Published online Nov 7, 2023. doi: 10.3748/wjg.v29.i41.5641
Peer-review started: July 27, 2023
First decision: September 26, 2023
Revised: October 7, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 7, 2023
Pembrolizumab combined with chemotherapy has been proven effective as first-line therapy in patients with advanced esophageal cancer. Few trials have assessed the safety and efficacy of this treatment in patients with locally advanced disease.
To analyze long-term outcomes of pembrolizumab in locally advanced or metastatic esophageal squamous cell carcinoma (ESCC) in the real world.
Patients with advanced ESCC admitted to our center from October 2019 to October 2021 were enrolled in this study. Clinical staging of the patients was based on the 8th edition of the American Joint Committee on Cancer TNM staging system. The patients received different treatments based on clinical stage. In brief, patients with locally advanced and resectable ESCC received neoadjuvant therapy combined with surgery. For those who were not candidates for resection, radical concurrent chemoradiotherapy plus pembrolizumab was more preferable. Patients with metastatic ESCC or who were unsuitable for radiotherapy under
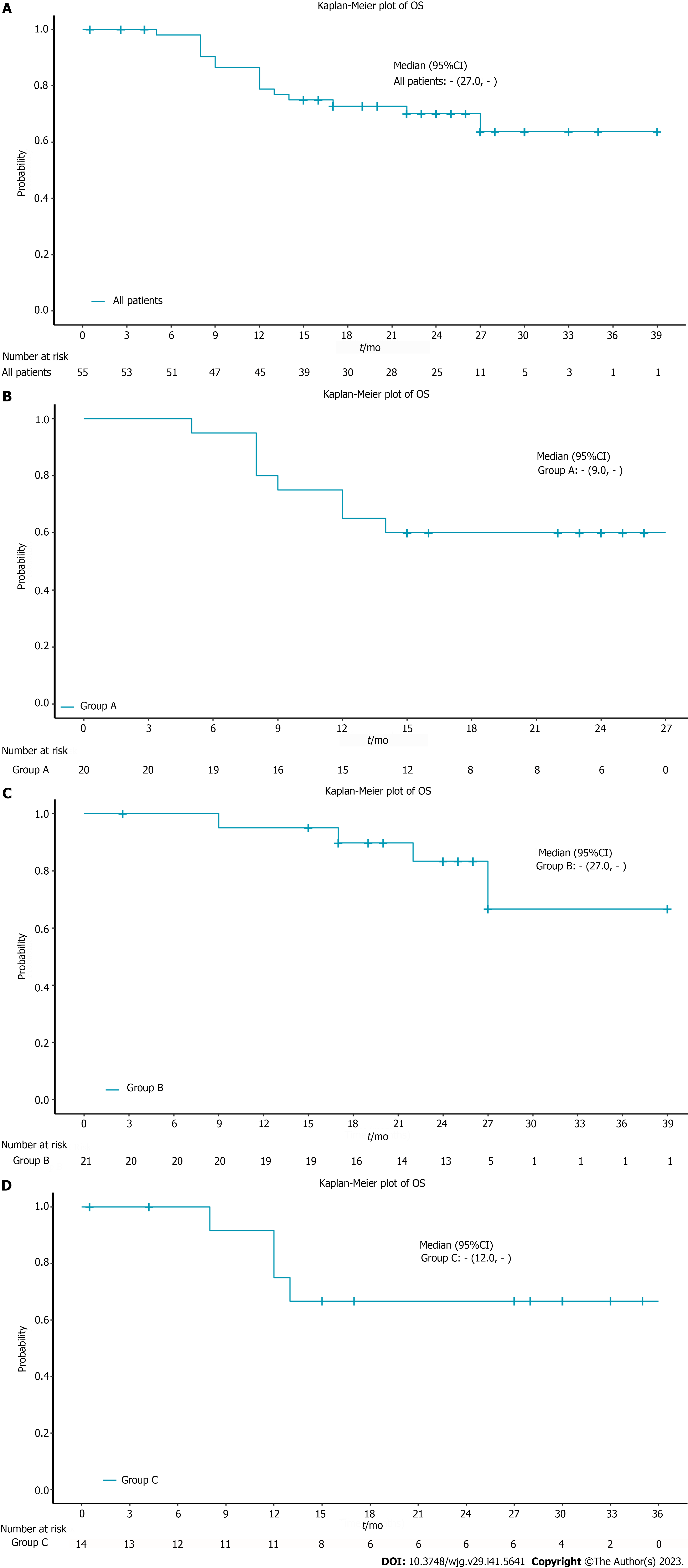
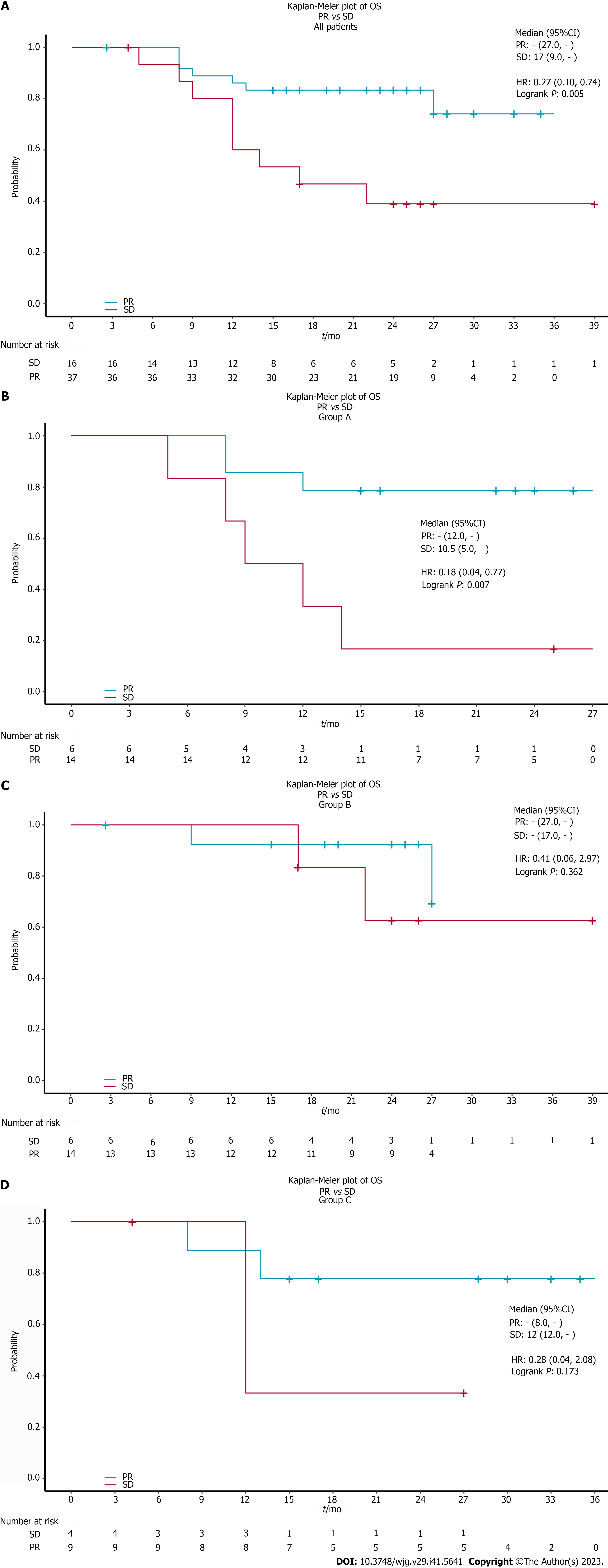
A total of 55 patients with advanced ESCC were enrolled in this retrospective, observational study. The median age was 61 years (range 44-74), with 47.3% (26/55) of the patients in stage IV and 45.5% of the patients had the tumor (25/55) located in the middle third of the esophagus. The median OS in all patients was not reached. The 12-mo OS rate among all patients was 78.8% and the 18-mo OS rate was 72.7%. 9 patients died due to tumor progression and 7 patients died due to treatment-related complications. The therapeutic effect evaluated at the interim evaluation was significantly reflected in the long-term outcome. Patients with complete response or partial response in all patients (P = 0.005) and in the chemoradiotherapy plus pembrolizumab group (P = 0.007) obtained a better prognosis than non-responders. A total of 20 patients (20/55, 36%) received immune maintenance therapy. Baseline peripheral blood biomarkers of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and neutrophil-to-(leukocyte-neutrophil) ratio did not predict the efficacy of ICIs.
Pembrolizumab combined with chemotherapy or radiotherapy resulted in favorable long-term survival in patients with locally advanced or metastatic ESCC, with safe and manageable long-term AEs.
Core Tip: Pembrolizumab combined with chemotherapy has been proven effective as first-line therapy in patients with metastatic esophageal cancer. Few trials have assessed the safety and efficacy of this treatment in patients with locally advanced disease. Our study showed that this treatment in patients with locally advanced or metastatic esophageal squamous cell carcinoma resulted in favorable long-term survival and manageable long-term adverse effects. Randomized phase III trials should be carried out for further verification.
- Citation: Wang HC, Huang X, Chen J, Li Y, Cong Y, Qu BL, Feng SQ, Liu F. Long-term efficacy and predictors of pembrolizumab-based regimens in patients with advanced esophageal cancer in the real world. World J Gastroenterol 2023; 29(41): 5641-5656
- URL: https://www.wjgnet.com/1007-9327/full/v29/i41/5641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i41.5641
