Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2259
Peer-review started: July 11, 2023
First decision: August 31, 2023
Revised: September 1, 2023
Accepted: September 7, 2023
Article in press: September 7, 2023
Published online: October 27, 2023
Textbook outcomes (TOs) have been used to assess the quality of surgical treatment for many digestive tumours but not ampullary carcinoma (AC).
To discuss the factors associated with achieving a TO and further explore the prognostic value of a TO for AC patients undergoing curative pancreaticoduodenectomy (PD).
Patients who underwent PD at the China National Cancer Center between 1998 and 2020 were identified. A TO was defined by R0 resection, examination of ≥ 12 Lymph nodes, no prolonged hospitalization, no intensive care unit treatment, no postoperative complications, and no 30-day readmission or mortality. Cox regression analysis was used to identify the prognostic value of a TO for overall survival (OS) and recurrence-free survival (RFS). Logistic regression was used to identify predictors of a TO. The rate of a TO and of each indicator were compared in patients who underwent surgery before and after 2010.
Ultimately, only 24.3% of 272 AC patients achieved a TO. A TO was indepen
Only approximately a quarter (24.3%) of AC patients achieved a TO following PD. A TO was independently related to favourable oncological outcomes in AC and should be considered as an outcome measure for the quality of surgery. Further multicentre research is warranted to better elucidate its impact.
Core Tip: Surgery has improved substantially with advances in surgical techniques, however we still lack an effective measure to evaluate the quality of surgery in ampullary carcinoma. As a composite metric, textbook outcome (TO) concluded the strengths of all indicators based on important short-term outcomes, which was more reliable and comprehensive than single outcome measure. Pancreaticoduodenectomy was still quite complicated and required a broad judgement to monitor and compare the quality of procedures. TO should be considered as an outcome measurement for the quality of surgery, our study will be helpful in completely and effectively evaluating the overall quality of surgical care, and even in the hospital administration.
- Citation: Zhang XJ, Fei H, Guo CG, Sun CY, Li ZF, Li Z, Chen YT, Che X, Zhao DB. Analysis of textbook outcomes for ampullary carcinoma patients following pancreaticoduodenectomy. World J Gastrointest Surg 2023; 15(10): 2259-2271
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2259.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2259
Ampullary carcinoma (AC) is a rare tumour constituting only 0.6%–0.8% of all digestive system malignancies[1], and the incidence of AC has increased over the last 2 decades[2]. Pancreaticoduodenectomy (PD) is one of the standard treatment strategies for curative purposes. The prognosis of AC patients is better than that of patients with other peri ACs[3], with a nearly 50% resection rate[4]. Surgery has improved substantially with advances in surgical techniques; however, there is still a lack an effective measure to evaluate the quality of surgery. Sun et al[5] found inflammatory index can be regarded as a more useful prognostic index and Gonzalez et al[3] established a nomogram to predict disease-specific survival; however, these method did not have intuitive indicators. Recently, textbook outcomes (TOs) have emerged and been applied in evaluating treatments for many tumours. To our knowledge, no previous study has explored the clinical value of a TO in AC patients.
The concept of the TO was first proposed by Kolfschoten et al[6] to investigate hospital variation in the Netherlands as a composite quality metric that encompassed several indicators of quality. Generally, individual quality metrics such as mortality and complications are applied to evaluate the quality of surgery[7,8]; however, these single indicators may lack practicality in reflecting the overall prognosis. As a composite metric, the TO represents the strength of all indicators based on important short-term outcomes and is thus more reliable and comprehensive than a single outcome measure[9,10]. Since the concept of the TO emerged in surgery for colon cancer, it has been defined for the treatment of many other tumours, such as gastroesophageal cancer and intrahepatic cholangiocarcinoma[9,11]. The definition of a TO follows the all-or-none principle[12] because partially favourable outcomes are not perfect postoperative outcomes.
Previous studies have successfully proven that a TO is associated with improved long-term survival in pancreatic adenocarcinoma patients who undergo PD[13-15], as well as for patients in other surgical fields[6,16,17]. Milbank considered PD to require a broad judgement to monitor and compare the quality of procedures[18]. Based on the above situation, the aims of this study were to propose a TO definition for AC patients and characterize the impact of a TO on survival. In addition, we assessed the factors associated with achieving a TO.
Patients who underwent surgery for AC between 1998 and 2020 in the China National Cancer Center were selected for analysis. Inclusion criteria: (1) Pathologically proved as AC; and (2) Patients were submitted to radical surgery. Patients with missing data necessary to define TO were excluded: R0 resection (n = 4), lymph nodes examined (n = 9), hospitalization (n = 8) intensive care unit (ICU) treatment (n = 6), postoperative complications (n = 10), tumor differentiation (n = 4). A total of 41 patients were excluded from analysis and 272 AC patients were included.
