Published online May 27, 2022. doi: 10.4240/wjgs.v14.i5.383
Peer-review started: November 10, 2021
First decision: January 9, 2022
Revised: January 17, 2022
Accepted: April 29, 2022
Article in press: April 29, 2022
Published online: May 27, 2022
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are a rare group of tumors originating from neuroendocrine cells of the digestive system. Their incidence has increased over the last decades. The specific pathogenetic mechanisms underlying GEP-NEN development have not been completely revealed. Unfunctional GEP-NENs are usually asymptomatic; some grow slowly and thus impede early diagnosis, which ultimately results in a high rate of misdiagnosis. Therefore, many GEP-NEN patients present with later staged tumors. Motivated hereby, research attention for diagnosis and treatment for GEP-NENs increased in recent years. The result of which is great progress in clinical diag
Core Tip: Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are a group of heterogeneous tumors arising from neuroendocrine cells of the digestive system. Researchers have achieved great improvements in diagnosis and treatment. This includes improved grading, identification of specific genetic mutations, functional imaging, and broad application of peptide receptor radionuclide therapy. Here, we systematically summarized the latest progress in diagnosis and treatment of GEP-NENs, thereby providing guidance for clinicians active in this field.
- Citation: Dai M, Mullins CS, Lu L, Alsfasser G, Linnebacher M. Recent advances in diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. World J Gastrointest Surg 2022; 14(5): 383-396
- URL: https://www.wjgnet.com/1948-9366/full/v14/i5/383.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i5.383
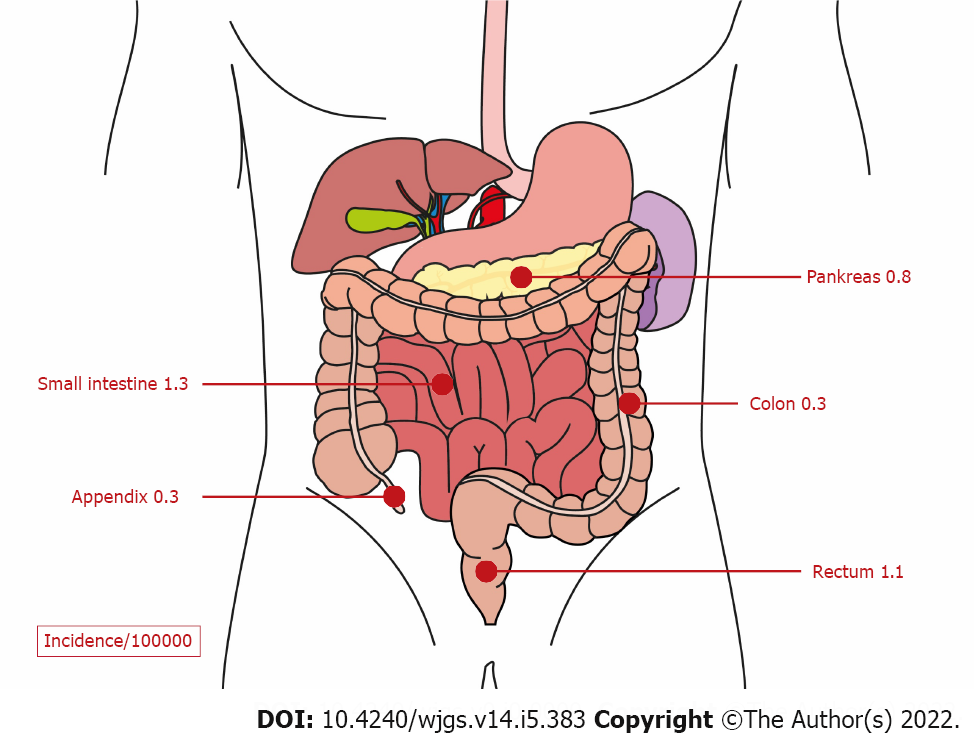
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) originate from neuroendocrine cells of the pancreas or the gastrointestinal tract. They represent the second most common cancer of the digestive system (Figure 1)[1]. The Surveillance, Epidemiology, and End Results (commonly known as SEER) 18 registry (2000-2012) revealed an increased incidence of GEP-NENs in the United States to 3.56/100000 inhabitants in the year 2012[2]. In European countries, the incidence also increased and was reported to be in the range of 1.33 to 2.33/100000 inhabitants[3,4]. Improvements in the detection methods have been identified as the most probable explanation for the increased incidence of GEP-NENs over the last decades[5]. These neoplasms are classified into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs). Moreover, depending on the hormone and amine secretion activity, GEP-NENs can be classified into functional and nonfunctional neoplasms[1,6]. Functional GEP-NENs produce hormones and amines, which cause specific clinical manifestations, such as hypoglycemia, refractory gastric ulcer, flushing, diarrhea, etc. However, immunohistochemical hormone staining is not sufficient for diagnosis[7].
Due to the clinical manifestations, functional GEP-NENs can frequently be diagnosed in early stages, what translates into a relatively good prognosis. In contrast, non-functional GEP-NENs are asymptomatic until distant metastases or mass effect cause late symptoms, such as intestinal obstruction[8]. The 2019 World Health Organization (WHO) classification of GEP-NENs consisted of the following categories: Grade 1, Grade 2, Grade 3, and NEC. This grading is based on the mitotic rate and/or the Ki-67 proliferation index, as listed in Table 1 below. The mitotic rate is determined by an immunohistochemistry method, in which 50 fields of 0.2 mm2 are counted. The Ki-67 proliferation index value is determined by counting more than 500 cells in the regions of highest labelling using scanning magnification. The NEN grade is assigned by the proliferation index of the two, which places the neoplasm in the higher-grade according to the classification. Mixed NENs consist of both neuroendocrine and non-neuroendocrine components and are poorly differentiated, and the neuroendocrine component has proliferation indexes in the same range as other NECs. This conceptual category however allows for respect of the fact that one or both components can also be well differentiated; if feasible, every component should be graded separately[9,10]. Surgery is still the mainstay of curative treatment for localized GEP-NENs[11]. Methods of clinical diagnosis and treatment have been continuously updated because of ongoing research and study activities. This review aims at systemically summarizing the latest research advances on diagnosis and treatment of GEP-NENs.
| Classification | Differentiation status | Ki-67 index | Mitotic rate |
| Grade 1, NET | Well differentiated | < 3% | < 2 |
| Grade 2, NET | Well differentiated | 3% to 20% | 2 to 20 |
| Grade 3, NET | Well differentiated | > 20% | > 20 |
| Small cell type, NEC | Poorly differentiated | > 20% | > 20 |
| Large cell type, NEC | Poorly differentiated | > 20% | > 20 |
| Mixed NEN | Well or poorlydifferentiated | Variable | Variable |
GEP-NENs present as very heterogeneous, both because of different organs of origin and because of different biological behavior; consequently, clinical symptoms are various. Especially functional GEP-NENs, which secrete specific hormones, cause characteristic clinical syndromes[12]. Insulinomas produce excessive amounts of insulin, thereby causing hypoglycemia. Excessive secretion of gastrin from functional gastrinomas often results in refectory and recurrent peptic ulcerations. Glucagonoma patients regularly present with recent diabetic mellitus as well as migratory necrolytic erythema caused by extremely high glucagon levels, whereas somatostatinoma patients will present with hyperglycemia and steatorrhea. Contrary to that, non-functional GEP-NENs do not cause specific clinical symptoms, and they are often only diagnosed during routine physical examinations[13].
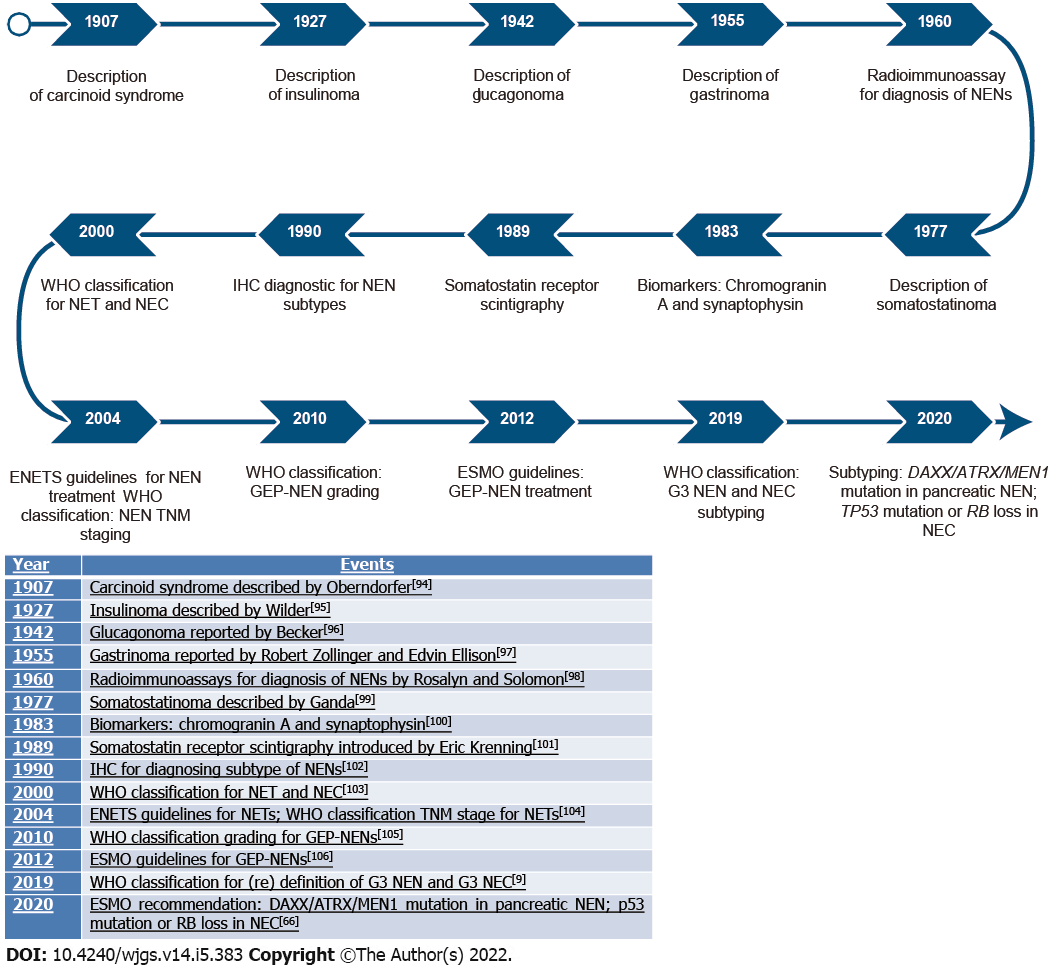
Diagnostic improvements over time are shown in Figure 2.
Chromogranin-A: Chromogranin-A (CgA) is a member of the chromogranin glycoprotein family and is physiologically secreted by neurons and neuroendocrine cells[14]. In clinical diagnosis, CgA is established as a universal routine diagnostic biomarker of neuroendocrine neoplasms. Sensitivity of CgA assays varies between 32% and 92%, depending on the NET type, secretory status, and tumor burden. The specificity can approach 100% if other diseases affecting serum CgA levels, such as kidney insufficiency and chronic atrophic gastritis, can be excluded[15].
Serotonin: Serotonin is assessed by measuring its degradation product, 5hydroxyindoleacetic acid (5-HIAA), in 24-h urine of patients with carcinoid symptoms[16]. A meta-analysis demonstrated that 5-HIAA can be a predictive biomarker for 1-year mortality rate of NEN patients[17]. However, since specific nutritious substances (such as eggplants, bananas, tomatoes, etc) and medications (such as nicotine, ephedrine, diazepam, etc) can affect 5-HIAA measurement, patients need to be guided to omit these substances.
Gastrin: Gastrinomas can result in elevation of serum gastrin levels. With excessive secretion of gastrin, patients will suffer from refractory peptic ulcers. Therefore, serum levels of gastrin are routinely measured in patients suspected to have gastrinomas. Criteria for diagnosis of Zollinger-Ellison syndrome as a result of gastrinomas are: At least 10-fold elevated serum gastrin levels and a gastric pH below 2.1. However, proton pump inhibitors (PPIs) can elevate serum gastrin levels. Patients receiving PPIs need to wean this medication for at least 1 wk before gastrin measurement[18].
Insulin: Insulin is measured for diagnosis of insulinomas after a 72-h gastric fasting. If, during fasting-induced hypoglycemia, serum insulin levels reach more than 3 mcIU/mL, serum pro-insulin levels rise above 5 pmol/L, and Cpeptide concentrations are at least 0.6 ng/mL, an insulinoma is a probable diagnosis; especially in patients with concurrent pancreatic mass[19].
Glucagon: Glucagon is measured in the blood of patients suspected to suffer from glucagonomas and meeting the following criteria: Recently diagnosed with diabetes mellitus, migratory necrolytic erythema, and a positive imaging confirmation of a gastroenteropancreatic mass[20].
In summary, although these serum molecular tests are in standard use for GEP-NEN differential diagnosis, a consensus conference of multinational experts repeated that a single biomarker to diagnose efficaciously and predict prognosis for patients with GEP-NENs would be beneficial[7].
Computed tomography and magnetic resonance imaging: Computed tomography and magnetic resonance imaging are conventional techniques used to determine localization and to evaluate neoplasm burden of GEP-NENs. Multiphase computed tomography (CT) or magnetic resonance imaging (MRI) scans are recommended to diagnose distant metastatic lesions[21,22], because GEP-NENs are highly vascularized and thus show the same resolution as the liver in conventional CT scanning. They can, however, be detected by either of these advanced imaging techniques. Similarly, contrast CT chest scanning is recommended for the evaluation of lung metastases. Small peritoneal, liver, and lymphatic metastases < 1 cm cannot be detected by CT analyses[23].
Functional imaging: Nowadays, functional somatostatin receptor (SSR) imaging is widely used in clinical diagnosis of NENs. Beside localizing tumors and selecting SSR-positive patients for specific therapies, it can be used to evaluate therapeutic responses[24]. Five subtypes of SSRs (SSR1 to SSR5) have been identified, and their molecular mechanisms of regulation and signaling have been elucidated[25]. The most prominent SSR subtype in GEP-NENs is SSR2, followed by SSR1 and SSR5; SSR3 and SSR4 are less frequently expressed[26]. Moreover, SSR2 and SSR5 are usually expressed in insulinomas[27].
The 68Ga-DOTA somatostatin analogues (SSA) imaging system consists of 68Ga-DOTA-Tyr3-octreotide (68Ga-DOTA-TOC), 68Ga-DOTA-Nal3-octreotide (68Ga-DOTA-NOC), and 68Ga-DOTA-Tyr3-octreotate (68Ga-DOTA-TATE). These different imaging agents display distinct affinities to variable SSRs. Compared to 111In-pentetreotide functional imaging, 68Ga-DOTA-SSA imaging has been shown to improve diagnosis and staging for NENs[28] and has become the imaging method of choice. 68Ga-DOTA-TOC shows a higher affinity to SSR-2, 68Ga-DOTA-NOC towards SSR-2, SSR-3, and SSR-5, whereas 68Ga-DOTA-TATE towards SSR-2 and SSR-5[29]. Clinicians are supposed to select appropriate imaging agents for specific NENs. 18Fluorodeoxyglucose (18FDG), a tracer for glucose metabolism, can indirectly assess metabolic activity of GEP-NENs. The ability of tumor cells to take up glucose is positively correlated with the tumor growth rate[30], which is in turn related to aggressiveness. Combining 18FDG-PET/CT with 18Ga-DOTA-TATE imaging is another functional imaging method for NENs[31]. Even for GEP-NENs with low or negative SSR expression, positive 18FDG PET/CT imaging denotes worse prognosis[32]. For the detection of tumor site and activity, the combination of SSR imaging and 18FDG imaging has proven to be complementary[33,34].
Endoscopy, ultrasonography, and endoscopic ultrasonography are also recommended for the diagnosis and treatment of GEP-NENs. For early-stage and smaller GEP-NENs, endoscopic resection should be taken into consideration when lymphatic metastases have been excluded by endoscopic ultrasonography (US) or imaging[35]. Endoscopic resection should be reserved for GEP-NENs with a diameter < 1 cm, superficial position, and low grading[35]. US can serve as the initial diagnostic approach for liver metastases. Moreover, it can guide the biopsy needle to collect tissues for histopathological assessment. Endoscopic US is currently the most sensitive diagnostic approach for pancreatic NENs and allows biopsy collection at the same time[36], whereas intraoperative US can detect tumors in liver and pancreas, otherwise not detected by imaging methods[37].
Histopathological examination is the gold standard for GEP-NEN diagnosis; both from biopsies and resected tissues. Hematoxylin and eosin staining is used to determine cytological and histomorphological indices, and immunohistochemical staining of CgA and synaptophysin are mandatory for differential diagnosis in pathological reports[38]. Immunohistochemical Ki-67 index determination and mitotic counts per mm2 are the basis of grade classification for GEP-NENs (see Table 1). According to the latest National Comprehensive Cancer Network (NCCN) guidelines, histological classification, the resection margin status, Tumor, Node, Metastasis (commonly known as TNM) stage, and the presence of vascular invasion are also mandatory in pathological reports, because these factors are significantly associated with patient prognosis[39].
For WHO grade 3 NENs, somatic mutations in the genes death domain associated protein (DAXX), multiple endocrine neoplasia type 1 (MEN1), and alpha thalassemia/intellectual disability syndrome X-linked (ATRX) are most frequent. Whereas, in NECs, mutations affect the genes retinoblastoma transcriptional corepressor 1 (RB1), mothers against decapentaplegic homolog 4 (SMAD4), and tumor protein p53 (TP53)[40,41]. This difference in the occurrence of somatic mutations can be exploited to discriminate GEP-NECs from WHO grade 3 GEP-NENs in challenging cases[42]. In addition, NECs of the small intestine often show mutations in the cyclin-dependent kinase inhibitor 1B (CDKN1B)[43], and lack of CDKN1B gene expression has been described as a negative prognostic factor in GEP-NENs[6,44]. Insulinoma-associated protein 1 (INSM1) has proven to be a specific and sensitive biomarker for diagnosing NECs[45,46].
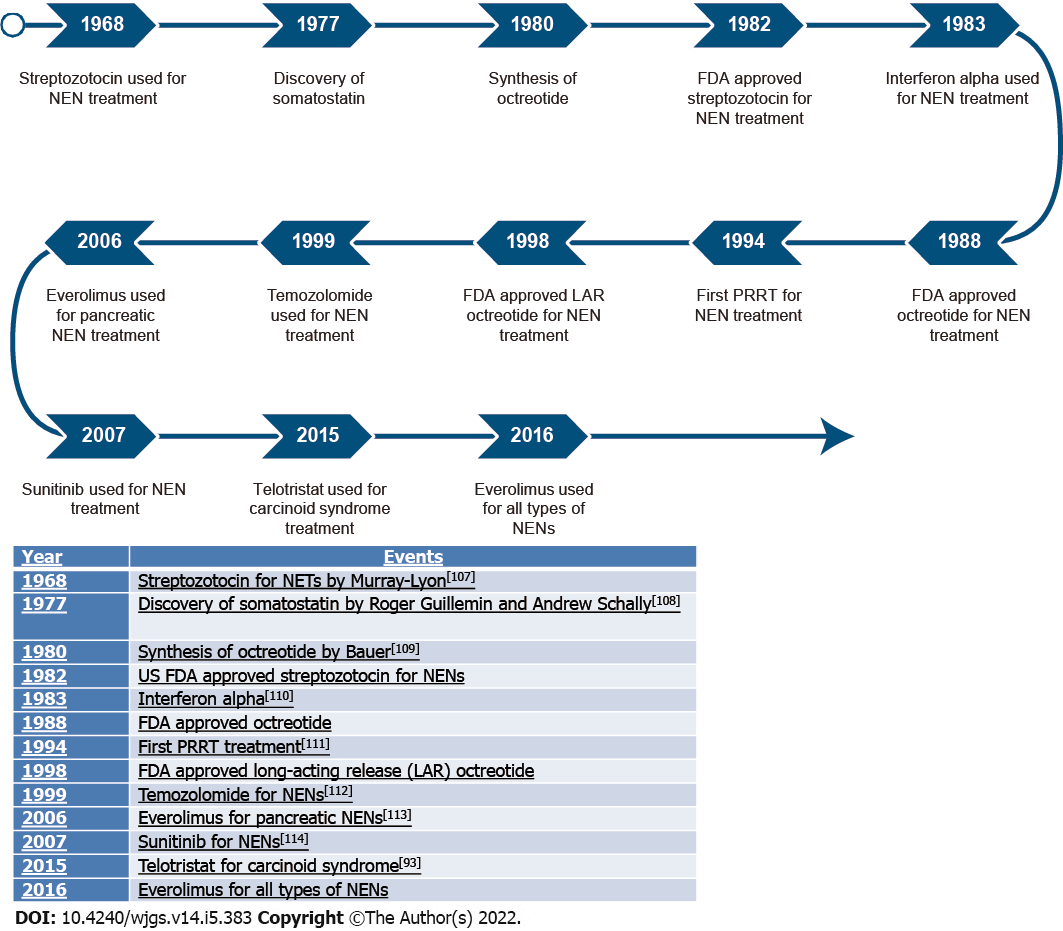
An overview of treatment developments is shown in Figure 3.
Surgical resection remains the sole curative form of therapy for patients with GEP-NENs[47]. Patients with local or locoregional GEP-NENs should be recommended for curative resection of the primary and the locoregional lymph nodes[48]. For patients with asymptomatic pancreatic NENs < 2 cm, a cautious surveillance with yearly imaging is recommended[49]. Patients with pancreatic NENs > 2 cm should receive pancreatectomy with regional lymphadenectomy[50]. Localized small intestinal NENs are resected radically, including removal of mesenteric lymph nodes[51]. This can also reduce the risk of associated comorbidities, such as intestinal obstruction. A clinical study including 581 patients operated on with metastatic NENs demonstrated that the median overall survival (OS) was 110.4 mo for curative resection. In comparison, resections resulting merely in debulking (OS: 89.2 mo) or performed in a palliative situation (OS: 50.0 mo) had significantly shorter OS rates (P < 0.001). Patients receiving cytoreductive surgery survived, in median, 89.2 mo, whereas when all metastatic lesions could be removed, the longest median survival of 112.5 mo could be reached (P < 0.001)[52]. Another clinical retrospective analysis of grade 3 GEP-NENs reported a 2-year OS rate after radical surgery of 64.5%, a 2-year progression-free survival (PFS) rate of 44.9%, and a median PFS of 14 mo[53]. Therefore, the 2021 NCCN guidelines6 recommended that, for small (< 2 cm) and low-grade NENs, surgery or close monitoring should be individualized. For large (> 2 cm) and higher-graded NENs, resection with negative margins and removal of regional lymph nodes should be conducted. Cytoreductive or debulking resection for distant metastases is recommended when more than 90% of the lesions can be removed safely, especially if patients present with serious hormonal symptoms[54,55].
Somatostatin: Somatostatin is a general endocrine “off-switch” due to its not only endocrine but also, exocrine, autocrine, and paracrine inhibitory effects. In the digestive system, somatostatin can inhibit bowel movements, decrease the blood flow of mesenteric vessels, inhibit gastrointestinal absorption as well as gallbladder contraction, and suppress hormone secretion[56]. The half-life of somatostatin is only 3 min, thus preventing its pharmacological use. Hence, SSAs with longer half-lives were developed to treat patients with GEP-NENs[57]. SSAs can control hormonal symptoms induced by GEP-NENs[58] by binding to SSRs, thereby preventing the activation. Currently, the most commonly used SSAs for GEP-NENs are octreotide and lanreotide. In the placebo-controlled, double-blind, prospective, and randomized study on the “effect of octreotide long-acting repeatable (LAR) in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID)” clinical trial, it was demonstrated that octreotide significantly delayed tumor progression time (LAR 14.3 mo vs placebo 6 mo)[59]. The controlled study of lanreotide anti-proliferative response in NEN (CLARINET) trial confirmed that lanreotide was associated with significantly higher 2-year PFS rates in patients with metastatic enteropancreatic NEN (65.1% in the lanreotide group vs 33.0% in the placebo group)[60]. In a phase III trial, pasireotide, a second generation SSA[61], was compared to octreotide. It prolonged the median PFS from 6.8 mo in the octreotide LAR control group to 11.8 mo in the pasireotide LAR group[62]. The guidelines of the European Neuroendocrine Tumor Society (ENETS) and the NCCN guidelines recommended SSAs as first-line therapeutic agents for GEP-NENs. For patients receiving LAR SSAs, cholecystectomy is recommended in case of cholecystitis and gallstones[63].
Interferon-α: Interferon-α (IFNα) has been used to inhibit hormone secretion and proliferation in NENs in the past decades[64]. The phase III clinical study of the Southwest Oncology Group compared octreotide LAR plus IFNα with octreotide LAR plus bevacizumab. Antitumor effectiveness was similar with median PFS of 15.4 mo and 16.6 mo, respectively[65]. When other available therapeutic options failed, IFNα could thus be taken into cautious consideration as a rescue antiproliferative therapy[66].
Mammalian target of rapamycin inhibitors: When the phosphatase and tension homolog protein is phosphorylated, a negative feedback regulation via phosphatidylinositide 3-kinase (PI3K) is normally activated, which inhibits cell proliferation and promotes cell apoptosis. However, the reduction of phosphatase and tension homolog messenger RNA expression stimulates activation of the PI3K-AKT-mammalian target of rapamycin (mTOR) pathway and can trigger tumor formation[67]. The key role of this signaling pathway in GEP-NEN development inspired mechanistic research with the aim to develop drugs targeting PI3K-Akt-mTOR[68,69]. Phase III clinical studies of RAD001 application for patients with advanced NEN (RADIANT)-3 and -4, lead to the approval of everolimus. This targeted inhibitor of mTOR with the capacity to delay NEN progression attained approval for treatment of GEP-NENs[70,71]. Both ENETS and NCCN guidelines recommend everolimus as a second or third-line drug for advanced GEP-NENs. In patients with insulinomas, everolimus showed the positive side-effect of stabilizing glycemic levels[72]. However, low expression of SSR2 in patients with insulinomas results in poor response to SSAs[73]. Even worse, SSA treatment of patients with insulinomas can exacerbate hypoglycemia due to an inhibition of glucagon[56,74]. Therefore, everolimus should be prioritized for patients with insulinomas.
Vascular endothelial growth factor receptors inhibitors: Sunitinib, a broadly acting tyrosine kinase inhibitor targeting vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors, has been affirmed to defer progression of pancreatic NENs in a phase III clinical trial75]. Sunitinib was thus included for treatment of advanced pancreatic NENs in the ENETS and NCCN guidelines. However, there is a lack of clinical data for the effects of sunitinib on gastroenteric NENs. The Grupo Espanol de Tumores Neuroendocrinos (GETNE 1509) phase II trial has proven that lenvatinib, another VEGFR inhibitor, achieved an overall response rate of 29.9% (44.2% in pancreatic and 16.4% in gastrointestinal NENs), a median response duration of 21.5 mo (19.9 mo in pancreatic and 33.9 mo in gastrointestinal NENs), a median PFS of 15.7 mo (15.6 mo and 15.7 mo respectively), and a median OS of 32 mo in the pancreatic NEN group. The median OS was not reached in the gastrointestinal NEN group. The phase III trial of surufatinib, a novel VEGFR inhibitor, in advanced extrapancreatic and pancreatic neuroendocrine tumors (SANET-ep and SANET-p) showed a meaningful improvement of PFS to 9.2 mo and 10.8 mo in the surufatinib groups vs 3.8 mo and 3.7 mo in the placebo groups for patients with advanced, progressive, well differentiated, extrapancreatic NENs, and advanced pancreatic NENs[76], respectively.
Immune checkpoint inhibitors, which target for example programmed death protein-1 (PD-1), its receptor programmed death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), showed promising antitumor efficacy in various tumor types[77]. In a phase IB study of the anti-PD-1 antibody pembrolizumab in advanced solid tumors (KEYNOTE-028), pembrolizumab monotherapy proved antitumor efficacy in patients with PD-L1-positive carcinoid and pancreatic NENs with high stable disease rates of 60% and 88%, respectively; however, only a disappointing objective response rate (ORR) of 12% and 6.3%, respectively[78]. In a subsequent phase II (KEYNOTE-158) study, pembrolizumab monotherapy had an ORR of only 3.7%, a median PFS of 4.1 mo and a median OS of 24.2 mo in patients with previously treated advanced well-differentiated NENs[79]. Pembrolizumab is also proposed for patients with tumor progression after previous treatment, tumors with high tumor mutational burden and no adequate alternative treatment regimens[80,81]. A phase II clinical trial of dual anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) inhibition in patients with nonpancreatic NENs reported an auspicious ORR of 44% (18 of 32 patients) with high-grade NENs. This trial demonstrated that dual immunotherapy preferentially plays a role in grade 3 NENs[82]. A similar phase II study (CA209-538) also verified the significant efficacy of combination immunotherapy with ipilimumab and nivolumab in high-grade NEN patients (the median PFS of 4.8 mo and the OS of 14.8 mo in all the patients with NENs)[83].
Peptide receptor radionuclide therapy is actually a kind of systemic and targeted radiotherapy in one[84]. SSAs are structured with a radioisotope [such as Yttrium-90 (90Y) and Lutetium-177 (177Lu)] via a chelating agent. The emitted radiation kills the cancer cells that express SSRs on the tumor cells’ surface[85]. 177Lu-DOTA-TATE was approved by the European Medicines Agency for the treatment of patients with GEP-NENs in 2017 and a year later by the American Food and Drug Administration[86,87]. In a comprehensive meta-analysis of 1920 patients with unresectable metastatic NENs receiving 177Lu-DOTATATE therapy from 18 studies, the ORR was between 29.1% and 30.6%, and the disease control rate was 74.1% to 81.1%[88].
For G1 and G2 pancreatic NENs, SSAs are recommended as first-line therapeutic regimen. When ineffective, however, both NCCN and ENETS guidelines recommend temozolomide combined with capecitabine or streptozotocin-based therapies. To date, there is no recommendation for systematic chemotherapy for G1 and G2 gastroenteric NENs from NCCN and ENETS. Similarly, no standard chemotherapeutic regimens are currently recommended for G3 NETs. The NORDIC NEC study demonstrated that NEC patients with Ki-67 < 55% were less sensitive to platinum-based chemotherapy than those with Ki-67 ≥ 55% (response rate: 15% vs 42%, respectively), yet survival times were better for patients with Ki-67 < 55% (14 mo vs 10 mo, respectively)[89]. Thus, ENETS and NCCN guidelines do not suggest platinum- but temozolomide-based chemotherapies for patients with Ki-67 < 65%. For grade 3 NEN patients with Ki-67 < 55%, temozolomide-based chemotherapies are recommended; whereas, patients with Ki-67 ≥ 55% should receive platinum-based regimens, such as cisplatin or carboplatin, both in combination with etoposide[90]. These regimens are also recommended for GEP-NEC patients in the 2021 NCCN guideline as first-line chemotherapy.
PPIs can control hypersecretion of gastric acid in patients with gastrinomas. However, related studies have proven that PPIs can lead to hypomagnesemia and vitamin B12 deficiency in patients with long-term use[91], suggesting a cautious use paired with regular control of magnesium and vitamin B12 levels.
Tryptophan hydroxylase is the rate-limiting enzyme for the conversion of tryptophan to serotonin. The tryptophan hydroxylase inhibitor telotristat can reduce the serotonin production. It is thus used in clinical practice to treat patients with refractory diarrhea resulting from a carcinoid syndrome[92] and it has been validated to normalize bowel movements and urinary levels of 5-HIAA[93].
In summary, the pathogenesis of GEP-NENs is still largely unclear. Multiple classification systems and treatment schedules have been accurately (re)defined thanks to the efforts of GEP-NEN experts. Because of the great improvement of detection technologies, an increasing number of suspicious patients can be diagnosed with GEP-NENs already at an early stage. Novel treatment approaches, including small molecule inhibitors, SSAs, and peptide receptor radionuclide therapy targeting GEP-NENs, have evolved remarkably. However, prospective research still needs to be conducted to confirm their efficacy. Also, many controversies concerning the therapy regimens for specific GEP-NENs of different types remain. Beside identifying and developing novel molecular targeted drugs, the rational combination of targeted, chemo-, and immunotherapy seems to be the future research direction in the field of GEP-NEN therapy.
All authors appreciate the excellent help from Burmeister J for the figure designs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Association for Cancer Research, No. EACR30750.
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hou XH, China; Pelaez-Luna M, Mexico S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X, Lv K, He X, Fang H, Jin K, Fei Y, Chen Y, Wang J, Liu H, Li H, Zhang H, He H, Zhang W. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann Surg. 2022;275:626-635. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Huguet I, Grossman AB, O'Toole D. Changes in the Epidemiology of Neuroendocrine Tumours. Neuroendocrinology. 2017;104:105-111. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Genus TSE, Bouvier C, Wong KF, Srirajaskanthan R, Rous BA, Talbot DC, Valle JW, Khan M, Pearce N, Elshafie M, Reed NS, Morgan E, Deas A, White C, Huws D, Ramage J. Impact of neuroendocrine morphology on cancer outcomes and stage at diagnosis: a UK nationwide cohort study 2013-2015. Br J Cancer. 2019;121:966-972. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, Chan J, Das S, Dickson PV, Fanta P, Giordano T, Halfdanarson TR, Halperin D, He J, Heaney A, Heslin MJ, Kandeel F, Kardan A, Khan SA, Kuvshinoff BW, Lieu C, Miller K, Pillarisetty VG, Reidy D, Salgado SA, Shaheen S, Soares HP, Soulen MC, Strosberg JR, Sussman CR, Trikalinos NA, Uboha NA, Vijayvergia N, Wong T, Lynn B, Hochstetler C. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:839-868. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, Meyer T, Moss SF, Washington K, Wolin E, Liu E, Goldenring J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435-e446. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Gorelik M, Ahmad M, Grossman D, Grossman M, Cooperman AM. Nonfunctioning Incidental Pancreatic Neuroendocrine Tumors: Who, When, and How to Treat? Surg Clin North Am. 2018;98:157-167. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Assarzadegan N, Montgomery E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2021;145:664-677. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Strosberg JR, Cheema A, Weber JM, Ghayouri M, Han G, Hodul PJ, Kvols LK. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg. 2012;256:321-325. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Fottner C, Ferrata M, Weber MM. Hormone secreting gastro-entero-pancreatic neuroendocrine neoplasias (GEP-NEN): When to consider, how to diagnose? Rev Endocr Metab Disord. 2017;18:393-410. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Bonds M, Rocha FG. Neuroendocrine Tumors of the Pancreatobiliary and Gastrointestinal Tracts. Surg Clin North Am. 2020;100:635-648. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26:791-802. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Marotta V, Zatelli MC, Sciammarella C, Ambrosio MR, Bondanelli M, Colao A, Faggiano A. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer. 2018;25:R11-R29. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, Pape UF, Perren A, Rindi G, Ruszniewski P, Scoazec JY, Welin S, Wiedenmann B, Ferone D; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Biochemical Markers. Neuroendocrinology. 2017;105:201-211. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Joish VN, Shah S, Tierce JC, Patel D, McKee C, Lapuerta P, Zacks J. Serotonin levels and 1-year mortality in patients with neuroendocrine tumors: a systematic review and meta-analysis. Future Oncol. 2019;15:1397-1406. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep. 2009;11:433-441. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709-728. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Sandru F, Carsote M, Albu SE, Valea A, Petca A, Dumitrascu MC. Glucagonoma: From skin lesions to the neuroendocrine component (Review). Exp Ther Med. 2020;20:3389-3393. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB. Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur J Endocrinol. 2004;151:15-27. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Cwikła JB, Buscombe JR, Caplin ME, Watkinson AF, Walecki J, Gorczyca-Wiśniewska E, Hilson AJ. Diagnostic imaging of carcinoid metastases to the abdomen and pelvis. Med Sci Monit. 2004;10 Suppl 3:9-16. [PubMed] [Cited in This Article: ] |
| 23. | Norlen O, Montan H, Hellman P, Stalberg P, Sundin A. Preoperative 68Ga-DOTA-Somatostatin Analog-PET/CT Hybrid Imaging Increases Detection Rate of Intra-abdominal Small Intestinal Neuroendocrine Tumor Lesions. World J Surg. 2018;42:498-505. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, Howe JR, Kulke MH, Kunz PL, Mailman J, May L, Metz DC, Millo C, O'Dorisio S, Reidy-Lagunes DL, Soulen MC, Strosberg JR. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med. 2018;59:66-74. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Reubi JC, Schonbrunn A. Illuminating somatostatin analog action at neuroendocrine tumor receptors. Trends Pharmacol Sci. 2013;34:676-688. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781-793. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Bertherat J, Tenenbaum F, Perlemoine K, Videau C, Alberini JL, Richard B, Dousset B, Bertagna X, Epelbaum J. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study. J Clin Endocrinol Metab. 2003;88:5353-5360. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, Delbeke D, Walker RC. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J Nucl Med. 2016;57:872-878. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Virgolini I, Gabriel M, Kroiss A, von Guggenberg E, Prommegger R, Warwitz B, Nilica B, Roig LG, Rodrigues M, Uprimny C. Current knowledge on the sensitivity of the (68)Ga-somatostatin receptor positron emission tomography and the SUVmax reference range for management of pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2016;43:2072-2083. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med. 2012;14:71-81. [PubMed] [Cited in This Article: ] |
| 31. | Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer. 2020;27:R67-R77. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978-985. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Has Simsek D, Kuyumcu S, Turkmen C, Sanlı Y, Aykan F, Unal S, Adalet I. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J Nucl Med. 2014;55:1811-1817. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Naswa N, Sharma P, Gupta SK, Karunanithi S, Reddy RM, Patnecha M, Lata S, Kumar R, Malhotra A, Bal C. Dual tracer functional imaging of gastroenteropancreatic neuroendocrine tumors using 68Ga-DOTA-NOC PET-CT and 18F-FDG PET-CT: competitive or complimentary? Clin Nucl Med. 2014;39:e27-e34. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Saund MS, Al Natour RH, Sharma AM, Huang Q, Boosalis VA, Gold JS. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol. 2011;18:2826-2832. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271-2277. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S, Fazio N, Giammarile F, Hicks RJ, Kjaer A, Krenning E, Kwekkeboom D, Lombard-Bohas C, O'Connor JM, O'Toole D, Rockall A, Wiedenmann B, Valle JW, Vullierme MP; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology. 2017;105:212-244. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense RT, Kulke M, Oeberg K, Rindi G, Sorbye H, Welin S; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology. 2017;105:196-200. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gönen M, Jensen RT, Kidd M, Kulke MH, Lloyd RV, Moran C, Moss SF, Oberg K, O'Toole D, Rindi G, Robert ME, Suster S, Tang LH, Tzen CY, Washington MK, Wiedenmann B, Yao J. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300-313. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40:1192-1202. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, Agaimy A, Sipos B, Zamboni G, Weichert W, Esposito I, Pfarr N, Klöppel G. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol. 2017;30:587-598. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Singhi AD, Klimstra DS. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology. 2018;72:168-177. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Francis JM, Kiezun A, Ramos AH, Serra S, Pedamallu CS, Qian ZR, Banck MS, Kanwar R, Kulkarni AA, Karpathakis A, Manzo V, Contractor T, Philips J, Nickerson E, Pho N, Hooshmand SM, Brais LK, Lawrence MS, Pugh T, McKenna A, Sivachenko A, Cibulskis K, Carter SL, Ojesina AI, Freeman S, Jones RT, Voet D, Saksena G, Auclair D, Onofrio R, Shefler E, Sougnez C, Grimsby J, Green L, Lennon N, Meyer T, Caplin M, Chung DC, Beutler AS, Ogino S, Thirlwell C, Shivdasani R, Asa SL, Harris CR, Getz G, Kulke M, Meyerson M. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45:1483-1486. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Kim HS, Lee HS, Nam KH, Choi J, Kim WH. p27 Loss Is Associated with Poor Prognosis in Gastroenteropancreatic Neuroendocrine Tumors. Cancer Res Treat. 2014;46:383-392. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Kim D, Viswanathan K, Goyal A, Rao R. Insulinoma-associated protein 1 (INSM1) is a robust marker for identifying and grading pancreatic neuroendocrine tumors. Cancer Cytopathol. 2020;128:269-277. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O'Dorisio TM, Halperin DM, Fishbein L, Eads J, Hope TA, Singh S, Salem R, Metz DC, Naraev BG, Reidy-Lagunes DL, Howe JR, Pommier RF, Menda Y, Chan JA. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:863-881. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Woltering EA, Voros BA, Beyer DT, Wang YZ, Thiagarajan R, Ryan P, Wright A, Ramirez RA, Ricks MJ, Boudreaux JP. Aggressive Surgical Approach to the Management of Neuroendocrine Tumors: A Report of 1,000 Surgical Cytoreductions by a Single Institution. J Am Coll Surg. 2017;224:434-447. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist. 2016;21:1191-1199. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Partelli S, Cirocchi R, Crippa S, Cardinali L, Fendrich V, Bartsch DK, Falconi M. Systematic review of active surveillance vs surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br J Surg. 2017;104:34-41. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Partelli S, Gaujoux S, Boninsegna L, Cherif R, Crippa S, Couvelard A, Scarpa A, Ruszniewski P, Sauvanet A, Falconi M. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg. 2013;148:932-939. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Lardière-Deguelte S, de Mestier L, Appéré F, Vullierme MP, Zappa M, Hoeffel C, Noaves M, Brixi H, Hentic O, Ruszniewski P, Cadiot G, Panis Y, Kianmanesh R. Toward a Preoperative Classification of Lymph Node Metastases in Patients with Small Intestinal Neuroendocrine Tumors in the Era of Intestinal-Sparing Surgery. Neuroendocrinology. 2016;103:552-559. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Chakedis J, Beal EW, Lopez-Aguiar AG, Poultsides G, Makris E, Rocha FG, Kanji Z, Weber S, Fisher A, Fields R. 343 - Surgery Provides Long-Term Survival in Patients with Metastatic Neuroendocrine Tumors Undergoing Resection for Non-Hormonal Symptoms. Journal of Gastrointestinal Surgery 2018; 23. [DOI] [Cited in This Article: ] |
| 53. | Merola E, Rinke A, Partelli S, Gress TM, Andreasi V, Kollár A, Perren A, Christ E, Panzuto F, Pascher A, Jann H, Arsenic R, Cremer B, Kaemmerer D, Kump P, Lipp RW, Agaimy A, Wiedenmann B, Falconi M, Pavel ME. Surgery with Radical Intent: Is There an Indication for G3 Neuroendocrine Neoplasms? Ann Surg Oncol. 2020;27:1348-1355. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Daskalakis K, Karakatsanis A, Hessman O, Stuart HC, Welin S, Tiensuu Janson E, Öberg K, Hellman P, Norlén O, Stålberg P. Association of a Prophylactic Surgical Approach to Stage IV Small Intestinal Neuroendocrine Tumors With Survival. JAMA Oncol. 2018;4:183-189. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Partelli S, Cirocchi R, Rancoita PMV, Muffatti F, Andreasi V, Crippa S, Tamburrino D, Falconi M. A Systematic review and meta-analysis on the role of palliative primary resection for pancreatic neuroendocrine neoplasm with liver metastases. HPB (Oxford). 2018;20:197-203. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Cives M, Strosberg J. The expanding role of somatostatin analogs in gastroenteropancreatic and lung neuroendocrine tumors. Drugs. 2015;75:847-858. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Stueven AK, Kayser A, Wetz C, Amthauer H, Wree A, Tacke F, Wiedenmann B, Roderburg C, Jann H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Baldelli R, Barnabei A, Rizza L, Isidori AM, Rota F, Di Giacinto P, Paoloni A, Torino F, Corsello SM, Lenzi A, Appetecchia M. Somatostatin analogs therapy in gastroenteropancreatic neuroendocrine tumors: current aspects and new perspectives. Front Endocrinol (Lausanne). 2014;5:7. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin Analogs in Clinical Practice: a Review. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Wolin EM, Jarzab B, Eriksson B, Walter T, Toumpanakis C, Morse MA, Tomassetti P, Weber MM, Fogelman DR, Ramage J, Poon D, Gadbaw B, Li J, Pasieka JL, Mahamat A, Swahn F, Newell-Price J, Mansoor W, Öberg K. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075-5086. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966-973. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Rinke A, Krug S. Neuroendocrine tumours - Medical therapy: Biological. Best Pract Res Clin Endocrinol Metab. 2016;30:79-91. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Yao JC, Guthrie KA, Moran C, Strosberg JR, Kulke MH, Chan JA, LoConte N, McWilliams RR, Wolin EM, Mattar B, McDonough S, Chen H, Blanke CD, Hochster HS. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol. 2017;35:1695-1703. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Capozzi M, VON Arx C, DE Divitiis C, Ottaiano A, Tatangelo F, Romano GM, Tafuto S; (On behalf of ENETS Center of Excellence Multidisciplinary Group for Neuroendocrine Tumors in Naples, Italy). Antiangiogenic Therapy in Pancreatic Neuroendocrine Tumors. Anticancer Res. 2016;36:5025-5030. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Wolin EM. PI3K/Akt/mTOR pathway inhibitors in the therapy of pancreatic neuroendocrine tumors. Cancer Lett. 2013;335:1-8. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Aristizabal Prada ET, Auernhammer CJ. Targeted therapy of gastroenteropancreatic neuroendocrine tumours: preclinical strategies and future targets. Endocr Connect. 2018;7:R1-R25. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016;34:3906-3913. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Agata, Matej, Hanna, Bujwid, Jakub, Wroński. Glycemic control in patients with insulinoma. Hormones (Athens, Greece) 2016. [DOI] [Cited in This Article: ] |
| 73. | Usukura M, Yoneda T, Oda N, Yamamoto Y, Takata H, Hasatani K, Takeda Y. Medical treatment of benign insulinoma using octreotide LAR: a case report. Endocr J. 2007;54:95-101. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Healy ML, Dawson SJ, Murray RM, Zalcberg J, Jefford M. Severe hypoglycaemia after long-acting octreotide in a patient with an unrecognized malignant insulinoma. Intern Med J. 2007;37:406-409. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Faivre S, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, Vinik A, Van Cutsem E, Bang YJ, Lee SH, Borbath I, Lombard-Bohas C, Metrakos P, Smith D, Chen JS, Ruszniewski P, Seitz JF, Patyna S, Lu DR, Ishak KJ, Raymond E. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol. 2017;28:339-343. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Xu J, Shen L, Bai C, Wang W, Li J, Yu X, Li Z, Li E, Yuan X, Chi Y, Yin Y, Lou W, Xu N, Bai Y, Zhang T, Xiu D, Wang X, Yuan Y, Chen J, Qin S, Jia R, Lu M, Cheng Y, Zhou Z, He J, Su W. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:1489-1499. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-249. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Mehnert JM, Bergsland E, O'Neil BH, Santoro A, Schellens JHM, Cohen RB, Doi T, Ott PA, Pishvaian MJ, Puzanov I, Aung KL, Hsu C, Le Tourneau C, Hollebecque A, Élez E, Tamura K, Gould M, Yang P, Stein K, Piha-Paul SA. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer. 2020;126:3021-3030. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res. 2020;26:2124-2130. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353-1365. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Merino DM, McShane LM, Fabrizio D, Funari V, Chen SJ, White JR, Wenz P, Baden J, Barrett JC, Chaudhary R, Chen L, Chen WS, Cheng JH, Cyanam D, Dickey JS, Gupta V, Hellmann M, Helman E, Li Y, Maas J, Papin A, Patidar R, Quinn KJ, Rizvi N, Tae H, Ward C, Xie M, Zehir A, Zhao C, Dietel M, Stenzinger A, Stewart M, Allen J; TMB Harmonization Consortium. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, Matrana M, Gatalica Z, Korn WM, Hayward J, McLeod C, Chen HX, Sharon E, Mayerson E, Ryan CW, Plets M, Blanke CD, Kurzrock R. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res. 2020;26:2290-2296. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, Jackett L, Lum C, Scott C, Nagrial A, Behren A, So JY, Palmer J, Cebon J. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin Cancer Res. 2020;26:4454-4459. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Kwekkeboom DJ, Krenning EP. Peptide Receptor Radionuclide Therapy in the Treatment of Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30:179-191. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Cives M, Strosberg J. Radionuclide Therapy for Neuroendocrine Tumors. Curr Oncol Rep. 2017;19:9. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Camus B, Cottereau AS, Palmieri LJ, Dermine S, Tenenbaum F, Brezault C, Coriat R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Salner AL, Blankenship B, Dunnack H, Niemann C, Bertsch H. Lutetium Lu-177 Dotatate Flare Reaction. Adv Radiat Oncol. 2021;6:100623. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Saravana-Bawan B, Bajwa A, Paterson J, McEwan AJB, McMullen TPW. Efficacy of 177Lu Peptide Receptor Radionuclide Therapy for the Treatment of Neuroendocrine Tumors: A Meta-analysis. Clin Nucl Med. 2019;44:719-727. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Sorbye H, Baudin E, Perren A. The Problem of High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumors, Neuroendocrine Carcinomas, and Beyond. Endocrinol Metab Clin North Am. 2018;47:683-698. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Corleto VD, Festa S, Di Giulio E, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21:3-8. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Pavel M, Gross DJ, Benavent M, Perros P, Srirajaskanthan R, Warner RRP, Kulke MH, Anthony LB, Kunz PL, Hörsch D, Weickert MO, Lapuerta P, Jiang W, Kassler-Taub K, Wason S, Fleming R, Fleming D, Garcia-Carbonero R. Telotristat ethyl in carcinoid syndrome: safety and efficacy in the TELECAST phase 3 trial. Endocr Relat Cancer. 2018;25:309-322. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Kulke MH, Hörsch D, Caplin ME, Anthony LB, Bergsland E, Öberg K, Welin S, Warner RR, Lombard-Bohas C, Kunz PL, Grande E, Valle JW, Fleming D, Lapuerta P, Banks P, Jackson S, Zambrowicz B, Sands AT, Pavel M. Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome. J Clin Oncol. 2017;35:14-23. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Oberndorfer S. Karzinoide Tumoren des Dunndarms. Frankfurt Z Path. 1907;1:426-432. [DOI] [Cited in This Article: ] |
| 95. | Wilder RM, Allan FN, Power MH, Robertson HE. Carcinoma of the islands of the pancreas: hyperinsulinism and hypoglycemia. J Am Med Dir Assoc. 1927;89:348-355. [DOI] [Cited in This Article: ] |
| 96. | Becker ER. THE IOWA ACADEMY OF SCIENCE. Science. 1942;95:651-652. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709-23; discussion, 724. [PubMed] [Cited in This Article: ] |
| 98. | Bloom SR, Bryant MG, Polak JM. Proceedings: Distribution of gut hormones. Gut. 1975;16:821. [PubMed] [Cited in This Article: ] |
| 99. | Ganda OP, Weir GC, Soeldner JS, Legg MA, Chick WL, Patel YC, Ebeid AM, Gabbay KH, Reichlin S. "Somatostatinoma": a somatostatin-containing tumor of the endocrine pancreas. N Engl J Med. 1977;296:963-967. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Lloyd RV, Wilson BS. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 1983;222:628-630. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716-731. [DOI] [Cited in This Article: ] |
| 102. | Solcia E, Rindi G, Capella C. Histochemistry in pathology. Edinburgh, London, New York: Churchill-Livingstone 1990: 397-409. [DOI] [Cited in This Article: ] |
| 103. | Evans EB, Eggers GW. World Health Organization International Histological Classification of Tumours: Histological Typing of Endocrine Tumours: Second Edition. Clinical Endocrinology. 2000;53:259-259. [DOI] [Cited in This Article: ] |
| 104. | Delellis RA, Lloyd RV, Heitz PU. World Health Organization Classification Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs. 2004. [DOI] [Cited in This Article: ] |
| 105. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. International Agency for Research on Cancer. 2010;. [Cited in This Article: ] |
| 106. | Oberg K, Akerström G, Rindi G, Jelic S; ESMO Guidelines Working Group. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v223-v227. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Murray-Lyon IM, Eddleston AL, Williams R, Brown M, Hogbin BM, Bennett A, Edwards JC, Taylor KW. Treatment of multiple-hormone-producing malignant islet-cell tumour with streptozotocin. Lancet. 1968;2:895-898. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495-1501. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | Bauer W, Briner U, Doepfner W, Haller R, Huguenin R, Marbach P, Petcher TJ, Pless. SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31:1133-1140. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | Oberg K, Funa K, Alm G. Effects of leukocyte interferon on clinical symptoms and hormone levels in patients with mid-gut carcinoid tumors and carcinoid syndrome. N Engl J Med. 1983;309:129-133. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Krenning EP, Kooij PP, Bakker WH, Breeman WA, Postema PT, Kwekkeboom DJ, Oei HY, de Jong M, Visser TJ, Reijs AE, et al. Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci. 1994;733:496-506. [DOI] [Cited in This Article: ] |
| 112. | Ekeblad S, Sundin A, Janson ET, Welin S, Granberg D, Kindmark H, Dunder K, Kozlovacki G, Orlefors H, Sigurd M, Oberg K, Eriksson B, Skogseid B. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986-2991. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311-4318. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [PubMed] [DOI] [Cited in This Article: ] |