Esophageal cancer is the seventh most common and the sixth leading cause of malignant tumor death worldwide[1]. In China, the incidence and mortality risk of esophageal cancer rank sixth and fourth, respectively[2]. The majority of esophageal cancer patients in China have esophageal squamous cell carcinoma (ESCC) which accounts for 90% of tissue types, and less than 10% have adenocarcinoma[3,4]. Most patients initially diagnosed with esophageal cancer have advanced disease, some patients have locally advanced disease which is inoperable, and some patients have metastases to other sites. Neoadjuvant chemoradiotherapy (nCRT) followed by surgery is the main treatment for resectable ESCC[5,6]. Radical concurrent chemoradiotherapy is an important treatment strategy for locally advanced unresectable patients[7]. For metastatic ESCC, systemic chemotherapy is the only treatment option[8,9]. In fact, for locally advanced or metastatic ESCC, treatment modalities are limited, progress in long-term survival is slow, and the efficacy is unsatisfactory. Data shows that the 5-year survival rate for locally advanced ESCC is no more than 30%. The 5-year survival rate for metastatic ESCC is less than 10%[10].
In recent years, immune checkpoint inhibitors (ICIs) combined with chemotherapy has made significant progress in the first-line treatment of advanced esophageal cancer[11-15]. In the randomized phase III KEYNOTE-590 study, ICIs therapy targeting programmed cell death protein 1 (PD-1), pembrolizumab combined with chemotherapy showed a significant survival advantage over chemotherapy alone in the first-line therapy. The median overall survival (OS) was more than 12 mo and the median progression-free survival (PFS) was 6.3 mo, significantly better than the median OS of 9.8 mo and the median PFS of 5.8 mo in the chemotherapy alone group. In addition, the safety was reliable[12]. For locally advanced patients treated with neoadjuvant therapy, a multicenter real-world study in China showed that the R0 resection rate reached 97.7% in combination with ICIs, and 25.5% of patients in the ICIs plus chemotherapy group and 42.3% of patients in the ICIs plus chemoradiotherapy group achieved pathologic complete response (pCR)[16]. Furthermore, some single-arm clinical trials have also investigated the application of ICIs combined with chemotherapy or concurrent chemoradiotherapy in the field of neoadjuvant therapy[17-20]. To date, the evidence for neoadjuvant treatment combined with ICIs remains inadequate, and results from large phase III clinical trials and long-term follow-up data are lacking. In unresectable locally advanced ESCC, a recent phase IB clinical study examined the efficacy and safety of the PD-1 inhibitor camrelizumab combined with radical radiotherapy for locally advanced ESCC which was intolerant to concurrent chemoradiotherapy[21]. The results showed that median OS and PFS were 16.7 mo and 11.7 mo, respectively. The 24-mo OS rate and PFS rate were 31.6% and 35.5%, respectively. The objective response rate (ORR) was 74%. Three randomized phase III studies (KEYNOTE-975, ESCORT-CRT and RATIONALE 311) are currently being conducted to further confirm the value of ICIs combined with concurrent chemoradiotherapy. Although these studies have demons
Based on this, our center conducted a real-world clinical study to examine the efficacy and safety of pembrolizumab in neoadjuvant therapy, concurrent chemoradiotherapy and first-line therapy for ESCC[25]. Early results showed that the combination with pembrolizumab demonstrated considerable ORR and acceptable adverse effects (AEs). We here report the long-term survival such as OS, disease-free survival (DFS) ,PFS, long-term toxicities and ICIs completion rates. We also assess the relationship between baseline blood cell composition indices such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), neutrophil-to-(leukocyte-neutrophil) ratio (dNLR) and long-term survival in order to determine the predictive factors of ICIs.