TO represents optimal oncologic care after PD for AC as a single composite measure. TO was achieved if the following indicators are fulfilled: The surgical margin was negative (R0 resection), ≥ 12 Lymph nodes examined (American Joint Committee on Cancer, eighth edition)[19], no prolonged hospitalization (< 75th percentile)[6,20], no ICU treatment, no postoperative complications, no 30-day readmission or mortality[13], hospitalization was defined as day of operation to day of discharge.
we counted the number of patients each of the indicator and calculated the cumulative proportion. The collected data was presented as frequencies and proportions, and was compared between the groups with and without TO using the Chi-square test. Cox regression analysis was used to identify if TO was an independent prognostic factor for overall survival (OS) and recurrence-free survival (RFS). Multivariable logistic regression was performed to determine the relationship between baseline characteristics and TO, factors with P < 0.2 in univariate analysis were included in the multivariate analysis, and odds ratios (OR) or hazard ratios (HR) and their 95% confidence intervals (95%CI) were reported. Survival curves of OS and RFS were plotted using the Kaplan–Meier method to determine the effect of TO on survival. We divided the patients into two groups by the year of surgery before and after 2010 to see the TO rate trend.
Follow-up was mainly conducted by telephone and though outpatient rechecks, other information was obtained by medical records and population death register information system. All data were analyzed with SPSS software (version 21; SPSS Inc., Chicago, IL, United States). Kaplan–Meier survival analyses were performed in R software (Version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria). P value < 0.05 was considered statistically significant.
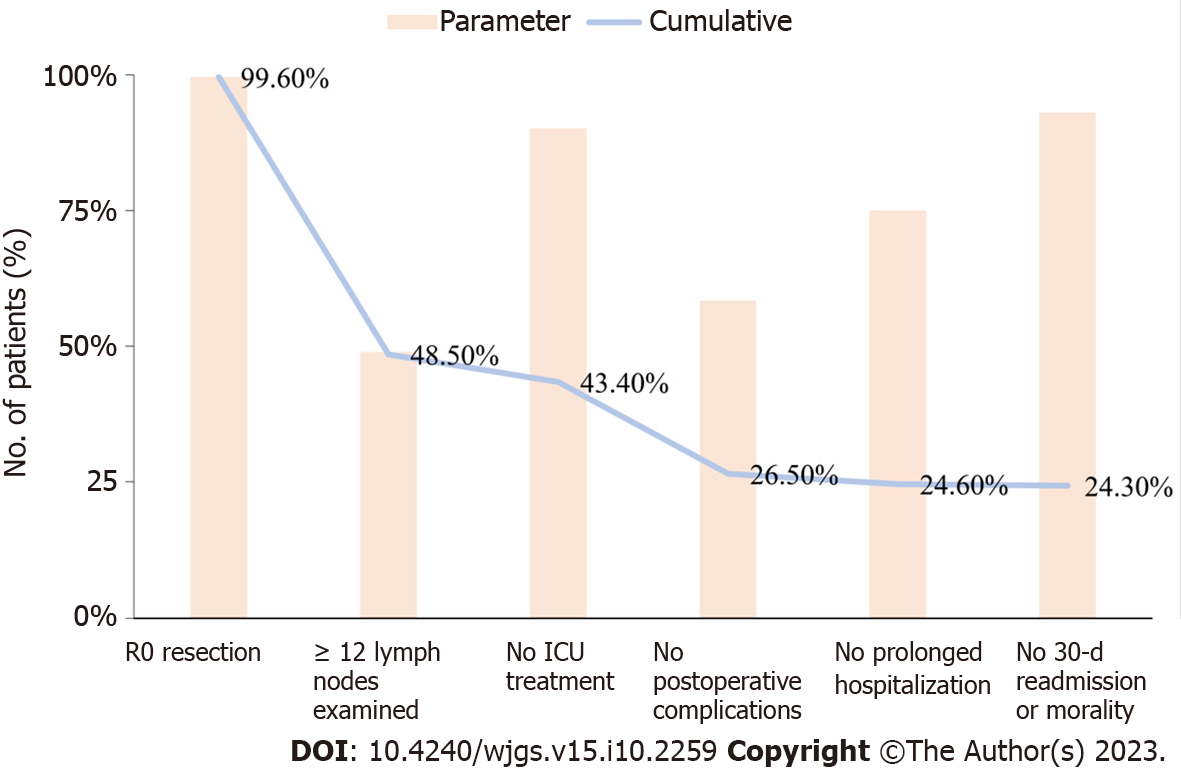
A total of 272 AC patients met the inclusion criteria. A TO was observed in 66 (24.3%) patients. Among the indicators used to define a TO, R0 resection (99.6%), no 30-day readmission or mortality (93.0%) and no ICU treatment (90.1%) were achieved easily, while Examination of ≥ 12 Lymph nodes (58.5%) and no postoperative complications (48.9%) were not achieved as easily. The data for the 6 TO indicators and the cumulative proportions are shown in Figure 1.
