Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.209
Peer-review started: November 22, 2023
First decision: December 8, 2023
Revised: December 16, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: February 15, 2024
Diabetes and thyroiditis are closely related. They occur in combination and cause significant damage to the body. There is no clear treatment for type-2 diabetes mellitus (T2DM) with Hashimoto's thyroiditis (HT). While single symptomatic drug treatment of the two diseases is less effective, combined drug treatment may improve efficacy.
To investigate the effect of a combination of vitamin D, selenium, and hypo-glycemic agents in T2DM with HT.
This retrospective study included 150 patients with T2DM and HT treated at The Central Hospital of Shaoyang from March 2020 to February 2023. Fifty patients were assigned to the control group, test group A, and test group B according to different treatment methods. The control group received low-iodine diet guidance and hypoglycemic drug treatment. Test group A received the control treatment plus vitamin D treatment. Test group B received the group A treatment plus selenium. Blood levels of markers of thyroid function [free T3 (FT3), thyroid stimulating hormone (TSH), free T4 (FT4)], autoantibodies [thyroid peroxidase antibody (TPOAB) and thyroid globulin antibody (TGAB)], blood lipid index [low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triacylglycerol (TG)], blood glucose index [fasting blood glucose (FBG), and hemoglobin A1c (HbA1c)] were measured pre-treatment and 3 and 6 months after treatment. The relationships between serum 25-hydroxyvitamin D3 [25 (OH) D3] level and each of these indices were analyzed.
The levels of 25 (OH) D3, FT3, FT4, and LDL-C increased in the order of the control group, test group A, and test group B (all P < 0.05). The TPOAB, TGAB, TC, TG, FBG, HbA1c, and TSH levels increased in the order of test groups B, A, and the control group (all P < 0.05). All the above indices were compared after 3 and 6 months of treatment. Pre-treatment, there was no divergence in serum 25 (OH) D3 level, thyroid function-related indexes, autoantibodies level, blood glucose, and blood lipid index between the control group, test groups A and B (all P > 0.05). The 25 (OH) D3 levels in test groups A and B were negatively correlated with FT4 and TGAB (all P < 0.05).
The combination drug treatment for T2DM with HT significantly improved thyroid function, autoantibody, and blood glucose and lipid levels.
Core Tip: Selenium yeast and active vitamin D can reduce thyroid-related antibodies in type-2 diabetes mellitus (T2DM) and Hashimoto's thyroiditis (HT) and improve thyroid function. Hypoglycemia drugs can lower blood sugar levels in patients and promote blood sugar stability. While most patients with T2DM and HT are currently treated with a single symptomatic drug, the effects are unsatisfactory. In this study, the combination of vitamin D and selenium yeast added to hypoglycemic agents to treat T2DM patients with HT showed a remarkable therapeutic effect.
- Citation: Feng F, Zhou B, Zhou CL, Huang P, Wang G, Yao K. Vitamin D, selenium, and antidiabetic drugs in the treatment of type 2 diabetes mellitus with Hashimoto's thyroiditis. World J Diabetes 2024; 15(2): 209-219
- URL: https://www.wjgnet.com/1948-9358/full/v15/i2/209.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i2.209
Diabetes is a chronic metabolic disease characterized by chronic hyperglycemia caused by a relative lack of insulin in the body. Its incidence increases annually, and approximately 90% of cases involve type-2 diabetes mellitus (T2DM)[1]. Thyroid disease is mainly characterized by dysfunction in thyroid hormone secretion. Hashimoto's thyroiditis (HT) is a typical autoimmune disease that has also shown an increasing incidence in recent years[2]. The main manifestations of HT are elevated levels of thyroid autoantibodies and goiter, which often lead to hypothyroidism with disease pro-gression. Diabetes and thyroiditis are closely related and often occur in combination. Foreign reports show that thyroid dysfunction has a higher prevalence in the diabetic population, at 12.5%-51.6%, which is two to three times that of other populations[3,4]. The study found that the incidence of HT in T2DM patients was significantly higher than in the general population[5]. The pathogenesis of T2DM and HT is believed to mainly involve insulin resistance, immune factors, infection, oxidative stress, genetics, leptin, molecular cytology, and other related factors; however, there is no clear consensus on the pathogenesis of T2DM with HT. Western medicine generally adopts symptomatic treatments for these two diseases, including hypoglycemic medications, improved thyroid function, and treatment of complications.