This single-arm, single-center, retrospective clinical study was conducted in the First Medical Center of Chinese PLA General Hospital. Patients who were initially diagnosed with locally advanced or metastatic esophageal cancer from October 1, 2019 to October 1, 2021 were included. Clinical staging of the patients was based on the 8th edition of the American Joint Committee on Cancer TNM staging system. According to the different clinical stages and treatment modalities, the patients were divided into different subgroups. ORR was defined as the proportion of total patients with CR or partial response (PR). OS was considered the time from definitive diagnosis to death by any cause. DFS was regarded as the period from definitive diagnosis to disease recurrence or death in operable patients. PFS was defined as the period from definitive diagnosis to disease recurrence or death in patients with inoperable locally advanced or metastatic esophageal cancer. Blood samples were obtained at baseline. The NLR was the total number of neutrophils divided by the lymphocyte count. The PLR was the platelet count divided by the lymphocyte count. The dNLR represented the total number of neutrophils divided by the difference between the total number of white blood cells and neutrophils. The study was approved by the Ethics Committee of the Chinese PLA General Hospital in line with the Declaration of Helsinki (as revised in 2013).
Neoadjuvant therapy plus surgery: Eligible patients were aged 18-75 years, initially diagnosed with operable locally advanced ESCC (T2-4N0M0 or T2-4N+M0), with Eastern Cooperative Oncology Group performance status score of 0 or 1, and life expectancy of at least 6 mo. Patients with active autoimmune disease, and a history of ICIs or chemotherapy treatment were excluded. The patients underwent neoadjuvant chemotherapy (lobaplatin combined with albumin-paclitaxel) plus ICIs (pembrolizumab), prior to surgery once every 3 wk for 2 or 3 cycles. Surgery was then performed after physical examination, laboratory tests, contrast-enhanced chest computed tomography (CT) and pulmonary function tests.
Chemoradiotherapy plus pembrolizumab: Patients were aged 18-75 years, locally advanced and inoperable esophageal cancer or limited to supraclavicular lymph node metastasis, with Eastern Cooperative Oncology Group performance status score of 0 or 1, life expectancy of at least 6 mo were included. Patients with active autoimmune disease, and a history of ICIs or chemotherapy treatment were excluded. They received radical chemoradiotherapy plus pembrolizumab. The patients underwent 2-4 cycles of induction therapy with a chemotherapy regimen (lobaplatin combined with albumin-paclitaxel) plus pembrolizumab. Radical radiotherapy or chemoradiotherapy was then given and an external irradiation technique was used. The total dose of radiotherapy was 54 Gy/30 F, 1.8 Gy each time, 5 times a week. On this basis, primary esophageal lesions and metastatic lymph nodes received 63 Gy/30 F. Pembrolizumab could be discontinued during radiotherapy due to safety concerns. After radiotherapy, pembrolizumab was used as maintenance therapy for a total of 2 years. Treatment was suspended if disease progression or intolerable toxicity occurred.
Chemotherapy plus pembrolizumab: Patients were aged 18-75 years, diagnosed with metastatic esophageal cancer or unsuitable for radiotherapy, with adequate organ function, Eastern Cooperative Oncology Group performance status score of 0 or 1, life expectancy of at least 6 mo were included. Patients with active autoimmune disease, and a history of ICI or chemotherapy treatment were excluded. A chemotherapy regimen (lobaplatin combined with albumin-paclitaxel) plus pembrolizumab was administered every 3 wk for a total of 4 cycles, and then pembrolizumab was given as maintenance therapy for 2 years.
Follow-up began at the time of the patient’s diagnosis and treatment in our hospital. The last follow-up was on December 1, 2022. Contrast-enhanced chest and abdominal CT, upper gastrointestinal contrast, ultrasound, and laboratory tests were routinely performed during the follow-up. Gastroscopy, positron emission tomography-CT and chest magnetic resonance imaging were also performed when necessary. Follow-up was conducted every 3 mo during the first 2 years and then every 6 mo thereafter. The patient’s physical condition and long-term AEs were assessed by consultation, telephone and other methods.
SAS 9.4 was used for all statistical analyses. The Kaplan-Meier method was used to estimate OS, DFS, PFS and their corresponding 95% confidence intervals (CIs). We divided patients into 3 subgroups according to the different treatment modalities (neoadjuvant treatment plus ICIs, radical chemoradiotherapy plus ICIs, chemotherapy plus ICIs). For the analysis of predictors of immunotherapy efficacy, we used a median cutoff value of 2.43 for NLR, 139.7 for PLR, and 1.72 for dNLR. The group with a larger cutoff value than the median cutoff value was defined as the high group, while the group with a smaller value than the median cutoff value was defined as the low group.