Patients were divided into a TO group (66 patients, 24.3%) and a non-TO group (206 patients, 75.7%). There were significant differences in the year of surgery, N stage, TNM stage and lymphovascular invasion (P < 0.05) between the two groups and no significant differences in sex, age, operation time, blood transfusion, tumour size, differentiation, CA199, T stage or adjuvant treatment (P > 0.05). Baseline characteristics for the TO and non-TO groups are presented in Table 1.
| Characteristic | Textbook outcome | No textbook outcome | P value | ||
| n = 66 | Percentage | n = 206 | Percentage | ||
| Year of surgery | < 0.001 | ||||
| 1998-2010 | 15 | 22.7% | 117 | 56.8% | |
| 2011-2020 | 51 | 77.3% | 89 | 43.2% | |
| Sex | 0.634 | ||||
| Male | 40 | 60.6% | 118 | 57.3% | |
| Female | 26 | 39.4% | 88 | 42.7% | |
| Age (yr) | 0.618 | ||||
| ≤ 60 | 42 | 63.6% | 124 | 60.2% | |
| > 60 | 24 | 36.4% | 82 | 39.8% | |
| Operation time (h) | 0.622 | ||||
| ≤ 6 | 45 | 68.2% | 147 | 71.4% | |
| > 6 | 21 | 31.8% | 59 | 28.3% | |
| Blood transfusion | 0.328 | ||||
| No | 35 | 53.0% | 95 | 46.1% | |
| Yes | 31 | 47.0% | 111 | 53.9% | |
| Tumor size (cm) | 0.155 | ||||
| ≤ 2.0 | 27 | 40.9% | 105 | 51.0% | |
| > 2.0 | 39 | 59.1% | 101 | 49.0% | |
| Differentiation | 0.369 | ||||
| Well | 10 | 15.2% | 47 | 22.8% | |
| Moderate | 29 | 43.9% | 88 | 42.7% | |
| Poor | 27 | 40.9% | 71 | 34.5% | |
| CA199 | 0.941 | ||||
| 0-40 | 23 | 34.8% | 74 | 35.9% | |
| > 40 | 37 | 56.1% | 111 | 53.9% | |
| unknown | 6 | 9.1% | 21 | 10.2% | |
| N stage | 0.038 | ||||
| N0 | 39 | 59.1% | 154 | 74.8% | |
| N1 | 23 | 34.8% | 41 | 19.9% | |
| N2 | 4 | 6.1% | 11 | 5.3% | |
| T stage | 0.585 | ||||
| T1 | 9 | 13.6% | 33 | 16.0% | |
| T2 | 26 | 39.4% | 67 | 32.5% | |
| T3 | 31 | 47.0% | 106 | 51.5% | |
| TNM stage | 0.034 | ||||
| I | 29 | 43.9% | 93 | 45.1% | |
| II | 10 | 15.2% | 58 | 28.2% | |
| III | 27 | 40.9% | 55 | 26.7% | |
| Lymphovascular invasion | 0.001 | ||||
| No | 40 | 60.6% | 166 | 80.6% | |
| Yes | 26 | 39.4% | 40 | 19.4% | |
| Adjuvant treatment | 0.223 | ||||
| No | 15 | 22.7% | 31 | 15.0% | |
| Yes | 16 | 24.2% | 43 | 20.9% | |
| Unknown | 35 | 53% | 132 | 64.1% | |
On Kaplan-Meier survival analysis, a TO was associated with better OS and RFS (all
Cox regression analysis showed that a TO was related to improved OS (HR: 0.443, 95%CI: 0.276-0.711, P = 0.001) and RFS (HR: 0.379, 95%CI: 0.228-0.629, P < 0.001) and that N1 stage disease (HR: 1.872, 95%CI: 1.178-2.977, P = 0.008) was an independent risk factor for OS. Regarding RFS, preoperative CA 199 Level > 40 (HR: 1.601, 95%CI: 1.025-2.501, P = 0.038), N1 stage disease (HR: 1.675, 95%CI: 1.006-2.789, P = 0.047) and lymphovascular invasion (HR: 1.892, 95%CI: 1.161-3.081, P = 0.010) were all independent risk factors. The detailed data are depicted in Tables 2 and 3.
| Characteristic | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Textbook outcome | ||||
| No | Reference | Reference | ||
| Yes | 0.598 (0.383-0.934) | 0.024 | 0.443 (0.276-0.711) | 0.001 |
| Year of surgery | ||||
| 1998-2010 | Reference | |||
| 2011-2020 | 1.095 (0.767-1.562) | 0.619 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 0.914 (0.641-1.303) | 0.619 | ||
| Age (yr) | ||||
| ≤ 60 | Reference | |||
| > 60 | 1.254 (0.875-1.797) | 0.218 | ||
| Operation time (h) | ||||
| ≤ 6 | Reference | |||
| > 6 | 1.259 (0.861-1.839) | 0.235 | ||
| Blood transfusion | ||||
| No | Reference | |||
| yes | 0.998 (0.702-1.417) | 0.990 | ||
| Tumor size (cm) | ||||
| ≤ 2.0 | Reference | Reference | ||
| > 2.0 | 1.396 (0.985-1.978) | 0.061 | 1.327 (0.919-1.917) | 0.131 |
| Differentiation | ||||
| Poor | Reference | Reference | ||
| Moderate | 1.077 (0.730-1.588) | 0.709 | 1.243 (0.830-1.863) | 0.291 |
| Well | 0.644 (0.389-1.065) | 0.086 | 1.026 (0.563-1.868) | 0.934 |
| CA199 | ||||
| 0-40 | Reference | Reference | ||
| > 40 | 1.495 (1.010-2.213) | 0.045 | 1.339 (0.885-2.026) | 0.168 |
| Unknown | 1.393 (0.741-2.619) | 0.303 | 2.022 (1.025-3.990) | 0.042 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.939 (1.303-2.886) | 0.001 | 1.872 (1.178-2.977) | 0.008 |
| N2 | 2.077 (1.002-4.305) | 0.049 | 1.850 (0.856-4.002) | 0.118 |
| T stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.092 (0.616-1.936) | 0.764 | 0.824 (0.441-1.542) | 0.546 |
| T3 | 2.230 (1.309-3.799) | 0.003 | 1.469 (0.771-2.799) | 0.243 |
| Lymphovascular invasion | ||||
| No | Reference | Reference | ||
| Yes | 1.528 (1.026-2.275) | 0.037 | 1.252 (0.797-1.966) | 0.330 |
| Adjuvant treatment | ||||
| No | Reference | |||
| Yes | 1.082 (0.624-1.876) | 0.780 | ||
| Unknown | 0.886 (0.548-1.431) | 0.620 | ||
| Characteristic | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Textbook outcome | ||||
| No | Reference | Reference | ||
| Yes | 0.607 (0.382-0.963) | 0.034 | 0.379 (0.228-0.629) | < 0.001 |
| Year of surgery | ||||
| 1998-2010 | Reference | |||
| 2011-2020 | 1.030 (0.703-1.509) | 0.879 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 1.009 (0.685-1.485) | 0.965 | ||
| Age (yr) | ||||
| ≤ 60 | Reference | |||
| > 60 | 0.972 (0.652-1.449) | 0.891 | ||
| Operation time (h) | ||||
| ≤ 6 | Reference | |||
| > 6 | 1.051 (0.689-1.603) | 0.818 | ||
| Blood transfusion | ||||
| No | Reference | |||
| Yes | 0.932 (0.638-1.363) | 0.717 | ||
| Tumor size (cm) | ||||
| ≤ 2.0 | Reference | Reference | ||
| > 2.0 | 1.540 (1.051-2.257) | 0.027 | 1.365 (0.909-2.048) | 0.133 |
| Differentiation | ||||
| Poor | Reference | Reference | ||
| Moderate | 1.112 (0.730-1.693) | 0.622 | 1.472 (0.946-2.290) | 0.086 |
| Well | 0.546 (0.307-0.974) | 0.040 | 1.002 (0.508-1.976) | 0.997 |
| CA199 | ||||
| 0-40 | Reference | |||
| > 40 | 1.751 (1.145-2.677) | 0.010 | 1.601 (1.025-2.501) | 0.038 |
| Unknown | 1.225 (0.584-2.568) | 0.591 | 1.646 (0.746-3.634) | 0.217 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.801 (1.170-2.771) | 0.008 | 1.675 (1.006-2.789) | 0.047 |
| N2 | 2.563 (1.173-5.604) | 0.018 | 1.807 (0.833-4.138) | 0.162 |
| T stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.152 (0.613-2.166) | 0.661 | 0.885 (0.446-1.754) | 0.726 |
| T3 | 2.488 (1.387-4.463) | 0.002 | 1.419 (0.709-2.842) | 0.323 |
| Lymphovascular invasion | ||||
| No | Reference | Reference | ||
| Yes | 2.002 (1.321-3.033) | 0.001 | 1.892 (1.161-3.081) | 0.010 |
| Adjuvant treatment | ||||
| No | Reference | |||
| Yes | 1.271 (0.730-2.215) | 0.397 | ||
| Unknown | 0.671 (0.405-1.112) | 0.122 | ||
Logistic regression revealed that a year of surgery between 2010 and 2020 (OR: 4.549, 95%CI: 2.064-10.028, P < 0.001) and N1 stage disease (HR: 2.251, 95%CI: 1.023-4.954, P = 0.044) were independently associated with lower odds of a TO. The results of the univariable and multivariable logistic regression analyses are shown in Table 4.
| Characteristic | Univariable analysis | Multivariable analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Year of surgery | ||||
| 1998-2010 | Reference | Reference | ||
| 2011-2020 | 4.470 (2.361-8.462) | < 0.001 | 4.549 (2.064-10.028) | < 0.001 |
| Sex | ||||
| Male | Reference | |||
| Female | 0.872 (0.495-1.535) | 0.634 | ||
| Age (yr) | ||||
| ≤ 60 | Reference | |||
| > 60 | 0.864 (0.487-1.534) | 0.618 | ||
| Operation time (h) | ||||
| ≤ 6 | Reference | |||
| > 6 | 1.163 (0.638-2.118) | 0.622 | ||
| Transfusion | ||||
| No | Reference | |||
| Yes | 0.758 (0.435-1.321) | 0.328 | ||
| Tumor size (cm) | ||||
| ≤ 2.0 | Reference | Reference | ||
| > 2.0 | 1.502 (0.856-2.633) | 0.156 | 1.728 (0.924-3.231) | 0.087 |
| Differentiation | ||||
| Poor | Reference | Reference | ||
| Moderate | 0.867 (0.471-1.595) | 0.646 | 1.194 (0.597-2.390) | 0.616 |
| Well | 0.559 (0.248-1.262) | 0.162 | 1.007 (0.360-2.812) | 0.990 |
| CA199 | ||||
| 0-40 | Reference | |||
| > 40 | 1.072 (0.590-1.950) | 0.819 | ||
| Unknown | 0.919 (0.331-2.551) | 0.872 | ||
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 2.215 (1.192-4.117) | 0.012 | 2.251 (1.023-4.954) | 0.044 |
| N2 | 1.436 (0.434-4.754) | 0.554 | 1.236 (0.314-4.864) | 0.762 |
| T stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.423 (0.599-3.380) | 0.424 | 1.205 (0.455-3.191) | 0.707 |
| T3 | 1.072 (0.464-2.481) | 0.870 | 0.449 (0.150-1.341) | 0.151 |
| Lymphovascular invasion | ||||
| No | Reference | Reference | ||
| Yes | 2.697 (1.477-4.927) | 0.001 | 1.483 (0.688-3.199) | 0.315 |
| Adjuvant treatment | ||||
| No | Reference | Reference | ||
| Yes | 0.769 (0.331-1.785) | 0.541 | 1.144 (0.450-2.912) | 0.777 |
| Unknown | 0.548 (0.267-1.126) | 0.102 | 1.459 (0.629-3.387) | 0.379 |
Fifteen (132, 11.4%) patients treated before 2010 and 52 (140, 36.4%) patients treated after 2010 achieved a TO. The TO rate significantly increased after 2010 (P < 0.001), mainly due to improvements in lymphadenectomy (P < 0.001) and 30-day readmission or mortality (P = 0.030). The detailed data are depicted in Table 5.
| Characteristic | OR (95%CI) | P value |
| R0 resection | - | - |
| ≥ 12 lymph nodes examined | 14.620 (5.323-40.156) | < 0.001 |
| No ICU treatment | 0.255 (0.052-1.258) | 0.093 |
| No postoperative complications | 3.375 (1.268-8.984) | 0.015 |
| No prolonged hospitalization | 2.057 (0.738-5.734) | 0.168 |
| No 30-d readmission or mortality | 6.399 (0.496-82.620) | 0.155 |
TOs are composite measures that represent ideal outcomes and have been used to assess the quality of surgical treatment for many digestive tumours. To our knowledge, this is the first study to define and examine a TO in the evaluation of outcomes in AC patients undergoing PD. We performed a hospital-based retrospective study of 272 patients undergoing curative surgery and found that only 24.3% achieved a TO. In addition, we found that a TO was independently associated with improved OS and RFS. The current study is important because it is the first to demonstrate that a TO is a potentially significant composite indicator for evaluating the quality of surgical treatment for AC.
Improving the quality of care remains a topic of interest for patients and physicians. As far back as 20 years ago, the Society for Thoracic Surgeons started a clinical audit to monitor their results[21]. Recently, TOs have become increasingly accessible for use in assessing the quality of surgical care as combinations of universal variables[17]. Prior studies have typically used isolated parameters to measure quality, such as prolonged hospitalization, morbidity, mortality and readmission[7,22,23]. However, the limitations of these individual metrics were gradually revealed with the progression of research, and they cannot reflect the quality of care completely[14,24,25]. On the other hand, hospitals might perform well in terms of one indicator and worse in terms of another[6,7]. Combining these isolated parameters to build a multidimensional metric might be a more accurate method for measuring quality[26]. As such, TOs are more reliable and comprehensive than single outcome measures, and the use of TOs might address different domains of surgical quality[27-29]. Of note, the all-or-none principle[12] could more accurately reflect desirable patient outcomes and align with ideal patient experiences. From this perspective, a TO is a much more patient-centred metric.
A TO directly reflects the short-term outcomes of rapid recovery and early discharge. However, assessment of long-term outcomes is equally important. Several studies have examined the relationship between a TO and survival among cancer patients. Kulshrestha et al[30] found that 37.2% of oesophageal cancer patients achieved a TO, which appeared to be associated with improved OS [Ed1]. Aquina et al[31] indicated that achieving a TO was related to better OS in the treatment of all eight kinds of cancer in the National Cancer Database. Consistently, similar results were found for PD in the treatment of pancreatic neuroendocrine tumours[32] and pancreatic adenocarcinoma[13-15,33]. Furthermore, we found that achieving a TO was independently associated with improved OS (HR: 0.443, 95%CI: 0.276-0.711, P = 0.001) and RFS (HR: 0.379, 95%CI: 0.228-0.629, P < 0.001). As such, achieving a TO is very significant, and these studies demonstrate the necessity and importance of improving surgical techniques and the quality of clinical care[34,35]. To this end, a TO is a reliable and valuable metric and should be applied in more clinical research.
Only approximately a quarter (24.3%) of patients achieved a TO in our research, meaning that adverse events occurred in a sizable fraction of patients. Previous studies on the achievement of a TO have shown large variations, with an average of 49% in colon cancer[6], 25.5% in intrahepatic cholangiocarcinoma[8], 32.1% in gastric cancer and 29.7% in oesophageal cancer patients[15]. Merath et al[36] found that TO rates varied from 11.1% to 69.6% after pancreatic surgery among hospitals. Aquina and associates[31] showed that the TO rate of pancreatic cancer patients was the lowest at 25% among that of all cancer patients. Similarly, only 16.8% of patients achieved a TO in the study by Sweigert and his colleagues[13]. In the present study, the achievement of a TO was mainly hampered by examination of ≥ 12 Lymph nodes (58.5%) and no postoperative complications (48.9%). The major hampering indicators in other studies include R0 resection[31], no prolonged hospital stay[36], and receipt of adjuvant chemotherapy within 12 wk[13,15], which showed considerable variation. As mentioned above, the wide variation among different hospitals further supports the superiority of TOs. Factors independently associated with a TO included a year of surgery between 2010 and 2020 (OR: 4.549, 95%CI: 2.064-10.028, P < 0.001) and N1 stage disease (OR: 2.251, 95%CI: 1.023-4.954, P = 0.044). Similar results were also found in pancreatic adenocarcinoma patients[13], which was mainly attributed to advancements in surgical techniques and the increasing number of examined lymph nodes. The proportion of AC patients with a TO remained low even at a large medical centre such as ours, which indicates great potential for improvement. Overall, a TO could be applied to guide quality improvement as a reliable metric[31].
Due to the observation of increasing trends in TO rates over the years (P < 0.05), we divided the patients into two groups by the year of surgery before and after 2010 and compared the trends of every indicator over time. Of note, the improvement in the TO rate was mainly attributed to the reduction in postoperative complications and increase in adequate lymphadenectomy, which indicated that there were significant advances in surgical techniques over time. However, Hyer et al[37] found that the improvement was mainly driven by a decline in mortality and prolonged hospital stay. Perioperative management should be further strengthened to better improve the quality of surgery.
There are several limitations to this study. First, the current study was a retrospective review of data from a large single centre, which might introduce the risk of selection bias. Second, our study had some missing data, such as estimated blood loss and details regarding adjuvant treatment. In addition, only a few patients underwent minimally invasive surgery, a subgroup analysis was not conducted. These factors could limit the generalizability of the study results. Third, no patients with T4 stage disease were included in this study due to the combined effects of the inclusion and exclusion criteria; therefore, the TO rate is possibly lower than described. Fourth, the TO definition was based on previous studies and is still in the early phase of development. Some indicators, such as patient satisfaction, social vulnerability[38] and hospital volume[39], which have been shown to affect the chances of achieving a TO, were not evaluated. There is an urgent need for a standard definition for a TO in AC patients who undergo PD.
In conclusion, only approximately a quarter (24.3%) of patients achieved TO in AC patients following PD and achieving TO was independently related to favorable oncological outcomes in AC. This study demonstrated that TO was a simple and reliable composite measure of ideal outcomes following PD which could completely and effectively evaluate the overall quality of surgical care. Further multicenter research is warranted to better elucidate its impact.
Textbook outcome (TO) is a composite measure that represents the ideal outcome and has been used to assess the quality of surgical treatment in many digestive tumors.
Lack of an effective measure to evaluate the quality of surgery for ampullary carcinoma (AC).
This study aimed to investigate the impact of TO on survival for AC patients following pancreaticoduodenectomy and the factors associated with achieving TO.
We defined the concept of TO in ampullary carcinoma and cox regression analysis was used to identify if TO was an independent prognostic factor for overall survival and recurrence free survival.
Only approximately a quarter (24.3%) of patients achieved TO and TO was independently related to favorable oncological outcomes in AC.
TO was a simple and reliable composite measure of ideal outcomes following pancreaticoduodenectomy.
Further multicenter research is warranted to better elucidate the impact of TO.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Shah OJ, India; Varma V, India S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Vanbiervliet G, Strijker M, Arvanitakis M, Aelvoet A, Arnelo U, Beyna T, Busch O, Deprez PH, Kunovsky L, Larghi A, Manes G, Moss A, Napoleon B, Nayar M, Pérez-Cuadrado-Robles E, Seewald S, Barthet M, van Hooft JE. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:429-448. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | de Jong EJM, Geurts SME, van der Geest LG, Besselink MG, Bouwense SAW, Buijsen J, Dejong CHC, Heij LR, Koerkamp BG, de Hingh IHJT, Hoge C, Kazemier G, van Laarhoven HWM, de Meijer VE, Mohammad NH, Strijker M, Timmermans KCAA, Valkenburg-van Iersel LBJ, Wilmink JW, Tjan-Heijnen VCG, de Vos-Geelen J; Dutch Pancreatic Cancer Group (DPCG). A population-based study on incidence, treatment, and survival in ampullary cancer in the Netherlands. Eur J Surg Oncol. 2021;47:1742-1749. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Gonzalez RS, Bagci P, Basturk O, Reid MD, Balci S, Knight JH, Kong SY, Memis B, Jang KT, Ohike N, Tajiri T, Bandyopadhyay S, Krasinskas AM, Kim GE, Cheng JD, Adsay NV. Intrapancreatic distal common bile duct carcinoma: Analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Mod Pathol. 2016;29:1358-1369. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Rostain F, Hamza S, Drouillard A, Faivre J, Bouvier AM, Lepage C. Trends in incidence and management of cancer of the ampulla of Vater. World J Gastroenterol. 2014;20:10144-10150. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Sun S, He C, Wang J, Huang X, Wu J, Li S. The prognostic significance of inflammation-based scores in patients with ampullary carcinoma after pancreaticoduodenectomy. BMC Cancer. 2020;20:981. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Kolfschoten NE, Kievit J, Gooiker GA, van Leersum NJ, Snijders HS, Eddes EH, Tollenaar RA, Wouters MW, Marang-van de Mheen PJ. Focusing on desired outcomes of care after colon cancer resections; hospital variations in 'textbook outcome'. Eur J Surg Oncol. 2013;39:156-163. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Parina RP, Chang DC, Rose JA, Talamini MA. Is a low readmission rate indicative of a good hospital? J Am Coll Surg. 2015;220:169-176. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Kneuertz PJ, Pitt HA, Bilimoria KY, Smiley JP, Cohen ME, Ko CY, Pawlik TM. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg. 2012;16:1727-1735. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Merath K, Chen Q, Bagante F, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Weiss MJ, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Guglielmi A, Itaru E, Cloyd JM, Pawlik TM. A Multi-institutional International Analysis of Textbook Outcomes Among Patients Undergoing Curative-Intent Resection of Intrahepatic Cholangiocarcinoma. JAMA Surg. 2019;154:e190571. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Tsilimigras DI, Sahara K, Moris D, Mehta R, Paredes AZ, Ratti F, Marques HP, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Weiss M, Bauer TW, Maithel SK, Pulitano C, Shen F, Koerkamp BG, Endo I, Pawlik TM. Assessing Textbook Outcomes Following Liver Surgery for Primary Liver Cancer Over a 12-Year Time Period at Major Hepatobiliary Centers. Ann Surg Oncol. 2020;27:3318-3327. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Poelemeijer YQM, Marang-van de Mheen PJ, Wouters MWJM, Nienhuijs SW, Liem RSL. Textbook Outcome: an Ordered Composite Measure for Quality of Bariatric Surgery. Obes Surg. 2019;29:1287-1294. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006;295:1168-1170. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Sweigert PJ, Eguia E, Baker MS, Paredes AZ, Tsilimigras DI, Dillhoff M, Ejaz A, Cloyd J, Tsung A, Pawlik TM. Assessment of textbook oncologic outcomes following pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2020;121:936-944. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kalagara R, Norain A, Chang YH, Stucky CC, Wasif N. Association of Textbook Outcome and Surgical Case Volume with Long-Term Survival in Patients Undergoing Surgical Resection for Pancreatic Cancer. J Am Coll Surg. 2022;235:829-837. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Sweigert PJ, Wang X, Eguia E, Baker MS, Kulshrestha S, Tsilimigras DI, Ejaz A, Pawlik TM. Does minimally invasive pancreaticoduodenectomy increase the chance of a textbook oncologic outcome? Surgery. 2021;170:880-888. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Busweiler LA, Schouwenburg MG, van Berge Henegouwen MI, Kolfschoten NE, de Jong PC, Rozema T, Wijnhoven BP, van Hillegersberg R, Wouters MW, van Sandick JW; Dutch Upper Gastrointestinal Cancer Audit (DUCA) group. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg. 2017;104:742-750. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Salet N, Bremmer RH, Verhagen MAMT, Ekkelenkamp VE, Hansen BE, de Jonge PJF, de Man RA. Is Textbook Outcome a valuable composite measure for short-term outcomes of gastrointestinal treatments in the Netherlands using hospital information system data? A retrospective cohort study. BMJ Open. 2018;8:e019405. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83:691-729. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Sweigert PJ, Eguia E, Baker MS, Link CM, Hyer JM, Paredes AZ, Tsilimigras DI, Husain S, Pawlik TM. Assessment of Cancer Center Variation in Textbook Oncologic Outcomes Following Colectomy for Adenocarcinoma. J Gastrointest Surg. 2021;25:775-785. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Clark RE. The development of The Society of Thoracic Surgeons voluntary national database system: genesis, issues, growth, and status. Best Pract Benchmarking Healthc. 1996;1:62-69. [PubMed] [Cited in This Article: ] |
| 22. | Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA. 2004;292:847-851. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Lawson EH, Zingmond DS, Stey AM, Hall BL, Ko CY. Measuring risk-adjusted value using Medicare and ACS-NSQIP: is high-quality, low-cost surgical care achievable everywhere? Ann Surg. 2014;260:668-77; discussion 677. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Siracuse JJ, Schermerhorn ML, Meltzer AJ, Eslami MH, Kalish JA, Rybin D, Doros G, Farber A; Vascular Study Group of New England. Comparison of outcomes after endovascular and open repair of abdominal aortic aneurysms in low-risk patients. Br J Surg. 2016;103:989-994. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, Buskens E, Grobbee DE, Blankensteijn JD; Dutch Randomized Endovascular Aneurysm Management (DREAM)Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607-1618. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Shahian DM, Edwards FH, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, O'Brien SM, Shewan CM, Dokholyan RS, Peterson ED; Society of Thoracic Surgeons Quality Measurement Task Force. Quality measurement in adult cardiac surgery: part 1--Conceptual framework and measure selection. Ann Thorac Surg. 2007;83:S3-12. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Li M, Zhang W, Jiang L, Yang J, Yan L. Fast track for open hepatectomy: A systemic review and meta-analysis. Int J Surg. 2016;36:81-89. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Chen Q, Olsen G, Bagante F, Merath K, Idrees JJ, Akgul O, Cloyd J, Dillhoff M, White S, Pawlik TM. Procedure-Specific Volume and Nurse-to-Patient Ratio: Implications for Failure to Rescue Patients Following Liver Surgery. World J Surg. 2019;43:910-919. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Nathan H, Yin H, Wong SL. Postoperative Complications and Long-Term Survival After Complex Cancer Resection. Ann Surg Oncol. 2017;24:638-644. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Kulshrestha S, Bunn C, Patel PM, Sweigert PJ, Eguia E, Pawlik TM, Baker MS. Textbook oncologic outcome is associated with increased overall survival after esophagectomy. Surgery. 2020;168:953-961. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Aquina CT, Hamad A, Becerra AZ, Cloyd JM, Tsung A, Pawlik TM, Ejaz A. Is Textbook Oncologic Outcome a Valid Hospital-Quality Metric after High-Risk Surgical Oncology Procedures? Ann Surg Oncol. 2021;28:8028-8045. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Heidsma CM, Hyer M, Tsilimigras DI, Rocha F, Abbott DE, Fields R, Smith PM, Poultsides GA, Cho C, Maithel SK, Pawlik TM; Other Members of the US Neuroendocrine Tumor Study Group. Incidence and impact of Textbook Outcome among patients undergoing resection of pancreatic neuroendocrine tumors: Results of the US Neuroendocrine Tumor Study Group. J Surg Oncol. 2020;121:1201-1208. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | van Roessel S, Mackay TM, van Dieren S, van der Schelling GP, Nieuwenhuijs VB, Bosscha K, van der Harst E, van Dam RM, Liem MSL, Festen S, Stommel MWJ, Roos D, Wit F, Molenaar IQ, de Meijer VE, Kazemier G, de Hingh IHJT, van Santvoort HC, Bonsing BA, Busch OR, Groot Koerkamp B, Besselink MG; Dutch Pancreatic Cancer Group. Textbook Outcome: Nationwide Analysis of a Novel Quality Measure in Pancreatic Surgery. Ann Surg. 2020;271:155-162. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Wu L, Tsilimigras DI, Paredes AZ, Mehta R, Hyer JM, Merath K, Sahara K, Bagante F, Beal EW, Shen F, Pawlik TM. Trends in the Incidence, Treatment and Outcomes of Patients with Intrahepatic Cholangiocarcinoma in the USA: Facility Type is Associated with Margin Status, Use of Lymphadenectomy and Overall Survival. World J Surg. 2019;43:1777-1787. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Idrees JJ, Merath K, Gani F, Bagante F, Mehta R, Beal E, Cloyd JM, Pawlik TM. Trends in centralization of surgical care and compliance with National Cancer Center Network guidelines for resected cholangiocarcinoma. HPB (Oxford). 2019;21:981-989. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Merath K, Chen Q, Bagante F, Beal E, Akgul O, Dillhoff M, Cloyd JM, Pawlik TM. Textbook Outcomes Among Medicare Patients Undergoing Hepatopancreatic Surgery. Ann Surg. 2020;271:1116-1123. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Hyer JM, Beane JD, Spolverato G, Tsilimigras DI, Diaz A, Paro A, Dalmacy D, Pawlik TM. Trends in Textbook Outcomes over Time: Are Optimal Outcomes Following Complex Gastrointestinal Surgery for Cancer Increasing? J Gastrointest Surg. 2022;26:50-59. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Azap RA, Paredes AZ, Diaz A, Hyer JM, Pawlik TM. The association of neighborhood social vulnerability with surgical textbook outcomes among patients undergoing hepatopancreatic surgery. Surgery. 2020;168:868-875. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Mehta R, Paredes AZ, Tsilimigras DI, Moro A, Sahara K, Farooq A, Dillhoff M, Cloyd JM, Tsung A, Ejaz A, Pawlik TM. Influence of hospital teaching status on the chance to achieve a textbook outcome after hepatopancreatic surgery for cancer among Medicare beneficiaries. Surgery. 2020;168:92-100. [PubMed] [DOI] [Cited in This Article: ] |