Iodine, selenium, and vitamin D are essential for thyroid hormone production in the human body. Deficiencies can cause changes in thyroid structure and function[6]. HT is often accompanied by vitamin D deficiency. In foreign literature, vitamin D deficiency in patients with HT was as high as 60.6% and was even lower in female patients[7]. Vitamin D levels are negatively correlated with thyroid-stimulating hormone levels. Patients with HT with insufficient or deficient vitamin D levels are more likely to have subclinical and clinical hypothyroidism than HT patients with normal vitamin D levels[8]. However, some studies have conflicting results regarding the effect of vitamin D on the incidence of HT[9,10]. Related literature reports that the occurrence of T2DM is relevant to changes in serum 25-hydroxyvitamin D3 [25 (OH) D3] levels. Supplementation with vitamin D increases serum 25 (OH) D3 levels[11]. Selenium supplementation can upregulate activated regulatory T cells' horizons and partially reduce thyroid autoantibodies' horizons[12,13]. Yu et al[14] explored the effect of the combined treatment of thyroxine and selenium on HT, and the results suggested that the combination of the two drugs was significantly better than thyroxine alone in preventing HT progression. However, few studies have reported the efficacy of combined treatments with vitamin D, selenium yeast, and hypoglycemic drugs in patients with T2DM and HT. This study explored the therapeutic effects of vitamin D, selenium, and vitamin D combined with selenium in patients with T2DM and HT. The study further examined 25 (OH) D3 indicators associated with combined T2DM and HT.
This retrospective study included 150 patients with T2DM and HT treated at The Central Hospital of Shaoyang between March 2020 and February 2023. According to the different treatment methods, the patients were split into test groups A and B and a control group, with 50 cases per group. The inclusion criteria were: Meeting the diagnostic criteria for T2DM[15] and in a stable condition; combined with HT and meeting the HT diagnostic criteria[16]: (1) Swollen and tough thyroid isthmus, (2) positivity for serum thyroid globulin antibody (TGAB) and thyroid peroxidase antibody (TPOAB); (3) thyroid ultrasound showing diffuse enlargement and hypoechoic thyroid gland; (4) thyroid fine needle puncture findings consistent with cytological changes of thyroiditis; and (5) thyroid function showing normal range of free T4 (FT4) and thyroid stimulating hormone (TSH) levels (< 10 Uiu/L), which was not treated after the initial diagnosis. Among them, (1), (2), (3) and (5) are necessary. If the case is atypical, (4) is required for diagnosis. No neurological diseases at study completion. The exclusion criteria were: (1) Type-1 diabetes mellitus; (2) severe infectious diseases and other autoimmune diseases; (3) heart, liver, kidney, and other serious diseases or malignant tumors; (4) pregnancy; (5) use of immunosuppressants, immune checkpoint inhibitors, or glucocorticoid drugs and a recent history of drugs affecting thyroid function; (6) history of thyroid trauma or surgical treatment combined with parathyroid dysfunction; and (7) chronic inflammation caused by other factors.
The control group received low-iodine diet guidance and hypoglycemic drug treatment. That is, saxagliptin tablets (Bristol-Myers Squibb Company, national drug approval number J20110029) were administered orally once daily (5 mg daily).
Test group A was administered oral vitamin D (Qingdao Double Whale Pharmaceutical Co., LTD., Sinopod H20113033, 4000 u/d) + hypoglycemic drug treatment in addition to the control group treatment[11].
Test group B was administered vitamin D + selenium yeast + hypoglycemic drug treatment. That is, based on test group A, oral selenium yeast (Mudanjiang Lingtai Pharmaceutical Co., LTD., Sinomedmedicine approval number: H10940161, 100 μg/time, 2 times/day). Treatment was discontinued in cases of adverse reactions, including cardiopulmonary events, allergies, or elevated blood calcium levels. All patients in each group were treated for 6 months [11,14].
(1) General information: Sex, age, and body mass index (BMI) were collected and recorded; (2) laboratory indicators: After 8 h of overnight fasting, the subjects were sent to the central laboratory for a venous blood sample the following morning. The samples were immediately stored at 4 °C. An automatic chemiluminescence analyzer (I2000SR, Abbott, United States) was used to detect serum 25 (OH) D3, thyroid function [TSH, free T3 (FT3), FT4], autoantibody (TGAB, TPOAB); automatic biochemical apparatus (Beckman Coulter, AU5800 model) determination of blood lipid index [low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triacylglycerol (TG)], blood glucose index [fasting blood glucose (FBG), hemoglobin A1c (HbA1c)]. These indicators were measured in all patients pre-treatment and after 3 and 6 months of treatment; and (3) the correlations between serum 25 (OH) D3 levels and each index in test groups A and B were analyzed.
IBM SPSS Statistics for Windows, version 26.0, was used to analyze the project data. Counting variables are expressed as n, (%) and compared by χ2 test. Continuous variables are reported as mean ± SD. One-way analysis of variance (ANOVA) was used to compare the three groups. If differences were observed, a pound-for-pair comparison was performed. Pearson's correlation analysis was used to analyze the relationships between serum 25 (OH) D3 levels and each index. The test level of statistical analysis was α = 0.05.
Comparisons of general data, such as sex, age, and BMI among the three groups (P > 0.05), are shown in Table 1.
| Group | Sex (male/female) | Age (yr) | BMI (kg/m2) |
| Control group (n = 50) | 23/27 | 53.78 ± 7.49 | 22.88 ± 2.31 |
| Test group A (n = 50) | 26/24 | 52.76 ± 7.88 | 23.07 ± 2.17 |
| Test group B (n = 50) | 22/28 | 52.52 ± 8.13 | 22.98 ± 2.30 |
| χ2/F value | 1.361 | 0.364 | 0.085 |
| P value | 0.715 | 0.695 | 0.918 |
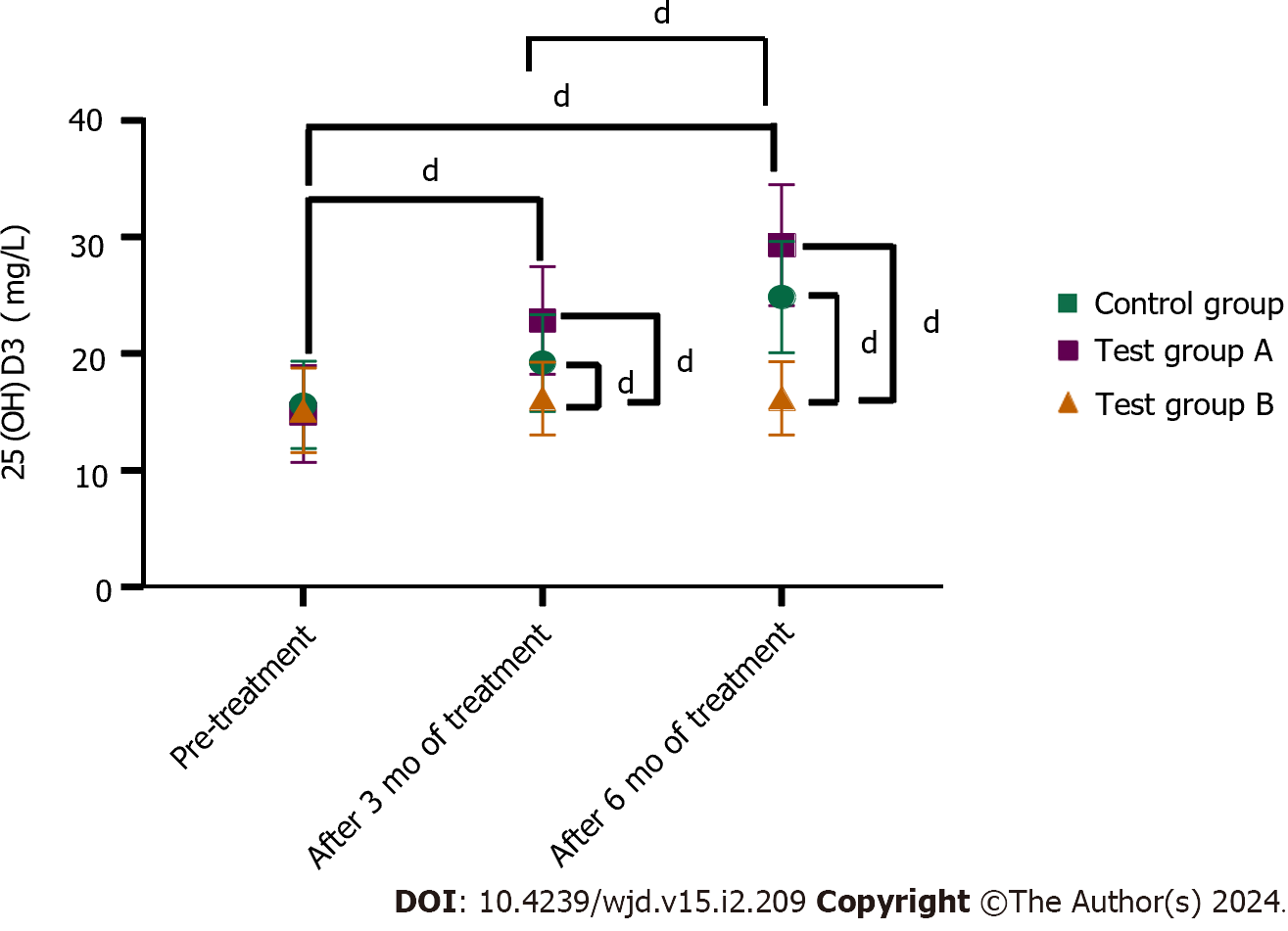
Pre-treatment, the 25 (OH) D3 levels in the control group and test groups A and B were 15.15 ± 3.64, 15.62 ± 3.75, and 14.85 ± 4.17 mg/L, respectively (P > 0.05 for the comparison between the three groups). After 3 months of treatment, the 25 (OH) D3 levels in test groups A and B were 19.24 ± 4.14 and 22.88 ± 4.60 mg/L, respectively, which were higher than that in the control group (16.18 ± 3.09 mg/L). Compared with test group A, the levels in test group B were higher (P < 0.05). After 6 months of treatment, the levels of 25 (OH) D3 in test groups A and B were 24.87 ± 4.75 and 29.31 ± 5.17 mg/L, respectively, both of which were higher than that of the control group (16.19 ± 3.14 mg/L). Compared with that in test group A, the level in test group B was higher (P < 0.05) (Figure 1).
After 3 and 6 months of treatment, The TSH levels of test groups A and B were lower than those of the control group. Compared with those in test group A, the values in group B were lower (P < 0.05). The FT3 and FT4 levels in test groups A and B were higher than those in the control group; compared with those in test group A, the levels in group B were higher (P < 0.05) (Table 2).
| Group | TSH (mU/L) | FT3 (pmol/L) | FT4 (pmol/L) | ||||||
| Pre-treatment | After 3 months of treatment | After 6 months of treatment | Pre-treatment | After 3 months of treatment | After 6 months of treatment | Pre-treatment | After 3 months of treatment | After 6 months of treatment | |
| Control group (n = 50) | 14.50 ± 2.30 | 13.29 ± 2.34 | 12.89 ± 2.18 | 2.39 ± 0.77 | 2.57 ± 0.83 | 2.65 ± 0.82 | 7.35 ± 1.35 | 8.27 ± 1.45 | 8.96 ± 2.05 |
| Test group A (n = 50) | 14.49 ± 2.23 | 8.37 ± 2.33a | 4.98 ± 1.45a | 2.43 ± 0.80 | 3.98 ± 0.86a | 5.21 ± 1.26a | 7.28 ± 1.38 | 11.27 ± 2.16a | 15.51 ± 2.40a |
| Test group B (n = 50) | 14.70 ± 2.34 | 8.54 ± 2.41a,b | 4.05 ± 1.27a,b | 2.48 ± 0.81 | 4.05 ± 0.90a,b | 5.47 ± 1.34a,b | 7.26 ± 1.26 | 11.87 ± 2.27a,b | 16.91 ± 2.73a,b |
| F value | 0.133 | 70.399 | 416.857 | 0.162 | 47.220 | 89.340 | 0.063 | 52.264 | 155.329 |
| P value | 0.875 | < 0.001 | < 0.001 | 0.850 | < 0.001 | < 0.001 | 0.939 | < 0.001 | < 0.001 |
Pre-treatment, the TPOAB levels of the control group, test groups A and B were 365.23 ± 87.26, 364.74 ± 86.78, and 365.76 ± 85.99 pmol/L, respectively (P > 0.05 for the comparison between all three groups). After 3 months of treatment, the TPOAB levels in test groups A and B were 78.26 ± 48.23 and 270.34 ± 46.25 pmol/L, respectively, both of which were lower than that of the control group (347.26 ± 79.56 pmol/L). Compared with that in test group A, the level in group B was lower (P < 0.05). After 6 months of treatment, the TPOAB levels in test groups A and B were 233.15 ± 41.26 and 201.23 ± 38.17 pmol/L, respectively, both of which were lower than that of the control group (318.23 ± 74.23) pmol/L. Compared with that in test group A, the level in group B was lower (P < 0.05) (Figure 2A).
Pre-treatment, the TGAB levels in the control group, test group A, and test group B were 138.29 ± 16.43, 139.22 ± 16.47, and 138.56 ± 16.73 U/mL, respectively (P > 0.05 for the comparison of all three groups). After 3 months of treatment, the TGAB levels in test groups A and B were 119.34 ± 12.05 and 117.23 ± 11.34 U/mL, respectively, both of which were lower than that of the control group (124.56 ± 15.03) U/mL. Compared with that in test group A, the level in group B was lower (P < 0.05). After 6 months of treatment, the TGAB levels in test groups A and B were 93.15 ± 11.23 and 89.37 ± 10.42 U/mL, respectively, both of which were lower than that of the control group (123.64 ± 14.34) U/mL. It was lower in test group B than in group A (P < 0.05) (Figure 2B).
After 3 and 6 months of treatment, the TC, TG FBG, and HbA1c levels in test groups A and B were lower than those in the control group, while these levels were lower in test group B than in group A (all P < 0.05). The LDL-C levels in test groups A and B were higher than in the control group. Compared with the test group A, the levels of group B were higher (all P < 0.05) (Tables 3 and 4).
| Group | TC (mU/L) | TG (pmol/L) | ||||
| Pre-treatment | After 3 months of treatment | After 6 months of treatment | Pre-treatment | After 3 months of treatment | After 6 months of treatment | |
| Control group (n = 50) | 5.13 ± 0.86 | 4.46 ± 0.81 | 4.16 ± 0.77 | 3.21 ± 1.02 | 2.97 ± 0.91 | 2.93 ± 0.91 |
| Test group A (n = 50) | 4.98 ± 0.89 | 3.76 ± 0.75a | 2.76 ± 0.68a | 3.17 ± 0.96 | 2.43 ± 0.71a | 1.98 ± 0.65a |
| Test group B (n = 50) | 4.96 ± 0.92 | 3.07 ± 0.68a,b | 2.40 ± 0.69a,b | 3.16 ± 1.05 | 2.08 ± 0.64a,b | 1.83 ± 0.60a,b |
| F value | 0.546 | 43.665 | 84.262 | 0.033 | 17.243 | 32.575 |
| P value | 0.581 | < 0.001 | < 0.001 | 0.967 | < 0.001 | < 0.001 |
| Group | LDL-C (pmol/L) | FBG(pmol/L) | HbA1c(%) | ||||||
| Pre-treatment | After 3 months of treatment | After 6 months of treatment | Pre-treatment | After 3 months of treatment | After 6 months of treatment | Pre-treatment | After 3 months of treatment | After 6 months of treatment | |
| Control group (n = 50) | 1.31 ± 0.43 | 1.43 ± 0.44 | 1.48 ± 0.45 | 13.52 ± 3.35 | 13.46 ± 3.33 | 13.37 ± 3.28 | 9.16 ± 1.55 | 8.90 ± 1.35 | 7.65 ± 1.26 |
| Test group A | 1.34 ± 0.43 | 1.76 ± 0.46a | 2.18 ± 0.50a | 13.69 ± 3.76 | 12.34 ± 3.82a | 12.98 ± 2.98a | 8.98 ± 1.58 | 8.55 ± 1.30 | 7.09 ± 1.15a,b |
| Test group B (n = 50) | 1.33 ± 0.40 | 1.87 ± 0.48a | 2.41 ± 0.58a,b | 13.60 ± 3.80 | 9.64 ± 1.45a | 7.30 ± 1.48a,b | 9.35 ± 1.52 | 8.20 ± 1.2a | 6.45 ± 1.10a,b |
| F value | 0.067 | 11.717 | 44.330 | 0.031 | 20.859 | 49.495 | 0.712 | 3.603 | 13.130 |
| P value | 0.935 | < 0.001 | < 0.001 | 0.970 | < 0.001 | < 0.001 | 0.492 | 0.030 | < 0.001 |
Test group A of serum 25 (OH) D3 levels and negatively correlated with FT4, TGAB level (P < 0.05). The other indices were not significantly correlated (P > 0.05) (Table 5).
| Index | 25 (OH) D3 | |
| r | P value | |
| TSH | 0.008 | 0.866 |
| FT3 | -0.027 | 0.853 |
| FT4 | -0.326 | 0.021 |
| TPOAB | -0.017 | 0.905 |
| TGAB | -0.322 | 0.021 |
| TC | -0.041 | 0.776 |
| TG | 0.021 | 0.143 |
| LDL-C | 0.177 | 0.218 |
| FBG | 0.111 | 0.444 |
| HbA1c | 0.035 | 0.810 |
Serum 25 (OH) D3 levels in test group B were negatively correlated with FT4 and TGAB levels (P < 0.05). The other indices were not significantly correlated (P > 0.05) (Table 6).
| Index | 25 (OH) D3 | |
| r | P value | |
| TSH | -0.205 | 0.866 |
| FT3 | -0.069 | 0.633 |
| FT4 | -0.291 | 0.040 |
| TPOAB | 0.107 | 0.459 |
| TGAB | -0.457 | 0.001 |
| TC | 0.003 | 0.985 |
| TG | 0.148 | 0.306 |
| LDL-C | -0.025 | 0.861 |
| FBG | 0.079 | 0.587 |
| HbA1c | 0.230 | 0.108 |
The onset of HT is insidious and difficult to detect. Its early clinical symptoms are not obvious. By the time the patient is diagnosed, there are already symptoms of hypothyroidism present. Early clinical symptoms are not obvious, and symptoms of hypothyroidism already exist when the condition is detected and diagnosed. The reduced secretion of thyroid hormones damages the physiological function and affects the normal life of patients[17,18]. Diabetes is a common endocrine disease in clinical settings. Diabetes combined with HT causes significant damage to the body. HT treatment mainly involves selenium, glucocorticoids, and a limited intake of iodine. Diabetes treatment is primarily targeted at aspects related to its pathogenesis[19]. The effects of single symptomatic treatments for the combination of these two diseases are unsatisfactory. However, combined treatments can improve treatment efficacy and patients' quality of life.
A large number of studies have confirmed that HT is closely related to trace elements, such as iodine and selenium[20-23]. Selenium is mainly present in the human body as selenium protein that participates in the synthesis and metabolism of thyroid hormone and can also be used as an antioxidant to reduce inflammation in HT patients[24]. However, the influence of selenium on the occurrence and development of HT is still controversial. Early studies have shown that selenium is ineffective in treating HT, and other studies have shown that selenium supplementation cannot enhance the immune function of healthy people[25]. However, in recent years, more and more studies have found that selenium supplementation can reduce the serum autoantibody TPOAB level of HT patients, and other studies have found that selenium can not only reduce the serum TPOAB level of patients but also reduce the serum TGAB level of patients[13,26,27]. Selenium mainly regulates the natural immune response through methionine sulfoxide reductase, and low selenium status can increase the incidence of thyroid diseases[28]. Wu et al's epidemiological study in China also confirmed that low selenium status was related to the increased risk of HT, and increasing the intake of trace element selenium could reduce the incidence of HT[29]. According to the available evidence, selenium supplementation appears to be associated with the downregulation of thyroid antibody titers and improvements in mood or general health[30].
However, whether there is a relationship between HT and vitamin D remains controversial. Recently, a review has shown that vitamin D deficiency is related to the pathophysiological process of HT, hypothyroidism, and thyroid autoimmunity to a certain extent[19]. A randomized controlled trial further confirmed the benefit of vitamin D supplementation in HT remission. 120 Newly diagnosed HT patients were randomly divided into two groups: Group 1 (intervention group) and group 2 (control group). Group 1 patients received 60000 IU of vitamin D3 per week and 500 mg of calcium tablets daily for 8 wk. Patients in group 2 were only supplemented with 500 mg calcium tablets daily for 8 wk, and the follow-up results after 3 months showed that compared with patients in group 2 (-16.6%), the TPOAB level in patients in group 1 was significantly decreased (-46.73%) (P = 0.028)[31]. In this study, after 3 and 6 months of treatment, the improvement of 25 (OH) D3 level, thyroid function index level, and autoantibody in trial group A and trial group B were better than those in the control group, and trial group B was better than trial group A (P < 0.05), indicating that the combined treatment of vitamin D, selenium and hypoglycemic drugs in T2DM patients with HT was more effective. It can be seen that supplementation of vitamin D and selenium yeast can increase the content of 25 (OH) D3 in the body, improving thyroid function and the level of autoantibodies in patients.
In a 2010 study, Muscogiuri et al[32] found that patients with vitamin D < 20 ng/mL had a higher incidence of autoimmune thyroiditis than those with vitamin D > 20 ng/mL and found a linear correlation between vitamin D3 and TPOAB. A large sample data by Choi et al[33] also showed that in the general population, the incidence of positive TPOAB was 10.1%, and in female patients, the level of vitamin D3 in TPOAB-positive people was lower than that in negative people. Studies have shown that polymorphisms of vitamin D receptors, such as BsmI and TaqI, play an important role in autoimmune thyroiditis[34]. Our study found that serum 25 (OH) D3 in groups A and B before treatment was negatively correlated with FT4 and TGAB (P < 0.05). That is, the lower the level of vitamin D3, the higher the risk of hypothyroidism. However, there are few studies on the relationship between 25 (OH) D3 and thyroid function. Since the thyroid antibodies in our study mainly include TPOAB and TGAB and thyroid function TSH, FT3, and FT4, we cannot rule out whether there is a linear correlation between vitamin D3 and other antibodies that cause hypothyroidism. More research on vitamin D3 and thyroid function is needed.
Saxagliptin is a commonly used clinical drug in the treatment of T2DM. It mainly inhibits the physiological activity of the DPP-4 enzyme, promotes the improvement of glucagon-like peptide-1 level, fully stimulates islet cells, and rationally increases the release of long-acting insulin, thereby reducing the blood glucose level of patients and achieving the effect of promoting the stability of blood glucose level[35-37]. Wang et al[38] randomly divided 25 obese subjects with impaired fasting glucose or impaired glucose tolerance with an average age of 45 years into 4 groups: Life intervention group, saxagliptin 2.5 mg group, saxagliptin 5 mg group, metformin 1500 mg group. Relevant parameters were measured at baseline, 4 wk, 12 wk, and 24 wk. The final study showed that the saxagliptin 5 mg group reduced subjects' FBG and HbA1c and significantly reduced blood glucose levels 2 h after meals after 24 wk of intervention. As we all know, dyslipidemia in T2DM patients is mainly manifested by increased levels of TC, TG, and LDL-C and decreased levels of LDL-C. Angellotti et al[39] found that vitamin D supplementation could significantly reduce serum TG levels in patients who did not take cholesterol-lowering drugs. Combined with the results of this study, it was found that the three groups of patients were treated with saxagliptin, but after 3 and 6 months of treatment, the levels of blood glucose indexes and lipid indexes of test group A and B were better than those of the control group, and test group B was better than test group A (P < 0.05). These results indicate that the combination of vitamin D, selenium, and hypoglycemic agents has a more significant effect on T2DM patients with HT. The reason may be that selenium yeast has an obvious inhibitory effect on thyroglobulin. After taking selenium yeast, the levels of the two antibodies can be reduced, which is conducive to improving hypothyroidism caused by HT. Studies have shown that selenoproteins also affect insulin secretion and its biosynthesis. Selenium exists in glutathione peroxidase, protects pancreatic β cells, prevents them from being oxidized, maintains the normal function of beta cells, promotes glucose metabolism, and plays a hypoglycemic role[24]. Appropriate selenium supplementation in T2DM patients can help the islets recover some functions and improve the condition of diabetes. Vitamin D in T2DM patients can effectively improve insulin resistance, promote insulin secretion, regulate blood sugar and lipid metabolism, and inhibit inflammation and oxidative stress. Tahrani et al[40] found that female T2DM patients with vitamin D deficiency had a higher HbA1c level, and after vitamin D supplementation, the Hba1c level was lower than before. Al-shahwan et al[41] supplemented 45 T2DM patients with 2000 IU of vitamin D per day, and the results showed that the level of vitamin D in T2DM patients increased and the degree of insulin resistance decreased significantly.
There are still some shortcomings in this study, such as single-center, retrospective, and sample size limitations, which may impact the results. The follow-up study will expand the region and sample for exploration to provide more comprehensive research support.
The combination of vitamin D, selenium, and oral hypoglycemic drugs in treating patients with T2DM and HT has a significant clinical effect, effectively improving thyroid function, autoantibodies, blood glucose, and blood lipid levels. The elevated 25 (OH) D3, FT4, and TGAB levels were reduced.
The pathogeneses of type-2 diabetes mellitus (T2DM) and Hashimoto's thyroiditis (HT) mainly involve insulin resistance, immune factors, infection, genetics, leptin, oxidative stress, molecular cytology, and other related fields; however, there is currently no clear consensus on the pathogenesis of the co-occurrence of these conditions. Symptomatic treatment for these two diseases, including hypoglycemic drugs and improvement in function, is generally performed clinically. Selenium yeast and active vitamin D can reduce thyroid-related antibody levels in T2DM and HT and improve thyroid function. Hypoglycemia drugs can reduce blood sugar levels in patients and promote blood sugar stability.
T2DM combined with HT may cause significant damage to the body. Currently, vitamin D amaryl, and selenium yeast are used in combination and applied to research in patients with T2DM combined HT rarely reported.
This article explored the therapeutic effect of vitamin D + selenium + hypoglycemic agents in patients with T2DM and HT and explored the serum 25-hydroxyvitamin D3 [25 (OH) D3] level and relations with related indicators.
The control group was administered low-iodine diet guidance and hypoglycemic drug treatment. Test group A was additionally administered vitamin D treatment, while test group B was administered selenium yeast treatment in addition to the treatment in test group A. All three groups were treated for 6 months.
The improvement ranges of 25 (OH) D3 level, thyroid function index level, autoantibody, blood glucose, and blood lipid levels in test groups A and B were better than those in the control group, and the improvement of test group B was better.
The combination of vitamin D, selenium, and oral hypoglycemic agents in the treatment of patients with T2DM and HT had a significant clinical effect and effectively improved thyroid function and autoantibody and blood glucose and blood lipid levels, increased 25 (OH) D3 levels, and decreased free T4 and thyroid globulin antibody levels in these patients.
The combination of vitamin D, selenium, and oral hypoglycemic agents for treating patients with T2DM and HT has obvious therapeutic effects and is worthy of clinical application.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manousaki D, Canada S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Ihnatowicz P, Drywień M, Wątor P, Wojsiat J. The importance of nutritional factors and dietary management of Hashimoto's thyroiditis. Ann Agric Environ Med. 2020;27:184-193. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Jayanthi R, Srinivasan AR. Biochemical isthmus [nexus] between type 2 diabetes mellitus and thyroid status-an update. Diabetes Metab Syndr. 2019;13:1173-1177. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Biondi B, Kahaly GJ, Robertson RP. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr Rev. 2019;40:789-824. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Han M, Wu H, Yang W, Chen J. Analysis of risk factors for the development of type 2 diabetes mellitus complicated with Hashimoto's thyroiditis. BMC Endocr Disord. 2022;22:173. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Dahiya V, Vasudeva N, Sharma S, Kumar A. Role of Dietary Supplements in Thyroid Diseases. Endocr Metab Immune Disord Drug Targets. 2022;22:985-996. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Cvek M, Kaličanin D, Barić A, Vuletić M, Gunjača I, Torlak Lovrić V, Škrabić V, Punda A, Boraska Perica V. Vitamin D and Hashimoto's Thyroiditis: Observations from CROHT Biobank. Nutrients. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, Barausse G, Bambara LM, Biasi D. Vitamin D and autoimmune rheumatic diseases. Clin Rheumatol. 2011;30:443-444. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Maciejewski A, Kowalczyk MJ, Herman W, Czyżyk A, Kowalska M, Żaba R, Łącka K. Vitamin D Receptor Gene Polymorphisms and Autoimmune Thyroiditis: Are They Associated with Disease Occurrence and Its Features? Biomed Res Int. 2019;2019:8197580. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Khozam SA, Sumaili AM, Alflan MA, Shawabkeh RAS. Association Between Vitamin D Deficiency and Autoimmune Thyroid Disorder: A Systematic Review. Cureus. 2022;14:e25869. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Hu Z, Chen J, Sun X, Wang L, Wang A. Efficacy of vitamin D supplementation on glycemic control in type 2 diabetes patients: A meta-analysis of interventional studies. Medicine (Baltimore). 2019;98:e14970. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Jiang H, Chen X, Qian X, Shao S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto's thyroiditis-A meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2022;47:767-775. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Hu Y, Feng W, Chen H, Shi H, Jiang L, Zheng X, Liu X, Zhang W, Ge Y, Liu Y, Cui D. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto's thyroiditis: A prospective randomized-controlled trial. Clin Transl Sci. 2021;14:1390-1402. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Yu L, Zhou L, Xu E, Bi Y, Hu X, Pei X, Jin G. Levothyroxine monotherapy versus levothyroxine and selenium combination therapy in chronic lymphocytic thyroiditis. J Endocrinol Invest. 2017;40:1243-1250. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391-397. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Klecha AJ, Barreiro Arcos ML, Frick L, Genaro AM, Cremaschi G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation. 2008;15:68-75. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Štefanić M, Tokić S, Suver Stević M, Glavaš-Obrovac L. Association of increased eomesodermin, BCL6, and granzyme B expression with major clinical manifestations of Hashimoto's thyroiditis - an observational study. Immunol Invest. 2018;47:279-292. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Liontiris MI, Mazokopakis EE. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients.Points that need more investigation. Hell J Nucl Med. 2017;20:51-56. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Vasiliu I, Ciobanu-Apostol DG, Armasu I, Bredetean O, Serban IL, Preda C. Protective role of selenium on thyroid morphology in iodine-induced autoimmune thyroiditis in Wistar rats. Exp Ther Med. 2020;20:3425-3437. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Duntas LH. The Role of Iodine and Selenium in Autoimmune Thyroiditis. Horm Metab Res. 2015;47:721-726. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Iddah MA, Macharia BN. Autoimmune thyroid disorders. ISRN Endocrinol. 2013;2013:509764. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Mojadadi A, Au A, Salah W, Witting P, Ahmad G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Santos JAR, Rama TA, da Silva DJL, Fernandes RJ, Zacca R. Supply of Antioxidants vs. Recruit Firefighters' Cellular Immune Status: A Randomized Double-Blinded Placebo-Controlled Parallel-Group Trial. Life (Basel). 2022;12. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Negro R, Attanasio R, Grimaldi F, Marcocci C, Guglielmi R, Papini E. A 2016 Italian Survey about the Clinical Use of Selenium in Thyroid Disease. Eur Thyroid J. 2016;5:164-170. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Wang W, Mao J, Zhao J, Lu J, Yan L, Du J, Lu Z, Wang H, Xu M, Bai X, Zhu L, Fan C, Zhang H, Shan Z, Teng W. Decreased Thyroid Peroxidase Antibody Titer in Response to Selenium Supplementation in Autoimmune Thyroiditis and the Influence of a Selenoprotein P Gene Polymorphism: A Prospective, Multicenter Study in China. Thyroid. 2018;28:1674-1681. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Brigelius-Flohé R, Flohé L. Selenium and redox signaling. Arch Biochem Biophys. 2017;617:48-59. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, Chen P, Zhuang G, Zhang Z, Peng X, Li H, Zhao Y, He X, Zeng G, Qin F, Hou P, Shi B. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J Clin Endocrinol Metab. 2015;100:4037-4047. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Esposito D, Rotondi M, Accardo G, Vallone G, Conzo G, Docimo G, Selvaggi F, Cappelli C, Chiovato L, Giugliano D, Pasquali D. Influence of short-term selenium supplementation on the natural course of Hashimoto's thyroiditis: clinical results of a blinded placebo-controlled randomized prospective trial. J Endocrinol Invest. 2017;40:83-89. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Chaudhary S, Dutta D, Kumar M, Saha S, Mondal SA, Kumar A, Mukhopadhyay S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian J Endocrinol Metab. 2016;20:391-398. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Muscogiuri G, Mari D, Prolo S, Fatti LM, Cantone MC, Garagnani P, Arosio B, Di Somma C, Vitale G. 25 Hydroxyvitamin D Deficiency and Its Relationship to Autoimmune Thyroid Disease in the Elderly. Int J Environ Res Public Health. 2016;13. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Choi YM, Kim WG, Kim TY, Bae SJ, Kim HK, Jang EK, Jeon MJ, Han JM, Lee SH, Baek JH, Shong YK, Kim WB. Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid. 2014;24:655-661. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Wang S, Wu Y, Zuo Z, Zhao Y, Wang K. The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a systematic review and a meta-analysis. Endocrine. 2018;59:499-505. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Subrahmanyan NA, Koshy RM, Jacob K, Pappachan JM. Efficacy and Cardiovascular Safety of DPP-4 Inhibitors. Curr Drug Saf. 2021;16:154-164. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Deacon CF, Lebovitz HE. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18:333-347. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Li JJ, Zhang P, Fan B, Guo XL, Zheng ZS. The efficacy of saxagliptin in T2DM patients with non-alcoholic fatty liver disease: preliminary data. Rev Assoc Med Bras (1992). 2019;65:33-37. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Wang Z, Xu D, Huang L, Zhang T, Wang J, Chen Q, Kong L, Zhou X. Effects of saxagliptin on glucose homeostasis and body composition of obese patients with newly diagnosed pre-diabetes. Diabetes Res Clin Pract. 2017;130:77-85. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Angellotti E, D'Alessio D, Dawson-Hughes B, Chu Y, Nelson J, Hu P, Cohen RM, Pittas AG. Effect of vitamin D supplementation on cardiovascular risk in type 2 diabetes. Clin Nutr. 2019;38:2449-2453. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Tahrani AA, Ball A, Shepherd L, Rahim A, Jones AF, Bates A. The prevalence of vitamin D abnormalities in South Asians with type 2 diabetes mellitus in the UK. Int J Clin Pract. 2010;64:351-355. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Al-Shahwan MA, Al-Othman AM, Al-Daghri NM, Sabico SB. Effects of 12-month, 2000IU/day vitamin D supplementation on treatment naïve and vitamin D deficient Saudi type 2 diabetic patients. Saudi Med J. 2015;36:1432-1438. [PubMed] [DOI] [Cited in This Article: ] |