A total of 55 patients with ESCC were enrolled in this study from October 1, 2019 to October 1, 2021 (Table 1). The majority of patients were male (43/55, 78.2%) and 12 patients were female. The median age was 61 years (range 44-74), with 47.3% (26/55) of the patients in stage IV and 45.5% of the patients had the tumor (25/55) located in the middle third of the esophagus.
| Characteristics | n (%) |
| Age (yr) | |
| Median | 61 |
| Range | 44-74 |
| Sex | |
| Male | 43 (78.2) |
| Female | 12 (21.8) |
| Tumor location | |
| Upper esophagus | 19 (34.5) |
| Middle esophagus | 25 (45.5) |
| Lower esophagus | 11 (20) |
| Clinical stage | |
| II | 10 (18.2) |
| III | 19 (34.5) |
| IV | 26 (47.3) |
| Subgroups | |
| Chemoradiotherapy plus pembrolizumab (group A) | 21 (38.2) |
| Neoadjuvant therapy plus surgery (group B) | 20 (36.4) |
| Chemotherapy plus pembrolizumab (group C) | 14 (25.5) |
Patients received different therapeutic regimens according to clinical stage. Among them, 21 patients received neoadjuvant treatment plus pembrolizumab followed by surgery. 20 patients with locally advanced inoperable and partial stage IV with supraclavicular lymph node metastasis were treated with radical chemoradiotherapy combined with pembrolizumab. The remaining patients who had metastatic esophageal cancer or were unsuitable for chemoradiotherapy received chemotherapy plus pembrolizumab.
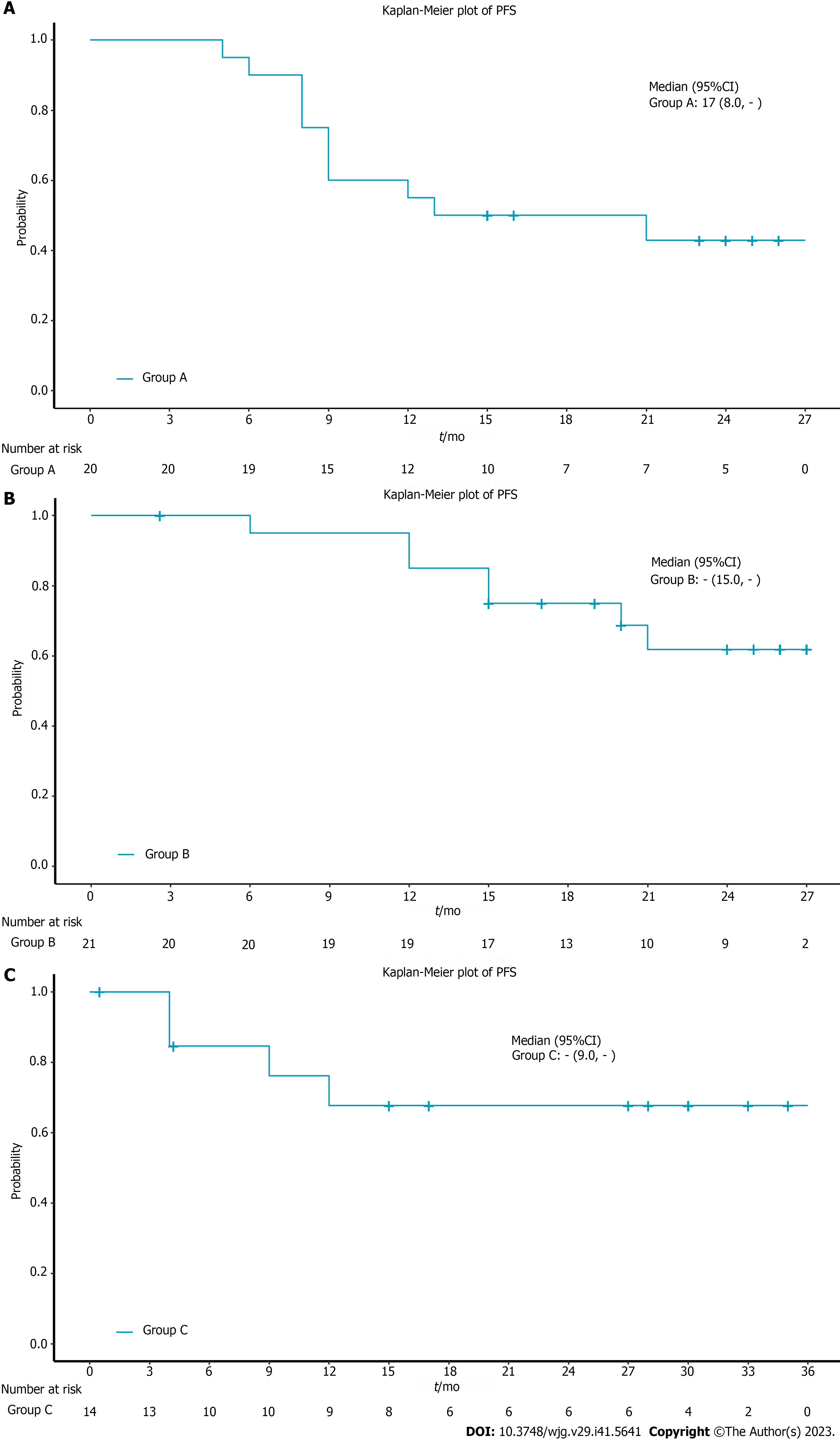
The median OS in all patients was not reached. The 12-mo OS rate in all patients was 78.8% and the 18-mo OS rate was 72.7% (Figure 1A). 9 patients died due to tumor progression and 7 died due to treatment-related complications. In the subgroup analysis, the 12-mo OS rate was 65% and the 18-mo OS rate was 60% in the chemoradiotherapy plus pembrolizumab group (Figure 1B). The 12-mo OS rate in the neoadjuvant treatment plus pembrolizumab group was 95% and the 18-mo OS rate was 89.7% (Figure 1C). In the chemotherapy plus pembrolizumab group, the 12-mo OS rate was 75% and the 18-mo OS rate was 66.7% (Figure 1D). The median OS for the 3 subgroups was not reached (Table 2). The 12-mo PFS rate was 55%, the 18-mo PFS rate was 50% and the median PFS was 17 mo in the chemoradiotherapy plus pembrolizumab group (Figure 2A). The 12-mo DFS rate was 85%, the 18-mo DFS rate was 75% and the median DFS was not reached in the neoadjuvant treatment plus pembrolizumab group (Figure 2B). The 12-mo PFS rate was 67.7%, the 18-mo PFS rate was 67.7% and the median PFS was not reached in the chemotherapy plus pembrolizumab group (Figure 2C, Tables 3 and 4).
| All patients (n = 55) | Group A (n = 20) | Group B (n = 21) | Group C (n = 14) | |
| Patients with event | 16 (29.1%) | 8 (40.0%) | 4 (19.0%) | 4 (28.6%) |
| Patients without event | 39 (70.9%) | 12 (60.0%) | 17 (81.0%) | 10 (71.4%) |
| Time to event (mo) | ||||
| Median | - | - | - | - |
| 95%CI | 27.0, - | 9.0, - | 27.0, - | 12.0, - |
| 25% and 75%-ile | 15.50, - | 10.50, - | 27.00, - | 12.50, - |
| Min-max | 0.5-39 | 5-26 | 2.6-39 | 0.5-35 |
| 12 mo probability (95%CI) | 78.8 (65.1-87.7) | 65.0 (40.3-81.5) | 95.0 (69.5-99.3) | 75.0 (40.8-91.2) |
| 18 mo probability (95%CI) | 72.7 (58.3-82.9) | 60.0 (35.7-77.6) | 89.7 (64.8-97.3) | 66.7 (33.7-86.0) |
| Group A (n = 20) | Group C (n = 14) | |
| Patients with event | 11 (55.0%) | 4 (28.6%) |
| Patients without event | 9 (45.0%) | 10 (71.4%) |
| Time to event (mo) | ||
| Median | 17 | - |
| 95%CI | 8.0, - | 9.0, - |
| 25% and 75%-ile | 8.50, - | 12.0, - |
| Min-max | 5-26 | 0.5-35 |
| 12 mo probability (95%CI) | 55.0 (31.3-73.5) | 67.7 (34.9-86.5) |
| 18 mo probability (95%CI) | 50.0 (27.1-69.2) | 67.7 (34.9-86.5) |
| Group B (n = 21) | |
| Patients with event | 7 (33.3%) |
| Patients without event | 14 (66.7%) |
| Time to event (mo) | |
| Median | - |
| 95%CI | 5.0, - |
| 25% and 75%-ile | 17.50, - |
| Min-max | 2.6-27 |
| 12 mo probability (95%CI) | 85.0 (60.4-94.9) |
| 18 mo probability (95%CI) | 75.0 (50.0-88.7) |
In addition, the therapeutic effect assessed at the interim evaluation was significant in the long-term outcome. Patients with ORR (CR or PR) in all patients (P = 0.005) (Figure 3A) and in the chemoradiotherapy plus pembrolizumab group (P = 0.007) (Figure 3B) obtained a better prognosis than non-responders. However, we did not find a tendency for benefit in the neoadjuvant therapy followed by surgery group (Figure 3C) and chemotherapy plus pembrolizumab group (Figure 3D).
In the chemoradiotherapy plus pembrolizumab group, 8 patients died (4 due to esophageal fistula, 1 due to liver failure, 2 due to tumor progression, and 1 due to lung infection). 5 patients developed disease progression (4 patients had recurrence in the radiotherapy targeted area of supraclavicular lymph node metastasis, esophageal lesion, mediastinal lymph node and 1 patient had liver metastasis). In the neoadjuvant treatment plus pembrolizumab group, 4 patients died, including 3 patients who died from tumor progression and 1 patient from a treatment-related complication. 7 patients had disease recurrence and metastasis, among whom 2 patients had local recurrence and 5 patients developed distant metastases. In the chemotherapy plus pembrolizumab group, 4 patients died (two from lung metastases and two from liver metastases) (Table 5).
| Patterns of recurrence | Immune maintenance therapy | ||
| Local | Distant organ | ||
| Group A (20) | 4 (20%) | 1 (5%) | 10 (50%) |
| Group B (21) | 2 (9%) | 5 (24%) | 3 (14.3%) |
| Group C (14) | 2 (14.3%) | 4 (28.6%) | 7 (50%) |
10 patients in the chemoradiotherapy plus pembrolizumab group (10/20, 50%), 3 patients in the neoadjuvant treatment plus pembrolizumab group (3/21, 14.3%) and 7 patients in the chemotherapy plus pembrolizumab group (7/14, 50%) received immune maintenance therapy. Rash occurred in 3 patients (3/20, 15%), 2 patients developed hypothyroidism (2/20, 10%), and 3 patients experienced pneumonia (3/20, 15%). To date, 6 patients have stopped immune maintenance therapy due to AEs (6/20, 30%) (Table 5).
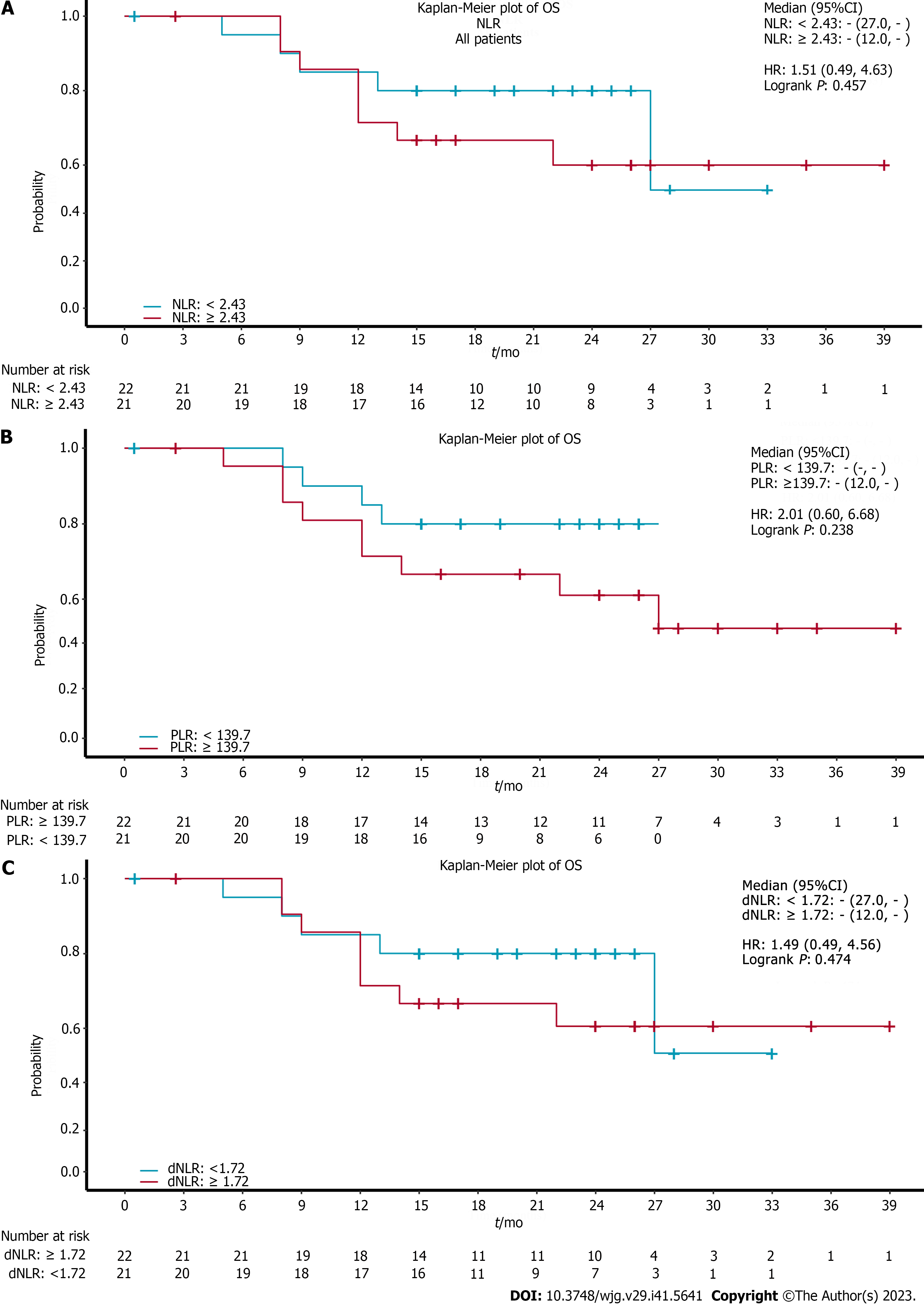
Figure 4 showed the relationship between the baseline NLR (Figure 4A), PLR (Figure 4B), dNLR (Figure 4C) and long-term survival outcomes following ICIs. These results suggested that baseline NLR < 2.43, dNLR < 1.72 and PLR < 139.7 indicated a trend in OS benefit compared with NLR > 2.43, dNLR > 1.72, and PLR > 139.7, although there were no statistically significant differences. The P values were 0.457, 0.474 and 0.238, respectively.
Our previous results showed that PD-1 inhibitor plus chemotherapy or chemoradiotherapy had a good ORR and manageable safety[25]. We used lobaplatin and albumin-paclitaxel as the chemotherapy regimen instead of cisplatin, as cisplatin has AEs on renal function. The trial proved that lobaplatin had favorable results in ESCC[26]. The present study reported the results of long-term follow-up.
For advanced ESCC, especially locally advanced disease, neoadjuvant chemotherapy plus immunotherapy followed by surgery or chemoradiotherapy combined with immunotherapy warrants further studies, as current clinical studies are confined to phase I-II trials, and long-term follow-up data are lacking. In the present study, relatively good long-term outcomes were achieved with tolerable side effects, and evidence for PD-1 inhibitor combined with chemotherapy or radiotherapy used in ESCC has been provided.
In this study, 21 patients received neoadjuvant therapy plus pembrolizumab followed by surgery. The results demonstrated that the 12-mo DFS rate was 85%, the 18-mo DFS rate was 75%, the 12-mo OS rate was 95% and the 18-mo OS rate was 89.7%. The median OS or DFS was not reached. The results of the NEOCRTEC 5010 study indicated that the 1-year OS rate in the nCRT group was 90% and the 2-year OS rate was 75.1%[5]. Our results were similar to those of the NEOCRTEC 5010 trial. However, during a median follow-up of 24 mo in our study, patients were found to have local recurrence in mediastinal lymph nodes, anastomotic stoma and retroperitoneal lymph nodes. Lung, pleural effusion, and supraclavicular lymph node metastases were found in 23.8% of patients (5/21). The 10-year pattern of recurrence and metastasis in the CROSS study showed that the proportion of isolated local recurrence in the neoadjuvant group was 8% (15/178). The percentage of patients with both local recurrence and distant metastasis were 13% (23/178). In addition, the ratio of patients with simple distant metastasis was 27% (48/178)[27]. In our study, the recurrence pattern was dominated by distant metastasis, but there was still a high local recurrence rate. Therefore, it remains unclear whether the local recurrence risk with neoadjuvant chemotherapy plus ICIs is non-inferior to neoadjuvant concurrent chemoradiotherapy. Furthermore, the relatively high recurrence rate in the short follow-up period in our study requires further verification in large clinical trials. In the CHECKMATE-577 trial, the median DFS for patients who did not reach pCR after nCRT was significantly better in the maintenance treatment group with nivolumab than in the placebo group[28]. In our study, the postoperative immune maintenance rate was only 14.3% (3/21), which may also be one of the reasons for the increased rate of distant metastasis. In the future, neoadjuvant therapy for locally advanced esophageal cancer requires continuous optimization of protocols to reduce the risk of distant metastasis and improve survival. In addition, on the premise of ensuring local control, eliminating radiotherapy to reduce AEs is also worth further exploration.
20 patients with unresectable locally advanced or limited supraclavicular lymph node metastases received chemoradiotherapy combined with pembrolizumab. The results showed that the 12-mo OS rate was 65%, the 18-mo OS rate was 60% and the median OS was not reached. The 12-mo PFS rate was 55%, the 18-mo PFS rate was 50% and the median PFS was 17 mo. The median survival time following radical concurrent chemoradiotherapy recommended by current guidelines was 18 mo, and the 2-year survival rate was about 40%[29]. The long-term survival in the radical chemoradiotherapy plus pembrolizumab group in our study was slightly better than that in the standard radical concurrent chemoradiotherapy group. The addition of ICIs to chemoradiotherapy likely increased the efficacy and prolonged survival. However, randomized phase III studies are needed to verify this. A phase IB clinical study is currently examining the efficacy and safety of the PD-1 inhibitor camrelizumab combined with radical radiotherapy in patients with locally advanced ESCC who are intolerant to concurrent chemoradiotherapy[21]. The median OS and PFS were 16.7 mo and 11.7 mo, respectively. The 24-mo OS rate and PFS rate were 31.6% and 35.5%, respectively. The ORR rate was 74%. Our results showed a more beneficial outcome, probably because we used the combination of radiotherapy and chemotherapy, which strengthened the intensity of treatment and improved survival outcomes. Studies have shown that the incidence rate of esophageal fistula caused by radiotherapy and chemotherapy is approximately 15%, of which T4 and esophageal stenosis increase the risk of fistula with a poor prognosis[30]. In our study, 20% (4/20) patients died from esophageal fistula. The patients with fistula in the radiotherapy plus pembrolizumab group were all T4 and the tumor was closely related to the trachea, which was suspected to have invasion. Furthermore, the radiotherapy dose in this group was 63 Gy, and the high radiotherapy dose was also the main cause of fistula. Studies have shown that higher 60 Gy did not improve long-term survival and simultaneously increased AEs[31]. Therefore, in the era of ICIs, for locally advanced patients with T3-T4, radiotherapy dose should be carefully selected and safety should be taken into account in the absence of clear evidence of benefit. The data and results of randomized phase III studies on the combination therapy of radiotherapy and ICIs are lacking at present. There are still some problems to be solved such as the timing of combination therapy, selection of the combination chemotherapy regimen, clinical target volume and so on. The results of KEYNOTE-975, ESCORT-CRT, RATIONALE-311 and other randomized phase III studies are expected.
A total of 14 patients in our study received chemotherapy combined with pembrolizumab. The 12-mo OS rate was 75% and the 18-mo OS rate was 66.7%. The 12-mo PFS rate and 18-mo PFS rate were 67.7%. In the randomized phase III JUPITER-06 study, toripalimab combined with chemotherapy significantly prolonged PFS in patients with a 42% reduction in the risk of disease progression and resulted in a significant benefit in median OS (17 mo vs 11 mo) compared with placebo plus chemotherapy. The 1-year PFS rate was 27.8% and the 1-year OS rate was 66% in the toripalimab-based group[11]. The long-term survival results in our study were better than those in the JUPITER-06 study, and even better than those in the radiotherapy plus pembrolizumab group. There are several possible reasons for this result: (1) The tumor burden in the chemotherapy combined with pembrolizumab group was relatively low. Some patients with stage III refused radiotherapy due to toxicity, while others had single liver metastasis or small nodules in lung metastasis; (2) In the radiotherapy plus pembrolizumab group, 4 patients died due to esophageal fistula after radiotherapy and survival outcomes were negatively affected; and (3) Small sample size and short follow-up time may have led to deviations in the results.
As indicators of systemic inflammation, the NLR, PLR and dNLR can reflect the microenvironment of inflammation. Neutrophils can promote tumor invasion and progression by secreting cytokines, vascular endothelial cell growth factors and chemokines[32]. However, lymphocytes play an important role in the immune system and can inhibit tumor proliferation[33]. Studies have reported that in patients with non-small cell lung cancer and a higher baseline NLR, ICIs had poor efficacy, which had a negative predictive value on PFS and OS[34]. Our study found that low baseline NLR, dNLR and PLR showed a trend for OS benefit, but a statistically significant difference was not observed. This result may have been limited by the small sample size. Thus, a larger sample size is needed to examine this issue in the future.
This study also had some limitations: (1) This was a single-arm, single-center retrospective clinical study, with a small number of patients and did not include a control group; (2) The follow-up period should have been longer, as there is a lack of 3-year and 5-year long-term survival outcomes; and (3) Prospective randomized controlled studies with long-term follow-up data are needed.
Our real-world results revealed that pembrolizumab combined with chemotherapy or radiotherapy resulted in a favorable long-term survival outcome in patients with locally advanced and metastatic esophageal cancer. Long-term toxicities associated with these regimens were manageable.
Although pembrolizumab combined with chemotherapy has been proven effective as first-line therapy in patients with advanced esophageal cancer, few trials have assessed the safety and efficacy of this treatment in patients with locally advanced disease.
Progress has been made in the immune checkpoint inhibitors combined with chemotherapy as the first-line treatment of advanced esophageal cancer. The efficacy and safety of pembrolizumab in locally advanced or metastatic esophageal squamous cell carcinoma (ESCC) in the real world were worth studying.
To analyze the long-term outcomes of pembrolizumab in locally advanced or metastatic ESCC in the real world.
This was a single-arm, single-center, retrospective clinical study. Patients who were initially diagnosed with locally advanced or metastatic esophageal cancer from October 1, 2019 to October 1, 2021 were included. According to the different clinical stages and treatment modalities, the patients were divided into different subgroups. Long-term survival outcomes were evaluated.
A total of 55 patients with ESCC were enrolled in this study from October 1, 2019 to October 1, 2021. The median overall survival (OS) in all patients was not reached. The 12-mo OS rate was 78.8% and the 18-mo OS rate was 72.7%. Nine patients died due to tumor progression and 7 patients died due to treatment-related complications.
Pembrolizumab combined with chemotherapy or radiotherapy resulted in favorable long-term survival in patients with locally advanced or metastatic ESCC, with safe and manageable long-term adverse effects.
It is necessary to explore the efficacy of pembrolizumab combined with chemotherapy or radiotherapy in patients with locally advanced or metastatic ESCC. Randomized phase III trials should be carried out for further verification of the efficacy.
We thank Dr. Run-Kun Yang from Merck Sharp and Dohme Medical Affairs for his scientific comments on the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koma YI, Japan; Shimokawa T, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 43963] [Article Influence: 14654.3] [Reference Citation Analysis (47)] |
| 2. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 1108] [Article Influence: 369.3] [Reference Citation Analysis (0)] |
| 3. | Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Colle R, Cohen R. [Epidemiology of microsatellite instability across solid neoplasms]. Bull Cancer. 2019;106:114-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J; AME Thoracic Surgery Collaborative Group. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796-2803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 505] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 6. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1292] [Cited by in F6Publishing: 1578] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 7. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1261] [Cited by in F6Publishing: 1306] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 8. | Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, Kato K, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Nakajima TE, Shitara K, Kawakami H, Narita Y, Yoshino T, Van Cutsem E, Martinelli E, Smyth EC, Arnold D, Minami H, Tabernero J, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:34-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Moehler M, Maderer A, Thuss-Patience PC, Brenner B, Meiler J, Ettrich TJ, Hofheinz RD, Al-Batran SE, Vogel A, Mueller L, Lutz MP, Lordick F, Alsina M, Borchert K, Greil R, Eisterer W, Schad A, Slotta-Huspenina J, Van Cutsem E, Lorenzen S. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann Oncol. 2020;31:228-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Rice TW, Ishwaran H, Blackstone EH, Hofstetter WL, Kelsen DP, Apperson-Hansen C; Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:913-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Chen X, Dai G, He J, Li J, Li G, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Tang P, Wang T, Wang W, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40:277-288.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 141] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 12. | Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 534] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 13. | Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, Li J, Fu Z, Gu K, Liu Z, Wu L, Zhang X, Feng J, Niu Z, Ba Y, Zhang H, Liu Y, Zhang L, Min X, Huang J, Cheng Y, Wang D, Shen Y, Yang Q, Zou J, Xu RH; ESCORT-1st Investigators. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326:916-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 266] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 14. | Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, Wang B, Sun G, Ji Y, Cao G, Liu H, Cui T, Li N, Qiu W, Li G, Hou X, Luo H, Xue L, Zhang Y, Yue W, Liu Z, Wang X, Gao S, Pan Y, Galais MP, Zaanan A, Ma Z, Li H, Wang Y, Shen L; ORIENT-15 study group. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 108] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 15. | Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386:449-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 344] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Tan L, Hu J, Li Y, Mao Y, Tian Z, Zhang B, Ma J, Li H, Chen C, Chen K, Han Y, Chen L, Liu J, Yu B, Yu Z, Li Z; Esophageal Cancer Committee of Chinese Anti-Cancer Association. Safety and efficacy of neoadjuvant treatment with immune checkpoint inhibitors in esophageal cancer: real-world multicenter retrospective study in China. Dis Esophagus. 2022;35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, Kitano S, Yachida S, Shiba S, Kitagawa Y. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol. 2020;16:1351-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, Meijer SL, Schokker S, Dings MPG, Bergman JJGHM, Haj Mohammad N, Ruurda JP, van Hillegersberg R, Mook S, Nieuwdorp M, de Gruijl TD, Soeratram TTD, Ylstra B, van Grieken NCT, Bijlsma MF, Hulshof MCCM, van Laarhoven HWM. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasibility Trial (PERFECT). Clin Cancer Res. 2021;27:3351-3359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 19. | Park SY, Hong MH, Kim HR, Lee CG, Cho JH, Cho BC, Kim DJ. The feasibility and safety of radical esophagectomy in patients receiving neoadjuvant chemoradiotherapy with pembrolizumab for esophageal squamous cell carcinoma. J Thorac Dis. 2020;12:6426-6434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, Wu Y, Feng X, Qi W, Chen K, Xiang J, Li J, Lerut T, Li H. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. 2021;144:232-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 21. | Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, Zhao J, Er P, Zhang T, Chen X, Wang Y, Jiang Y, Wang Q, Zhang B, Qian D, Wang J, Zhou D, Ren X, Yu Z, Zhao L, Yuan Z, Wang P, Pang Q. Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma. Oncologist. 2021;26:e1110-e1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Wu X, Han R, Zhong Y, Weng N, Zhang A. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Cancer Cell Int. 2021;21:356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Wang X, Zhang B, Chen X, Mo H, Wu D, Lan B, Li Q, Xu B, Huang J. Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR-1210), a novel anti-PD-1 antibody. Thorac Cancer. 2019;10:1395-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Guo JC, Lin CC, Lin CY, Hsieh MS, Kuo HY, Lien MY, Shao YY, Huang TC, Hsu CH. Neutrophil-to-lymphocyte Ratio and Use of Antibiotics Associated With Prognosis in Esophageal Squamous Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Anticancer Res. 2019;39:5675-5682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Zhang P, Hou X, Cai B, Yu W, Chen J, Huang X, Li Y, Zeng M, Ren Z, Gabriel E, Qu B, Liu F. Efficacy and safety of combined treatment with pembrolizumab in patients with locally advanced or metastatic esophageal squamous cell carcinoma in the real world. Ann Transl Med. 2022;10:708. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 26. | Yan MH, Liu F, Qu BL, Cai BN, Yu W, Dai XK. Induction chemotherapy with albumin-bound paclitaxel plus lobaplatin followed by concurrent radiochemotherapy for locally advanced esophageal cancer. World J Gastrointest Oncol. 2021;13:1781-1790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch OR, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Spillenaar Bilgen EJ, van der Sangen MJC, Rozema T, Ten Kate FJW, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS Study Group. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol. 2021;39:1995-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 257] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 28. | Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M; CheckMate 577 Investigators. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384:1191-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 704] [Article Influence: 234.7] [Reference Citation Analysis (0)] |
| 29. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 645] [Cited by in F6Publishing: 808] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 30. | Pao TH, Chen YY, Chang WL, Chang JS, Chiang NJ, Lin CY, Lai WW, Tseng YL, Yen YT, Chung TJ, Lin FC. Esophageal fistula after definitive concurrent chemotherapy and intensity modulated radiotherapy for esophageal squamous cell carcinoma. PLoS One. 2021;16:e0251811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Hulshof MCCM, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, Nuyttens JJME, van der Sangen MJC, Jeene PM, Reinders JG, van Berge Henegouwen MI, Thano A, van Hooft JE, van Laarhoven HWM, van der Gaast A. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study). J Clin Oncol. 2021;39:2816-2824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 32. | Zhi X, Jiang K, Shen Y, Su X, Wang K, Ma Y, Zhou L. Peripheral blood cell count ratios are predictive biomarkers of clinical response and prognosis for non-surgical esophageal squamous cell carcinoma patients treated with radiotherapy. J Clin Lab Anal. 2020;34:e23468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3108] [Cited by in F6Publishing: 3298] [Article Influence: 274.8] [Reference Citation Analysis (0)] |
| 34. | Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, Qian X, Li Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69:1813-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |