identifying risk factors (including extensive disease, low-grade dysplasia, colonic strictures, post-inflammatory polyps, primary sclerosing cholangitis, and family history of CRC) and protective factors (including colonoscopic surveillance, 5-aminosalicylic acid, thiopurines, and smoking).
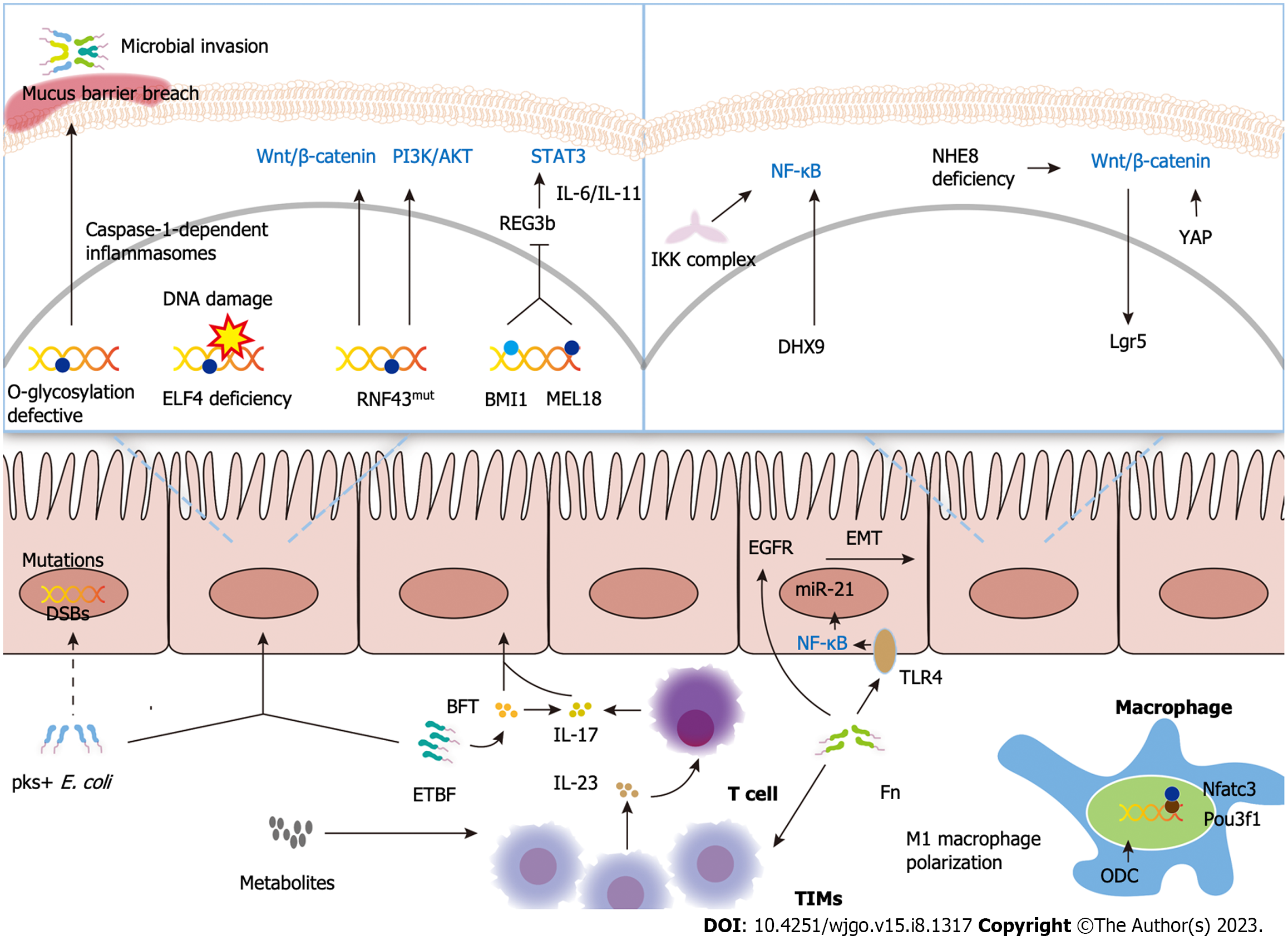
The main classical pathways recognized for CRC carcinogenesis are the adenoma-carcinoma sequence, the serrated pathway, and the inflammatory pathway. The “adenoma-carcinoma sequence” hypothesis suggests that the accumulation of genetic and epigenetic abnormalities drives the transformation of normal cells into adenomas that progress to CRC, explaining the pathogenesis of most CRCs. However, as a specific subtype of CRC, CAC, which develops progressively in patients with IBD, has a unique pathogenesis. Here, the pathogenesis and recent advances underlying CAC are reviewed by three mechanisms: Genetics and epigenetics, immunity and inflammation, and microbiota, illustrated in Figure 1.
DNA is the basis of genetic information, and maintaining its integrity is essential for life and health. In the early 1900s, scientists developed the idea that “at the molecular level, tumors are the result of damage to cellular DNA”. DNA damage, such as genetic mutations, double-strand breaks (DSBs), and oxidative stress, can be caused by endogenous (e.g., reactive oxygen species) and exogenous factors (e.g., UV light). According to the widely accepted theory, DSBs and oxidative stress are closely related to cancers. DSB is one of the most critical and dangerous types of DNA damage that, if not repaired, can lead to cell death. Oxidative stress to DNA and DSBs might drive colitis-associated colorectal carcinogenesis in IBD patients[18]. In contrast to UC, CAC has a unique mutational profile. It is notable that mutations in NFKBIZ are of high frequency in UC but are rarely found in CAC, which suggests a discrete mechanism in colorectal carcinogenesis[19]. A study investigating somatic mutations in CAC found high frequencies of RNF43 mutations in CAC somatic cells. RNA-Seq analysis revealed elevated c-Myc and target gene expression in RNF43-mutated tumors, suggesting that RNF43 is a driver of colorectal tumorigenesis[20]. In addition, the proto-oncogene BMI1 and its target anti-oncogene Reg3b were identified as being closely associated with the development of CAC. A separate series of in vitro and in vivo studies demonstrated that BMI1 expression was elevated in CAC patients, that high BMI1 expression was associated with a lower response rate to antitumor necrosis factor α (TNF-α) therapy[21] and that BMI1 and its homologue MEL18 promoted cancer by inhibiting Reg3b expression[22].
Epigenetics is important for tumor initiation. The hypothesis of “epigenetic triggers in cancer initiation” is that once endogenous or environmental stimuli trigger epigenetic initiation in cancer-initiating cells, this leads to the development and progression of tumors[23]. ELF4, a member of the E-Twenty-Six domain transcription factor family, is involved in the regulation of a variety of DNA damage repair mechanisms. ELF4 suppression caused by methylation of the promoter region is prevalent in UC and CAC, supporting the “epigenetic triggers in cancer initiation” hypothesis[24]. One study by Emmett and colleagues evaluated DNA methylation patterns in CAC and sporadic CRC and found that several genes were highly methylated in CAC, such as MINT1, MYOD, and the promoter regions of EYA4 and ESR[25]. In addition to DNA methylation, defects in DNA glycosylation have also been implicated in the pathogenesis of CAC. A breakdown of the colonic mucus barrier mediated by impaired O-glycosylation expression was identified to result in spontaneous CAC in mice[26]. Based on these findings, genetics and epigenetics are involved in the development of CAC.
The most comprehensively studied proinflammatory and protumor pathways in CAC are the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), β-catenin-mediated Wnt signaling, signal transducer and activator of transcription 3 (STAT3)/interleukin-6 (IL-6), and IL-23/T helper 17 cell (Th17) pathways. It has been well established that activation of intestinal NF-κB signaling promotes colitis-associated carcinogenesis[27]. DHX9 was overexpressed in CRC tissue and CAC mouse models. DHX9 was found to promote NF-κB-mediated transcriptional activity and enhance the expression of downstream targets of NF-κB, thereby promoting colorectal carcinogenesis[28]. In the early stages of colitis-associated carcinogenesis, the Wnt pathway is activated in 50% of cases[29]. Recent studies support the involvement of the WNT signaling pathway in the pathogenesis of CAC. The Wnt/β-catenin signaling pathway is activated through the deletion of NHE8, a multifunctional protein expressed in colon stem cells, leading to increased expression of Lgr5 in colon tissue, which is a target gene for Wnt signaling and ultimately exhibits a favorable outcome of colitis-associated tumorigenesis[30]. Excess yes-associated protein 1 triggers the Wnt/β-catenin signaling pathway and promotes colitis-associated tumorigenesis[31]. Furthermore, upregulation of IL-23 and IL-17 has been demonstrated in clinical CRC specimens and CRC mouse models. It has been shown that barrier disruption due to genetic damage leads to the invasion of microbial products, triggering IL-23- and IL-17-driven inflammatory infiltration of tumors, which in turn drives tumor growth[32].
Both the innate and adaptive immune systems play important roles in the development and progression of CAC. Macrophages are the most well-known member of the intrinsic immune system. Owing to the known potential of Pou3f1 in regulating immune responses and the immune system, investigators have investigated the role of Pou3f1 in colitis-associated colorectal tumorigenesis. Reduced secretion of inflammatory mediators in macrophages by knocking out Pou3f1 inhibited CAC development[33]. Ornithine decarboxylase in macrophages promotes colon carcinogenesis by impairing the M1 immune response[34]. Tumor-infiltrating T cells have been demonstrated to contribute to CAC immunosurveillance. Interestingly, the incidence of CAC was found to increase after an appendectomy, which may accelerate tumor development by inhibiting T-cell initiation and subsequently reducing cancer immune surveillance, as well as by dysbiosis of intestinal microbes and impairment of the intestinal barrier[35,36].
There are 3 main microbial hypotheses associated with the pathogenesis of CAC, including the “α-bug” hypothesis[37], the “driver-passenger” hypothesis[38], and the “common ground” hypothesis. According to the “common ground” hypothesis, exogenous and endogenous factors (unhealthy diet, exogenous contaminants, and chronic inflammation) create a “leaky gut” that allows pathogens to become highly permeable across cells and internalize bacteria, leading to chronic inflammation and morphological changes[39]. Accumulated evidence supports a significant role for the microbiota in CRC development and progression, focusing on Escherichia coli (E. coli), Bacteroides fragilis (B. fragilis), and Fusobacterium nucleatum (F. nucleatum).
E. coli can produce colibactin, which is encoded by the pathogenicity island polyketide synthesis (pks) gene. E. coli carrying the pks island causes DSBs and activation of the DNA damage checkpoint pathway, which may be a predisposing factor for CRC development[40]. A novel area of research confirmed a distinct mutational signature with unique single-base substitution, insertion, and deletion mutations in CRC caused by colibactin from pks+E. coli[41]. However, there appeared to be no significant differences between pks+E. coli and the prevalence of CRC or colorectal adenoma lesion patients in a Japanese prospective cohort study[42] and a Canadian cohort study[43]. Adherent invasive E. coli (AIEC)-associated genes, including chitinase 3-like1, carcinoembryonic antigen-related cell adhesion molecule 6, and claudin-2, as well as the protein expression of these genes, were found to be upregulated in CAC samples, suggesting an underlying link between AIEC and CAC[44].
Unlike pks+ E. coli, no unique mutational signature was produced by enterotoxigenic B. fragilis (ETBF). Allen et al[45] performed whole-exome sequencing and whole-genome sequencing and found that errors in DNA mismatch repair and homologous recombination DNA damage repair were involved in the pathogenesis of ETBF-induced CRC. Notably, a unique ETBF-associated colonic immune infiltrate was observed in a CRC murine model. The combined effect of IL-17 and B. fragilis toxin, specifically secreted by ETBF on colon epithelial cells, ultimately led to the suppression of T-cell proliferation[46].
In addition, F. nucleatum may contribute to the development and progression of CRC in several ways. It promotes epithelial-mesenchymal transition through the epidermal growth factor receptor signaling pathway, thereby accelerating the progression of CAC[47]; it recruits tumor-infiltrating immune cells, leading to a proinflammatory microenvironment conducive to colorectal carcinogenesis[48]; and it activates the NF-κB signaling pathway by activating Toll-like receptor 4 signaling, thereby upregulating microRNA-21 expression and leading to accelerated tumor progression[49]. Further efforts must be made to unravel the complexity between the microbiota and CAC.
Surveillance colonoscopy in patients with IBD may allow earlier detection of colorectal neoplasms and therefore improve prognosis. Research from the Cochrane Library has confirmed a significant reduction in mortality associated with CAC in the surveillance group compared to the non-surveillance group[50]. Given the evidence, surveillance colonoscopy is warranted in patients with IBD, especially those at high risk for CAC. To standardize the management of patients after the onset of IBD, many guidelines or expert consensus have been published to guide endoscopic surveillance in patients with IBD (Table 1)[51-64]. There is considerable variation in the recommendations of these guidelines, which detail the timing of surveillance colonoscopy, surveillance intervals, and new screening modalities.
| Society | Disease type | Initiation | Risk categories | Surveillance intervals | Endoscopic selection of dysplasia detection |
| ACG, 2019; Guideline[51] | UC | 8 yr; concomitant PSC: From diagnosis | No specific recommendation | UC: 1-3 yr; concomitant PSC: 1 yr | Dye spray chromoendoscopy with methylene blue or indigo carmine; white-light endoscopy with narrow-band imaging |
| ACG, 2018; Guideline[56] | CD | No specific recommendation | Colonoscopy with chromoendoscopy: high risk for colorectal neoplasia1 | ||
| AGA, 2021; expert consensus[52] | IBD | 8-10 yr; after a negative screening colonoscopy: 1-5 yr; concomitant PSC: From diagnosis | No specific recommendation | High risk for developing colorectal dysplasia2, persistent moderate-severe pouchitis, and/or pre-pouch ileitis: At least 1 yr | Dye spray chromoendoscopy; high-definition endoscopy with virtual chromoendoscopy |
| AOCC and APAG, 2021; expert consensus[57] | IBD | 8 yr | UC patients with LGD in flat mucosae: In 3-6 mo | No specific recommendation | |
| BSG, 2019; guideline[58] | IBD | 8 yr; concomitant PSC: From diagnosis | Lower risk: Extensive colitis with no active inflammation; colitis affecting < 50% of the colon; intermediate risk: extensive colitis with mildly active inflammation; post-inflammatory polyps; CRC in an FDR older than 50 yr; higher risk: Extensive colitis with moderate-to-severely active inflammation; stricture or dysplasia in last 5 yr; history of PSC (including after orthotopic liver transplantation); CRC in a FDR younger than 50 yr | Lower risk: 5 yr; intermediate risk: 3 yr; higher risk: 1 yr | High-definition colonoscopy with chromoendoscopy |
| CCA, 2018; Guideline[59] | IBD | 8-10 yr | Lower risk: Quiescent disease and no other risk factors; intermediate risk: Quiescent disease without high risk factors; family history of CRC in an FDR; higher risk: Chronic active inflammation; prior colorectal dysplasia; evidence of intestinal damage with foreshortened tubular colon, colonic stricture, or pseudopolyps; PSC; family history of CRC younger than 50 yr | Lower risk: 5 yr; Intermediate risk: 3 yr; higher risk: 1 yr | Colonoscopy with chromoendoscopy |
| CSG, 2018; Chinese consensus[53] | IBD | 8-10 yr | No specific recommendation | UC: 8-10 yr; montreal type E2: 2 yr (15 yr after the onset of the disease); montreal type E3: 2 yr (8-10 yr after the onset of the disease); 1 yr (after 20 yr); concomitant PSC: 1 yr | No specific recommendation |
| ECCO, 2017; guideline[55] | UC | Over 8 yr | Lower risk: Neither intermediate nor high-risk features; intermediate risk: Extensive colitis with mild or moderate active inflammation; post-inflammatory polyps; CRC in a FDR older than 50 yr; higher risk: Extensive colitis with severe active inflammation; stricture or dysplasia in last 5 yr; PSC | Lower risk: 5 yr; intermediate risk: 2-3 yr; higher risk: 1 yr | High-definition endoscopy; chromoendoscopy with targeted biopsies |
| ECCO, 2019; guideline[60,61] | IBD | No specific recommendation | Same with BSG Guideline (2019) | Lower risk: 5 yr; intermediate risk: 2-3 yr; higher risk: 1 yr | |
| JSG, 2020; Guideline[62] | IBD | 8 yr | No specific recommendation | Targeted biopsies | |
| NCCN, 2022; Guideline[63] | IBD | 8 yr | Low risk: No active inflammation; high risk: Extensive colitis with active inflammation; dysplasia; PSC; family history of CRC younger than 50 yr | Low risk: 2-3 yr; high risk: 1 yr; HGD or piecemeal resection: 3-6 mo | High-definition white light endoscopy; colonoscopy with chromoendoscopy |
| NICE, 2022; guideline[64] | IBD | UC but not proctitis alone or CD involving more than one segment of the colon: 10 yr | Same with BSG guideline (2019) | Low risk: 5 yr; intermediate risk: 3 yr; high risk: 1 yr | Colonoscopy with chromoendoscopy |
| WGO, 2015; guideline[54] | IBD | 8 yr | No specific recommendation | Magnification and chromoendoscopy | |
The initiation of surveillance colonoscopy and endoscopic options for dysplasia is basically consistent across gui
It is difficult to make a clear macroscopic distinction between CAC and sporadic CRC. In contrast to sporadic CRC, CAC appears to have distinguishing clinicopathologic features, evolving from a polymorphous dysplastic lesion rather than a polypoid adenoma[66]. It is characterized by the lack of tumor histologic heterogeneity, tumor necrosis, Crohn’s-like reaction, the presence of mucin, and signet ring cell differentiation and tumor well differentiation[67].
Previous viewpoints supported IBD patients with non-adenoma-like dysplasia-related lesions or masses were recommended to undergo colectomy, whereas IBD patients with adenoma-like dysplasia-related lesions or masses could be safely managed with polypectomy and continued surveillance in the absence of flat dysplasia elsewhere in the colon[68]. The mainstream consensus is that endoscopic resection should be considered for all clearly delineated dysplastic-appearing lesions without evidence of invasive cancer or significant submucosal fibrosis. Endoscopic mucosal resection or endoscopic submucosal dissection may be considered for complex lesions not amenable to standard polypectomy, such as large and highly irregular lesions[52].
After a long course of the disease, a large number of IBD patients opt for surgery. Based on a long-term follow-up study of Australian IBD patients, the cumulative incidence of colectomy was estimated to be 15%, 26%, and 31% at 10, 20, and 30 years in patients with UC, whereas the cumulative incidence of resection was 32%, 43%, and 53% at 5, 10, and 15 years in patients with CD[69]. Indications for surgery in patients with IBD vary among guidelines (Table 2)[70-72]. The Chinese consensus recommends surgery for UC combined with massive hemorrhage, intestinal perforation, malignancy or high suspicion of malignant lesions, CD complications, and ineffective medical treatment[53]. The World Gastroenterology Organization (WGO) guidelines recommend different procedures for patients with UC (e.g., consider segmental resection in elderly patients with localized neoplasms or extensive comorbidities) and CD (e.g., temporary diverting ileostomy/colostomy for severe perianal fistula) in different states[54].
| Society | Disease type | Absolute indication (surgery is recommended) | Relative indication (surgery can be considered) |
| ACG, 2019; guideline[51] | UC | Dysplasia in UC is not resectable or is multifocal | Moderately to severely active UC who are refractory or intolerant to medical therapy |
| ACG, 2018; guideline[56] | CD | No statements are provided | Intra-abdominal abscess |
| AGA, 2021; expert consensus[52] | IBD | Unresectable visible dysplasia or invisible multifocal or high-grade dysplasia on histology | No statements are provided |
| AOCC and APAG, 2021; expert consensus[57] | IBD | No statements are provided | |
| BSG, 2019; guideline[58] | UC | Patients with acute severe UC who have not responded within 7 d of rescue therapy with infliximab or ciclosporin, or those with deterioration or complications before that time (including toxic megacolon, severe hemorrhage or perforation): Subtotal colectomy and ileostomy, with preservation of the rectum; patients who have chronic active symptoms despite optimal medical therapy: Surgical resection of the colon and rectum | |
| CD | Localized ileocaecal CD for those failing or relapsing after initial medical therapy, or in those preferring surgery to the continuation of drug therapy: Lparoscopic resection; patients with small bowel CD strictures shorter than 10 cm: Strictureplasty/resection; patients with severe perianal CD refractory to medical therapy: Fecal stream diversion | ||
| ASCRS, 2020[71]; guideline | CD | Patients with severe acute colitis who do not adequately respond to medical therapy or who have signs or symptoms of impending or actual perforation; patients with a free perforation: surgical resection of the perforated segment | Patients who demonstrate an inadequate response to, develop complications from or are nonadherent with medical therapy; patients with symptomatic small-bowel or anastomotic strictures that are not amenable to medical therapy and/or endoscopic dilation; patients with strictures of the colon that cannot be adequately surveyed endoscopically: Resection; patients with penetrating Crohn’s disease with abscess formation; patients with enteric fistulas that persist despite appropriate medical therapy |
| CSG 2018; Chinese consensus[53] | UC | Massive hemorrhage, perforation, malignancy, and high suspicion of malignant pathology | Severe UC that is refractory to active medical treatment, and toxic megacolon refractory to medical treatment should; undergo surgical intervention early; poor efficacy of medical treatment and/or adverse drug reactions that have seriously affected patients’ quality of life |
| CD | CD complications1, ineffective medical treatment2 | No statements are provided | |
| ECCO, 2019; guideline[70] | UC | No statements are provided | Refractory and corticosteroid-dependent patients; patients with UC and a minimally affected rectum |
| ECCO, 2020; guideline[72] | CD | Patients with refractory pancolonic Crohn’s disease without a history of perianal disease: Restorative proctocolectomy with IPAA; patients with a single involved colonic segment in CD: Segmental colectomy; patients with limited, nonstructuring, ileocaecal CD (diseased terminal ileum < 40 cm): Laparoscopic resection; Small-bowel strictures related to CD: Strictureplasty; patients with short (< 5 cm) strictures of the terminal ileum in CD: Endoscopic balloon dilatation or surgery; patients with CD and complex perianal fistulae: Ligation of the intersphincteric fistula tract | |
| JSG, 2020; guideline[62] | IBD | In severe cases of IBD and those with cancer or dysplasia; patients with symptoms caused by the primary disease that do not improve with medical treatment, side effects of medication, and extraintestinal complications (especially pyoderma gangrenosum) | |
| WGO, 2015; guideline[54] | UC | Medical treatment is not completely successful or in the presence of dysplasia | |
| CD | Surgery should be considered as an alternative to medical treatment early in the disease course for short-segment CD limited to the distal ileum | ||
Importantly, surgery appears to increase the risk of developing cancer. Patients with IBD have a higher risk of developing CRC after segmental colonic resection for CRC. During the 3-year follow-up period after initial surgery, 1.6% of IBD patients developed CRC compared to 0.7% of non-IBD patients. Furthermore, 6.3% of IBD patients developed tumors compared to 2.3% of non-IBD patients during the 15-year follow-up period after initial surgery[73]. Consequently, no guidelines recommend prophylactic colectomy to prevent cancer.
According to the widely accepted concepts, aminosalicylic acid (ASA) has been an essential drug in the treatment of mild to moderate UC. Several studies have investigated its potential value in the chemoprevention of CAC. Long-term use of 5-ASA compounds has been shown to reduce the risk of UC-associated CRC in both human[74] and animal models[75]. Three meta-analyses[76-78] and a large epidemiological study[79] confirmed a protective association between the application of 5-aminosalicylate compounds and CRC. However, in another meta-analysis of nonreferral populations, there appeared to be no protective effect of 5-ASA on CAC[80].
Despite some conflicting results, most studies have confirmed the chemopreventive effect of 5-ASA in preventing CAC. The chemopreventive effects of 5-ASA vary for different disease classifications and drug types: The risk of CAC is greatly reduced in UC patients but not significantly in CD patients; mesalazine significantly reduces the risk of CAC, but salazosulfapyridine does not show any protective effect[76,77]. The dose-effect relationship on the protective effect of mesa
The antineoplastic effect of thiopurines is debatable due to inconsistent results across studies. A cohort study in the Netherlands found a significant protective effect of thiopurines on the risk of advanced neoplasia[82]. However, data from a meta-analysis[83] and a case–control study[84] have demonstrated that thiopurine use was not associated with a significantly lower risk of colorectal neoplasia. In addition, the effectiveness of thiopurines varies in different disease classifications and geographical regions. Thiopurine treatment was associated with a reduced risk of colorectal neoplasia in European studies but not in African and Asian studies; thiopurine treatment also reduces the risk of CAC, which is significant in patients with UC but not in patients with CD[85].
Although thiopurines may reduce the risk of CAC, they are also associated with an increased risk of cancer[86]. There was no excess risk of developing any cancers in individuals with older-onset IBD in a population-based study[87]. Nevertheless, a nested case-control study has identified that exposure to thiopurines for over 5 years is related to a significantly higher risk of nonmelanoma skin cancer and lymphoproliferative disorders[86] but unrelated to a higher risk of melanoma or colorectal cancer[88]. Therefore, given this evidence, the American College of Gastroenterology (ACG) guidelines recommended that chemopreventive medical therapy alone is not an appropriate way to prevent UC-associated CRC and is not a substitute for colonoscopy[51].
In addition to thiopurines, given the therapeutic effect of thalidomide in moderate UC and moderate CD, its efficacy in CAC is under investigation. Lu et al[89] demonstrated that thalidomide suppressed macrophage polarization in the tumor microenvironment, which not only relieved colonic inflammation to promote mucosal healing but also inhibited the development of CAC. These findings may shed light on the potential use of thalidomide for the chemoprevention of CAC.
Biological agents commonly used in the guidelines for the treatment of IBD include antitumor necrosis factor-α (anti-TNFα) agent (Infliximab), α4β7 integrin antibody (Vedolizumab), Janus kinase (JAK) inhibitor (Tofacitinib), and IL-12/IL-23 antagonist (Ustekinumab)[70]. The guidelines recommend these drugs as an alternative treatment for moderate to severe UC and moderate to severe CD. However, biological agents appear to have no chemopreventive effect on CAC. Two population-based studies, one from France[87] and the other from Canada[88], found no association between anti-TNFα exposure and CRC. Concerns about the carcinogenic risk of antineoplastic drugs are of significant importance. For
| Population | Agent | Efficacy of candidate chemopreventive drugs | Type of study |
| CAC[77] | 5-ASA | Protective factors | Meta-analysis |
| CAC[78] | 5-ASA | Protective factors | Meta-analysis |
| CAC[76] | Mesalamine; Sulfasalazine | Protective factors; No statistical effect | Meta-analysis |
| CAC[80] | 5-ASA | No statistical effect | Meta-analysis |
| CAC[81] | Mesalamine | Protective factors | Case-control study |
| CAC[84] | 5-ASA | Protective factors | Case-control study |
| GI cancer in IBD[91] | 5-ASA | Protective factors | Cohort study |
| CAC[79] | 5-ASA | Protective factors | Nested case-control study |
| CAC[87] | 5-ASA | No statistical effect | A population-based study |
| CAC[85] | Thiopurines | Protective factors | Meta-analysis |
| CAC[83] | Thiopurines | No statistical effect | Meta-analysis |
| CAC[84] | Thiopurines | No statistical effect | Case-control study |
| Advanced neoplasia in IBD1[82] | Thiopurines | Protective factors | Cohort study |
| CRC[88] | Immunomodulators Anti-TNF-α agents | No statistical effect No statistical effect | Nested case-control study |
| CRC[92] | Folic acid | No statistical effect | Meta-analysis |
| CRC[93] | Folic acid | No statistical effect | Meta-analysis |
| CAC[94] | Non-aspirin NSAIDs | No statistical effect | Meta-analysis |
| CRC[95] | Vitamin D | No statistical effect | Meta-analysis |
| Colorectal adenomas[96] | Calcium intake as a food and dairy product | Significantly decrease | Meta-analysis |
| CRC[93] | Calcium | No statistical effect | Meta-analysis |
| CAC[97] | Statin | No statistical effect | Cohort study |
Recent attention has focused on the anticancer activity of probiotics, despite the lack of data from large clinical trials. Although the protective effect of probiotics on CAC has not yet been reported, the use of probiotic supplements has been indicated to significantly reduce the risk of postoperative complications in patients undergoing CRC surgery[98]. Importantly, some studies appear to form the basis of future explorations into probiotics for the treatment of CAC. As mentioned earlier, genomic instability has been linked to carcinogenesis in IBD. Bifidobacterium infantis has been demonstrated to alleviate colonic inflammation by activating DNA repair pathways and enhancing genomic stability[99]. Lactic acid bacteria, which have been reported to be successfully used in managing sporadic CRC[100], have also been shown to have a potential chemopreventive effect on CAC. One study by Silveira et al[101] established the CAC murine model and found that Lactobacillus bulgaricus downregulated certain cytokine levels in the intestine and tumors and inhibited tumor growth.
Traditional Chinese medicines (TCMs) are a unique but helpful health resource in China. Many classic TCMs have a long history of adjunct therapy in patients with mild to moderate IBD and are still in use today[102]. Qingchang Wenzhong decoction[103] and Sini decoction[104], effective TCM prescriptions, have been indicated to inhibit colitis-associated carcinogenesis, possibly through the improvement of intestinal flora dysbiosis and the intestinal barrier.
Improving microbial dysbiosis by modulating the gut microbiota is a novel strategy for the prevention and treatment of CRC. As mentioned above, probiotics have shown great potential in the prevention and treatment of CRC. Other approaches to gut microbiota modulation, such as prebiotics, postbiotics, and fecal microbiota transplantation (FMT), are theoretically promising for the prevention and treatment of CRC. Despite the lack of clinical evidence, two clinical trials of FMT in CRC are underway. It is anticipated that these drugs are effective in the prevention and treatment of CRC.
Compared to sporadic CRC, CAC has a unique carcinogenic process. Regular colonoscopic surveillance is an effective way to improve prognosis. There is no consensus on the role of immunomodulators and biological agents in the chemoprevention of CAC. Currently, ASA is the only effective chemopreventive agent. TCM, probiotics, and other gut microbiota modulators seem to be promising strategies for the prevention and treatment of CRC. Recent advances in CAC are based on the rapid development of various types of omics in the postgenomic era. Existing studies have attempted to explore the pathogenesis of CAC from different perspectives, including genetics, immunology, and microbiology. However, due to the complexity and sophistication of life activities, more efforts are needed to restore the findings from different levels to the process of cellular carcinogenesis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gazouli M, Greece; Iizuka M, Japan; Sulbaran MN, Brazil S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, Ekbom A, Askling J. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561-7; quiz 1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, Ekbom A, Sørensen HT, Ludvigsson JF. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 234] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 3. | Lu C, Schardey J, Zhang T, Crispin A, Wirth U, Karcz KW, Bazhin AV, Andrassy J, Werner J, Kühn F. Survival Outcomes and Clinicopathological Features in Inflammatory Bowel Disease-associated Colorectal Cancer: A Systematic Review and Meta-analysis. Ann Surg. 2022;276:e319-e330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 5. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 374] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 7. | Wan Q, Zhao R, Xia L, Wu Y, Zhou Y, Wang Y, Cui Y, Shen X, Wu XT. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol. 2021;147:1077-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Jewel Samadder N, Valentine JF, Guthery S, Singh H, Bernstein CN, Wan Y, Wong J, Boucher K, Pappas L, Rowe K, Bronner M, Ulrich CM, Burt RW, Curtin K, Smith KR. Colorectal Cancer in Inflammatory Bowel Diseases: A Population-Based Study in Utah. Dig Dis Sci. 2017;62:2126-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1955] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 10. | Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Gong W, Lv N, Wang B, Chen Y, Huang Y, Pan W, Jiang B. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study. Dig Dis Sci. 2012;57:503-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Zhou Q, Shen ZF, Wu BS, Xu CB, He ZQ, Chen T, Shang HT, Xie CF, Huang SY, Chen YG, Chen HB, Han ST. Risk of Colorectal Cancer in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2019;2019:5363261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Clifford GM, Georges D, Shiels MS, Engels EA, Albuquerque A, Poynten IM, de Pokomandy A, Easson AM, Stier EA. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int J Cancer. 2021;148:38-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 185] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 14. | Castaño-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther. 2014;39:645-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Samadder NJ, Valentine JF, Guthery S, Singh H, Bernstein CN, Leighton JA, Wan Y, Wong J, Boucher K, Pappas L, Rowe K, Burt RW, Curtin K, Smith KR. Family History Associates With Increased Risk of Colorectal Cancer in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17:1807-1813.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | O'Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, Heitman SJ, Hilsden RJ, Brenner DR. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:1229-1240.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 93] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 17. | Wijnands AM, de Jong ME, Lutgens MWMD, Hoentjen F, Elias SG, Oldenburg B; Dutch Initiative on Crohn and Colitis (ICC). Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-analysis. Gastroenterology. 2021;160:1584-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 18. | Frick A, Khare V, Paul G, Lang M, Ferk F, Knasmüller S, Beer A, Oberhuber G, Gasche C. Overt Increase of Oxidative Stress and DNA Damage in Murine and Human Colitis and Colitis-Associated Neoplasia. Mol Cancer Res. 2018;16:634-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kakiuchi N, Yoshida K, Uchino M, Kihara T, Akaki K, Inoue Y, Kawada K, Nagayama S, Yokoyama A, Yamamoto S, Matsuura M, Horimatsu T, Hirano T, Goto N, Takeuchi Y, Ochi Y, Shiozawa Y, Kogure Y, Watatani Y, Fujii Y, Kim SK, Kon A, Kataoka K, Yoshizato T, Nakagawa MM, Yoda A, Nanya Y, Makishima H, Shiraishi Y, Chiba K, Tanaka H, Sanada M, Sugihara E, Sato TA, Maruyama T, Miyoshi H, Taketo MM, Oishi J, Inagaki R, Ueda Y, Okamoto S, Okajima H, Sakai Y, Sakurai T, Haga H, Hirota S, Ikeuchi H, Nakase H, Marusawa H, Chiba T, Takeuchi O, Miyano S, Seno H, Ogawa S. Frequent mutations that converge on the NFKBIZ pathway in ulcerative colitis. Nature. 2020;577:260-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Fujita M, Matsubara N, Matsuda I, Maejima K, Oosawa A, Yamano T, Fujimoto A, Furuta M, Nakano K, Oku-Sasaki A, Tanaka H, Shiraishi Y, Mateos RN, Nakai K, Miyano S, Tomita N, Hirota S, Ikeuchi H, Nakagawa H. Genomic landscape of colitis-associated cancer indicates the impact of chronic inflammation and its stratification by mutations in the Wnt signaling. Oncotarget. 2018;9:969-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Yamada M, Sakurai T, Komeda Y, Nagai T, Kamata K, Minaga K, Yamao K, Takenaka M, Hagiwara S, Matsui S, Watanabe T, Nishida N, Kashida H, Kudo M. Clinical Significance of Bmi1 Expression in Inflammatory Bowel Disease. Oncology. 2017;93 Suppl 1:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Liu X, Wei W, Li X, Shen P, Ju D, Wang Z, Zhang R, Yang F, Chen C, Cao K, Zhu G, Chen H, Chen L, Sui J, Zhang E, Wu K, Wang F, Zhao L, Xi R. BMI1 and MEL18 Promote Colitis-Associated Cancer in Mice via REG3B and STAT3. Gastroenterology. 2017;153:1607-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Vicente-Dueñas C, Hauer J, Cobaleda C, Borkhardt A, Sánchez-García I. Epigenetic Priming in Cancer Initiation. Trends Cancer. 2018;4:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Du H, Xia H, Liu T, Li Y, Liu J, Xie B, Chen J, Cao L, Liu S, Li S, Wang P, Wang D, Zhang Z, Guo X, Wu A, Li M, You F. Suppression of ELF4 in ulcerative colitis predisposes host to colorectal cancer. iScience. 2021;24:102169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Emmett RA, Davidson KL, Gould NJ, Arasaradnam RP. DNA methylation patterns in ulcerative colitis-associated cancer: a systematic review. Epigenomics. 2017;9:1029-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, Cui Y, Li Y, McDaniel JM, McGee S, Chen W, Huycke MM, Houchen CW, Zenewicz LA, West CM, Chen H, Braun J, Fu J, Xia L. Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice. Gastroenterology. 2016;151:152-164.e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1859] [Cited by in F6Publishing: 1912] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 28. | Liu S, He L, Wu J, Wu X, Xie L, Dai W, Chen L, Xie F, Liu Z. DHX9 contributes to the malignant phenotypes of colorectal cancer via activating NF-κB signaling pathway. Cell Mol Life Sci. 2021;78:8261-8281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Claessen MM, Schipper ME, Oldenburg B, Siersema PD, Offerhaus GJ, Vleggaar FP. WNT-pathway activation in IBD-associated colorectal carcinogenesis: potential biomarkers for colonic surveillance. Cell Oncol. 2010;32:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 27] [Reference Citation Analysis (0)] |
| 30. | Xu H, Li J, Chen H, Ghishan FK. NHE8 Deficiency Promotes Colitis-Associated Cancer in Mice via Expansion of Lgr5-Expressing Cells. Cell Mol Gastroenterol Hepatol. 2019;7:19-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, Zhao X, Zhi F. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018;9:153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 32. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 961] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 33. | Lin Y, Wang D, Zhao H, Li D, Li X, Lin L. Pou3f1 mediates the effect of Nfatc3 on ulcerative colitis-associated colorectal cancer by regulating inflammation. Cell Mol Biol Lett. 2022;27:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 34. | Singh K, Coburn LA, Asim M, Barry DP, Allaman MM, Shi C, Washington MK, Luis PB, Schneider C, Delgado AG, Piazuelo MB, Cleveland JL, Gobert AP, Wilson KT. Ornithine Decarboxylase in Macrophages Exacerbates Colitis and Promotes Colitis-Associated Colon Carcinogenesis by Impairing M1 Immune Responses. Cancer Res. 2018;78:4303-4315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Shi F, Liu G, Lin Y, Guo CL, Han J, Chu ESH, Shi C, Li Y, Zhang H, Hu C, Liu R, He S, Guo G, Chen Y, Zhang X, Coker OO, Wong SH, Yu J, She J. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene. 2023;42:530-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 20] [Reference Citation Analysis (0)] |
| 36. | Collard MK, Tourneur-Marsille J, Uzzan M, Albuquerque M, Roy M, Dumay A, Freund JN, Hugot JP, Guedj N, Treton X, Panis Y, Ogier-Denis E. The Appendix Orchestrates T-Cell Mediated Immunosurveillance in Colitis-Associated Cancer. Cell Mol Gastroenterol Hepatol. 2023;15:665-687. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 37. | Sears CL, Pardoll DM. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis. 2011;203:306-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 575] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 39. | Yu LC. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 40. | Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 688] [Cited by in F6Publishing: 730] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 41. | Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C, Manders F, Dalmasso G, Stege PB, Paganelli FL, Geurts MH, Beumer J, Mizutani T, Miao Y, van der Linden R, van der Elst S; Genomics England Research Consortium, Garcia KC, Top J, Willems RJL, Giannakis M, Bonnet R, Quirke P, Meyerson M, Cuppen E, van Boxtel R, Clevers H. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 522] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 42. | Iwasaki M, Kanehara R, Yamaji T, Katagiri R, Mutoh M, Tsunematsu Y, Sato M, Watanabe K, Hosomi K, Kakugawa Y, Ikematsu H, Hotta K, Kunisawa J, Wakabayashi K, Matsuda T. Association of Escherichia coli containing polyketide synthase in the gut microbiota with colorectal neoplasia in Japan. Cancer Sci. 2022;113:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Oliero M, Hajjar R, Cuisiniere T, Fragoso G, Calvé A, Dagbert F, Loungnarath R, Sebajang H, Schwenter F, Wassef R, Ratelle R, De Broux É, Richard CS, Santos MM. Prevalence of pks + bacteria and enterotoxigenic Bacteroides fragilis in patients with colorectal cancer. Gut Pathog. 2022;14:51. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 44. | Kinugasa T, Tsunoda T, Mizoguchi E, Okada T, Sudo T, Kawahara A, Akiba J, Akagi Y. Chitinase 3-like 1, Carcinoembryonic Antigen-related Cell Adhesion Molecule 6, and Ectopic Claudin-2 in the Carcinogenic Processes of Ulcerative Colitis. Anticancer Res. 2022;42:4119-4127. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 45. | Allen J, Rosendahl Huber A, Pleguezuelos-Manzano C, Puschhof J, Wu S, Wu X, Boot C, Saftien A, O'Hagan HM, Wang H, van Boxtel R, Clevers H, Sears CL. Colon Tumors in Enterotoxigenic Bacteroides fragilis (ETBF)-Colonized Mice Do Not Display a Unique Mutational Signature but Instead Possess Host-Dependent Alterations in the APC Gene. Microbiol Spectr. 2022;10:e0105522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Thiele Orberg E, Fan H, Tam AJ, Dejea CM, Destefano Shields CE, Wu S, Chung L, Finard BB, Wu X, Fathi P, Ganguly S, Fu J, Pardoll DM, Sears CL, Housseau F. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017;10:421-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 47. | Yu MR, Kim HJ, Park HR. Fusobacterium nucleatum Accelerates the Progression of Colitis-Associated Colorectal Cancer by Promoting EMT. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1659] [Cited by in F6Publishing: 1603] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 49. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 455] [Cited by in F6Publishing: 576] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 50. | Bye WA, Nguyen TM, Parker CE, Jairath V, East JE. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2017;9:CD000279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 783] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 52. | Murthy SK, Feuerstein JD, Nguyen GC, Velayos FS. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology. 2021;161:1043-1051.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 53. | Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis. 2021;22:298-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 54. | Bernstein CN, Eliakim A, Fedail S, Fried M, Gearry R, Goh KL, Hamid S, Khan AG, Khalif I, Ng SC, Ouyang Q, Rey JF, Sood A, Steinwurz F, Watermeyer G, LeMair A; Review Team:. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: Update August 2015. J Clin Gastroenterol. 2016;50:803-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 1094] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 56. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 716] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 57. | Ran Z, Wu K, Matsuoka K, Jeen YT, Wei SC, Ahuja V, Chen M, Hu PJ, Andoh A, Kim HJ, Yang SK, Watanabe M, Ng SC, Hibi T, Hilmi IN, Suzuki Y, Han DS, Leung WK, Sollano J, Ooi CJ, Qian J. Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol. 2021;36:637-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 58. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1162] [Article Influence: 232.4] [Reference Citation Analysis (0)] |
| 59. | Guidelines CCASC, Party W. Clinical practice guidelines for surveillance colonoscopy. Sydney: Cancer Council Australia 2018. [cited 5 July 2023]. Available from: https://cancerwa.asn.au/wp-content/uploads/2022/07/2020-11-26-Short-form-guidelines-Colonoscopy-surveillance-Summary-of-recommendations-Mar19.pdf. [Cited in This Article: ] |
| 60. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 817] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 61. | Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 208] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 62. | Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, Saruta M, Hirai F, Hata K, Hiraoka S, Esaki M, Sugimoto K, Fuji T, Watanabe K, Nakamura S, Inoue N, Itoh T, Naganuma M, Hisamatsu T, Watanabe M, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 175] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 63. | Ness RM. Updates in Screening Recommendations for Colorectal Cancer. J Natl Compr Canc Netw. 2022;20:603-606. [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 64. | Colorectal cancer prevention: colonoscopic surveillance in adults with ulcerative colitis, Crohn’s disease or adenomas. London: National Institute for Health and Care Excellence (NICE); 2022-Sep-20 . [PubMed] [Cited in This Article: ] |
| 65. | Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, Thomas-Gibson S, Saunders BP, Graham TA, Hart AL. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol. 2015;110:1022-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 66. | Svrcek M, Borralho Nunes P, Villanacci V, Beaugerie L, Rogler G, De Hertogh G, Tripathi M, Feakins R; H-ECCO group. Clinicopathological and Molecular Specificities of Inflammatory Bowel Disease-Related Colorectal Neoplastic Lesions: The Role of Inflammation. J Crohns Colitis. 2018;12:1486-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Liu X, Goldblum JR, Zhao Z, Landau M, Heald B, Pai R, Lin J. Distinct clinicohistologic features of inflammatory bowel disease-associated colorectal adenocarcinoma: in comparison with sporadic microsatellite-stable and Lynch syndrome-related colorectal adenocarcinoma. Am J Surg Pathol. 2012;36:1228-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T 3rd, McLeod R, Burgart LJ, Allen J, Brill JV; AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 358] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 69. | Selinger CP, Andrews JM, Titman A, Norton I, Jones DB, McDonald C, Barr G, Selby W, Leong RW; Sydney IBD Cohort Study Group. Long-term follow-up reveals low incidence of colorectal cancer, but frequent need for resection, among Australian patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:644-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Spinelli A, Panis Y, Doherty G. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis. 2022;16:2-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 258] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 71. | Lightner AL, Vogel JD, Carmichael JC, Keller DS, Shah SA, Mahadevan U, Kane SV, Paquette IM, Steele SR, Feingold DL. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surgical Management of Crohn's Disease. Dis Colon Rectum. 2020;63:1028-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, Doherty G, El-Hussuna A, Ellul P, Fiorino G, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gisbert JP, Gomollon F, González Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Kucharzik T, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Stassen L, Torres J, Uzzan M, Vavricka S, Verstockt B, Zmora O. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J Crohns Colitis. 2020;14:155-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 253] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 73. | Birch RJ, Burr N, Subramanian V, Tiernan JP, Hull MA, Finan P, Rose A, Rutter M, Valori R, Downing A, Morris EJA. Inflammatory Bowel Disease-Associated Colorectal Cancer Epidemiology and Outcomes: An English Population-Based Study. Am J Gastroenterol. 2022;117:1858-1870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 74. | Qiu X, Ma J, Wang K, Zhang H. Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: a systematic review with meta-analysis. Oncotarget. 2017;8:1031-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 75. | Clapper ML, Gary MA, Coudry RA, Litwin S, Chang WC, Devarajan K, Lubet RA, Cooper HS. 5-aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2008;14:1341-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | OʼConnor A, Packey CD, Akbari M, Moss AC. Mesalamine, but Not Sulfasalazine, Reduces the Risk of Colorectal Neoplasia in Patients with Inflammatory Bowel Disease: An Agent-specific Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2015;21:2562-2569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Bonovas S, Fiorino G, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1179-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 78. | Zhao LN, Li JY, Yu T, Chen GC, Yuan YH, Chen QK. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis. PLoS One. 2014;9:e94208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573-1578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 80. | Nguyen GC, Gulamhusein A, Bernstein CN. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: a meta-analysis of non-referral populations. Am J Gastroenterol. 2012;107:1298-304; quiz 1297, 1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 81. | Tang J, Sharif O, Pai C, Silverman AL. Mesalamine protects against colorectal cancer in inflammatory bowel disease. Dig Dis Sci. 2010;55:1696-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | van Schaik FD, van Oijen MG, Smeets HM, van der Heijden GJ, Siersema PD, Oldenburg B. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut. 2012;61:235-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 83. | Jess T, Lopez A, Andersson M, Beaugerie L, Peyrin-Biroulet L. Thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1793-1800.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 84. | Carrat F, Seksik P, Colombel JF, Peyrin-Biroulet L, Beaugerie L; CESAME Study Group. The effects of aminosalicylates or thiopurines on the risk of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:533-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Lu MJ, Qiu XY, Mao XQ, Li XT, Zhang HJ. Systematic review with meta-analysis: thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:318-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, Simon T, Maynadié M, Hermine O, Faivre J, Carrat F; CESAME Study Group. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 748] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 87. | Cheddani H, Dauchet L, Fumery M, Charpentier C, Marie Bouvier A, Dupas JL, Pariente B, Peyrin-Biroulet L, Savoye G, Gower-Rousseau C. Cancer in Elderly Onset Inflammatory Bowel Disease: A Population-Based Study. Am J Gastroenterol. 2016;111:1428-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Kopylov U, Vutcovici M, Kezouh A, Seidman E, Bitton A, Afif W. Risk of Lymphoma, Colorectal and Skin Cancer in Patients with IBD Treated with Immunomodulators and Biologics: A Quebec Claims Database Study. Inflamm Bowel Dis. 2015;21:1847-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Lu J, Liu D, Tan Y, Li R, Wang X, Deng F. Thalidomide Attenuates Colitis and Is Associated with the Suppression of M1 Macrophage Polarization by Targeting the Transcription Factor IRF5. Dig Dis Sci. 2021;66:3803-3812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-α therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:447-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 91. | Scharl S, Barthel C, Rossel JB, Biedermann L, Misselwitz B, Schoepfer AM, Straumann A, Vavricka SR, Rogler G, Scharl M, Greuter T. Malignancies in Inflammatory Bowel Disease: Frequency, Incidence and Risk Factors-Results from the Swiss IBD Cohort Study. Am J Gastroenterol. 2019;114:116-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Carroll C, Cooper K, Papaioannou D, Hind D, Tappenden P, Pilgrim H, Booth A. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Aliment Pharmacol Ther. 2010;31:708-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, Maguire C, Hind D, Tappenden P. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010;14:1-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 94. | Burr NE, Hull MA, Subramanian V. Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease? World J Gastroenterol. 2016;22:3679-3686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Emmanouilidou G, Kalopitas G, Bakaloudi DR, Karanika E, Theocharidou E, Germanidis G, Chourdakis M. Vitamin D as a chemopreventive agent in colorectal neoplasms. A systematic review and meta-analysis of randomized controlled trials. Pharmacol Ther. 2022;237:108252. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 96. | Emami MH, Salehi M, Hassanzadeh Keshteli A, Mansourian M, Mohammadzadeh S, Maghool F. Calcium and dairy products in the chemoprevention of colorectal adenomas: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62:7168-7183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Shah SC, Glass J, Giustino G, Hove JRT, Castaneda D, Torres J, Kumar A, Elman J, Ullman TA, Itzkowitz SH. Statin Exposure Is Not Associated with Reduced Prevalence of Colorectal Neoplasia in Patients with Inflammatory Bowel Disease. Gut Liver. 2019;13:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Chen Y, Qi A, Teng D, Li S, Yan Y, Hu S, Du X. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol. 2022;26:425-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Han T, Hu X, Li K, Zhang D, Zhang Y, Li J. Bifidobacterium infantis Maintains Genome Stability in Ulcerative Colitis via Regulating Anaphase-Promoting Complex Subunit 7. Front Microbiol. 2021;12:761113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Azcárate-Peril MA, Sikes M, Bruno-Bárcena JM. The intestinal microbiota, gastrointestinal environment and colorectal cancer: a putative role for probiotics in prevention of colorectal cancer? Am J Physiol Gastrointest Liver Physiol. 2011;301:G401-G424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 101. | Silveira DSC, Veronez LC, Lopes-Júnior LC, Anatriello E, Brunaldi MO, Pereira-da-Silva G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J Gastroenterol. 2020;26:6782-6794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 102. | Yang L, Luo H, Tan D, Zhang S, Zhong Z, Wang S, Vong CT, Wang Y. A recent update on the use of Chinese medicine in the treatment of inflammatory bowel disease. Phytomedicine. 2021;92:153709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 103. | Ren L, Zhang Z, Zhao W, Zhao B, Chen X, Wang Y, Chen Z, Ye J, Yang Y, Cao P. Qingchang Wenzhong Decoction Prevents the Occurrence of Intestinal Tumors by Regulating Intestinal Microbiota and Gasdermin E. Front Physiol. 2022;13:917323. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 104. | Wang Y, Zhang X, Li J, Zhang Y, Guo Y, Chang Q, Chen L, Wang Y, Wang S, Song Y, Zhao Y, Wang Z. Sini Decoction Ameliorates Colorectal Cancer and Modulates the Composition of Gut Microbiota in Mice. Front Pharmacol. 2021;12:609992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |