Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2052
Peer-review started: December 9, 2016
First decision: December 29, 2016
Revised: January 6, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: March 21, 2017
To determine whether infection in patients with acute severe alcoholic hepatitis (AAH) treated with corticosteroids is associated with increased mortality.
Consecutive patients with AAH were treated with steroids and recruited to the study. Clinically relevant infections (body temperature > 38 °C or < 36 °C for more than 4 h, ascitic neutrophil count > 0.25 ×109/L, consolidation on chest radiograph or clinically relevant positive microbiological culture of bodily fluid) were recorded prospectively. Clinical and laboratory parameters were recorded and survival at 90 d and 6 mo was determined. Univariate analysis of factors associated with 90-d mortality was performed and significant variables included in a multivariate analysis.
Seventy-two patients were included in the final analysis (mean age 47.9 years, 26% female, mean discriminant function 53.0). Overall mortality in the group occurred in 15 (21%), 23 (32%) and 31 (43%) at day 28, day 90 and 1 year respectively. 36 (50%) had a clinically relevant infection during their hospitalisation (23 after initiation of steroids). The median time to development of incident infection after commencement of steroids was 10 d. The commonest site of infection was ascites (31%) and bacteraemia (31%) followed by urinary tract (19%) and respiratory tract (8%). Forty-one separate organisms were isolated in 33 patients; the most frequent genus was Escherichia (22%) and Enterococcus (20%). Infection was not associated with 90-d or 1 year mortality but was associated with higher creatinine, model for end-stage liver disease and Lille score. Baseline urea was the only independent predictor of 90-d mortality.
Clinically relevant infections are common in patients with AAH but are not associated with increased 90-d or 1 year mortality.
Core tip: Corticosteroids are the only treatment shown to improve outcome in patients with acute severe alcoholic hepatitis (AAH) but may be associated with increased rates of infection and mortality. In this prospective cohort study of patients with AAH treated with corticosteroids rates of clinically relevant infections were accurately documented. Half of the study participants developed an infection during their hospitalisation with the commonest sites being ascites and bacteraemia. Infection was associated with higher creatinine, model for end-stage liver disease and lille score but not with higher 90-d or 1 year mortality. Infection is common in patients with AAH but is not associated with increased mortality.
- Citation: Dhanda AD, Sinha A, Hunt V, Saleem S, Cramp ME, Collins PL. Infection does not increase long-term mortality in patients with acute severe alcoholic hepatitis treated with corticosteroids. World J Gastroenterol 2017; 23(11): 2052-2059
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2052.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2052
Alcoholic hepatitis is an acute syndrome characterised by recent onset jaundice and coagulopathy in a patient with a history of prolonged and heavy alcohol consumption[1]. Despite improved recognition of and research interest in the condition, mortality remains high in patients with acute severe alcoholic hepatitis (AAH; traditionally defined as having a discriminant function > 32[2]) with 90 d and 1 year mortality of 29% and 56% respectively[3]. Data regarding cause of death is challenging to capture but a Danish registry study suggests that early mortality (within 84 d) is mostly liver related (58%) or due to infection (20%) while late mortality is also contributed to by cancer and alcohol and 16% is still due to infection[4]. Other than abstinence from alcohol the only treatment with a proven short-term survival benefit is corticosteroids (steroids)[5]. However, this benefit may be outweighed by the increased risk of infection posed by steroid treatment. Although some randomised controlled trials (RCTs) have reported higher rates of infection in steroid treated patients[3,6], it remains controversial whether increased infections result in increased mortality.
Increased risk of mortality was clearly described in a prospective cohort study in which infections that developed after initiation of steroid treatment were associated with increased 2 mo mortality[7]. However, adequately treated infections prior to commencement of steroids were not associated with increased mortality risk. A retrospective cohort analysis also demonstrated that infection at presentation or during hospitalisation was associated with increased 1 year mortality risk on univariate but not multivariate analysis[8]. However, only 43% of the cohort received steroids and the interaction between steroids and infection was not investigated. A recent sub-analysis from the United Kingdom STOPAH trial data found that prednisolone treatment was associated with increased risk of infection in the post-treatment period and that incident infection increased 28- and 120-d mortality but this was independent of steroid treatment[9]. A meta-analysis of data from 12 RCTs including a steroid arm did not demonstrate any increased infection or mortality risk associated with steroids except with the occurrence of fungal infections, which were uncommon (9 out of 1062 patients)[10].
The inconsistency of these data may be explained by poor recording of infections in clinical trials (which do not always specify prospective collection of infection information) and due to insufficient detail in retrospective analyses. Here, we add to the existing literature with a single centre prospective cohort of patients with AAH including long-term follow-up data.
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the NHS Health Research Authority (07/Q2007/09). Written informed consent was obtained from participants or, where they lacked capacity, assent was obtained from a personal or nominated consultee.
Consecutive patients admitted to University Hospitals Bristol NHS Foundation Trust with AAH from October 2007 to September 2015 were prospectively recruited to this study. AAH was defined as new onset jaundice (within the previous 3 mo) with serum bilirubin > 80 μmol/L and coagulopathy in a heavy drinker [more than 10 units alcohol (80 g ethanol) daily in males and 7.5 units (60 g ethanol) in females within the previous 4 wk]. Additionally, discriminant function[2] was greater than 32.
Clinically relevant infections were recorded at first presentation and prospectively during hospital admission and were defined as a body temperature > 38 °C or < 36 °C for more than 4 h, ascitic neutrophil count > 0.25 × 109/L, consolidation on chest radiograph or clinically relevant positive microbiological culture of bodily fluid.
All patients were screened for infection on admission to hospital with chest radiograph, urinalysis, ascitic fluid analysis (where ascites was present) and peripheral blood cultures if body temperature was greater than 38 °C. In patients with temperature < 38 °C and negative infection screen, oral prednisolone was commenced at a dose of 40 mg daily and continued for 28 d. Patients with a positive infection screen or body temperature > 38 °C were treated with broad spectrum intravenous antibiotics according to Trust protocol for at least 48 h before converting to oral antibiotics. In these patients the 28 d course of prednisolone was only started after temperature < 38 °C had been recorded for at least 48 h.
Patients who developed clinically relevant infection after initiation of steroid treatment (incident infections) were treated within 12 h with intravenous broad spectrum antibiotics according to Trust protocol for at least 48 h before being converted to oral antibiotics. Steroids were not discontinued during or after incident infections except in those in whom active treatment was withdrawn and palliative care was initiated.
Routine laboratory data were collected at baseline, day 7 and 28 of steroid treatment and survival status was recorded at day 90, 6 and 12 mo. Survival was determined by accessing Trust databases which are linked to community databases and where necessary by direct contact with the patient’s General Practitioner. Alcohol consumption at follow-up was determined by face-to-face or telephone consultations carried out by the Alcohol Liaison Team at regular intervals after hospital discharge.
Patients who did not receive prednisolone or in whom investigators were blinded to their treatment (where they also participated in the United Kingdom STOPAH clinical trial) were excluded from this analysis.
Patient characteristics, baseline laboratory parameters, day 7 serum bilirubin levels and composite scores [discriminant function (DF), Glasgow Alcoholic Hepatitis Score (GAHS), Model for End-stage Liver Disease (MELD) and Lille score[11]] were compared between survivors and non-survivors at day 90 by univariate analysis with Mann-Whitney U tests for continuous data and Fisher Exact tests for categorical data. Terms that were found to be significant at the 5% level of significance were then included in a multivariate regression model which used survival at day 90 as the dependent variable.
Kaplan-Meier survival analysis was also performed at day 90 and 1 year. Survival was compared between patients with clinically relevant infection on admission, post-steroid initiation and at any time by log-rank test.
A total of 116 participants were recruited to the study; 44 were excluded as treatment allocation was blinded due to participation in the STOPAH trial (n = 42) or they did not receive prednisolone (n = 2; 1 with concurrent active hepatitis C infection and 1 with a borderline DF which improved to less than 32 in 24 h without treatment). Therefore 72 patients were included in the final analysis (mean age 47.9 years, 26% female, mean DF 53.0; Table 1). Overall mortality in the group occurred in 15 (21%), 23 (32%) and 31 (43%) at day 28, day 90 and 1 year respectively.
| Age | 47.9 ± 10.6 |
| Male (%) | 74 |
| Baseline CRP (mg/L) | 33 ± 26.8 |
| Baseline bilirubin (μmol/L) | 294 ± 142 |
| Baseline albumin (g/L) | 25 ± 7.7 |
| Baseline INR | 1.9 ± 0.5 |
| Baseline PT (s) | 19.2 ± 4.6 |
| Baseline urea (μmol/L) | 3.9 ± 2.7 |
| Baseline creatinine (μmol/L) | 90 ± 56.6 |
| Baseline WBC (× 109/L) | 9.1 ± 4.5 |
| Day 7 bilirubin (μmol/L) | 251 ± 174 |
| Baseline DF | 53.0 ± 24.4 |
| Baseline GAHS | 8.1 ± 1.4 |
| Baseline MELD | 22.3 ± 6.7 |
| Lille score | 0.403 ± 0.350 |
| Day 28 survival (%) | 21 |
| Day 90 survival (%) | 32 |
| 12 mo survival (%) | 43 |
During the period of recruitment to the STOPAH trial in our centre (April 2011 to December 2013), 27 patients who met the STOPAH trial selection criteria were not recruited to it either due to patient choice or because steroids were commenced prior to screening and were hence included in the present study. These patients represent a similar population to the study participants recruited outside the STOPAH trial recruitment period in terms of age (49 vs 47, P = 0.87), gender (26% vs 27% female, P = 1.0) and disease severity (DF 53.7 vs 52.7, P = 0.46). Additionally, there were no statistical differences between patients in the current study and those recruited to the STOPAH clinical trial from our centre (n = 42). Age was similar (48 vs 51, P = 0.14) as was DF (53.0 vs 53.1, P = 0.88), 90 d mortality (23% vs 32%, P = 0.48) and 1 year mortality (43% vs 42%, P = 1.0).
In total 36 patients (50%) had a clinically relevant infection during their hospital stay with 8 (11%) on admission, 7 (10%) prior to initiation of steroids and 23 (32%) after initiation of steroids (including 2 who also had a separate infection on admission). On admission, bacteraemia was present in 3 patients (all Escherichia coli), spontaneous bacterial peritonitis (SBP) in 2, respiratory infection in 1 and urinary tract infection and SBP in 1. No obvious source of infection could be identified in 1 patient.
Of the 7 patients who developed a clinically relevant infection after admission but prior to initiation of steroids 3 were due to bacteraemia, 2 urinary tract infections, 1 gastrointestinal (GI) infection and 1 in whom no proven source of infection was found.
In those that developed incident infections after initiation of steroids there were 9 cases of SBP, 4 urinary tract infections, 2 respiratory infections, 5 bacteraemias and 5 infections at other sites (2 ear infections, 2 GI tract infections and 1 cellulitis). A source of infection was not determined in 2 patients. Four of these patients had non-concurrent infections at more than one site.
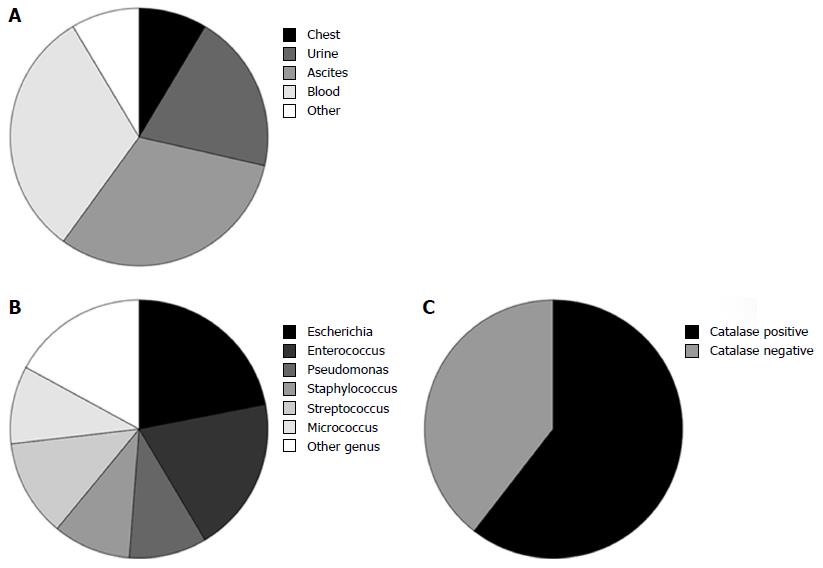
Overall bacteraemia and SBP were the most common sites of infection (11 each) followed by urinary tract (7) and respiratory tract (3; Figure 1A and Supplementary Table). The median time to commencement of steroids from hospital admission was 2 d (mean 2.4 d) in all patients. In those with infection identified at the time of admission the median time to commencement of steroids was 3 d (mean 5.0). The median time to the first incident infection after commencement of steroids was 10 d (range 2-42 d).
The causative bacterial genus was isolated from 41 separate clinically relevant infections in 33 individual patients. The commonest genus was Escherichia (9; 22%) followed by Enterococcus (8, 20%), Streptococcus (5; 12%) and Staphylococcus, Pseudomonas and Micrococcus (4 each; 10%; Figure 1B). Seven patients had non-concurrent infections with more than 1 organism and in 3 patients 2 organisms were identified from the same specimen. Four other infective organisms were identified: Acinetobacter (2 cases), Clostridium difficile (2 cases), Haemophilus and norovirus. Of the 36 patients with clinically relevant infections, 23 (64%) had infections with catalase positive bacteria including 2 patients who had infections with both catalase negative and positive organisms (Figure 1C).
Clinically relevant infection was not significantly associated with biochemical or haematological markers of infection at baseline or day 7 of steroid treatment: baseline C-reactive protein was 36 vs 30 g/L (P = 0.40), baseline white blood count (WBC) was 9.9 vs 8.3 × 109/L (P = 0.14) and day 7 WBC was 13.4 x109/L vs 10.5 × 109/L (P = 0.08) in patients with infection vs no infection respectively. However, presence of clinically relevant infection was associated with higher creatinine (105 vs 73 μmol/L, P = 0.01), MELD (23.9 vs 20.6, P = 0.04) and Lille score (0.51 vs 0.28, P = 0.01) than those without infection.
Univariate analysis of patient characteristics, baseline and day 7 biochemistry and composite scores identified age, baseline urea and baseline GAHS as significantly different between survivors and non-survivors at day 90 (Table 2). GAHS was excluded from multivariate analysis due to co-linearity with the other variables. Applying age and urea to a multivariate regression model identified only urea as an independent predictor of 90-d outcome (P = 0.01; Table 3 and Supplementary Table).
| Day 90 outcome | P value | ||
| Survivor (n = 49) | Non-survivor (n = 23) | ||
| Age (yr) | 45 | 53 | 0.01 |
| Gender (% male) | 73 | 74 | 1.00 |
| Sepsis on admission (%) | 10 | 13 | 0.50 |
| Sepsis at any time (%) | 51 | 48 | 1.00 |
| Sepsis after steroids (%) | 33 | 30 | 1.00 |
| CRP (mg/L) | 30 | 40 | 0.15 |
| Baseline bilirubin (μmol/L) | 301 | 279 | 0.52 |
| Baseline albumin (g/L) | 25 | 24 | 0.38 |
| Baseline INR | 1.8 | 2 | 0.14 |
| Baseline PT (s) | 18.6 | 20.5 | 0.17 |
| Baseline urea (μmol/L) | 3.2 | 5.5 | < 0.01 |
| Baseline creatinine (μmol/L) | 90 | 91 | 0.93 |
| Baseline WBC (× 109/L) | 9 | 10 | 0.44 |
| Day 7 bilirubin (μmol/L) | 244 | 254 | 0.67 |
| Baseline DF | 51.1 | 57.2 | 0.41 |
| Baseline GAHS | 7.8 | 8.7 | 0.04 |
| Baseline MELD | 21.7 | 23.7 | 0.23 |
| Lille score | 0.38 | 0.46 | 0.38 |
| R2 | Adjusted R2 | B | Std. Error | F | Sig | |
| Model | 0.205 | 0.182 | 0.425 | 8.87 | < 0.001 | |
| Constant | -0.351 | 0.234 | 0.138 | |||
| Urea | 0.054 | 0.020 | 0.010 | |||
| Age | 0.010 | 0.005 | 0.071 |
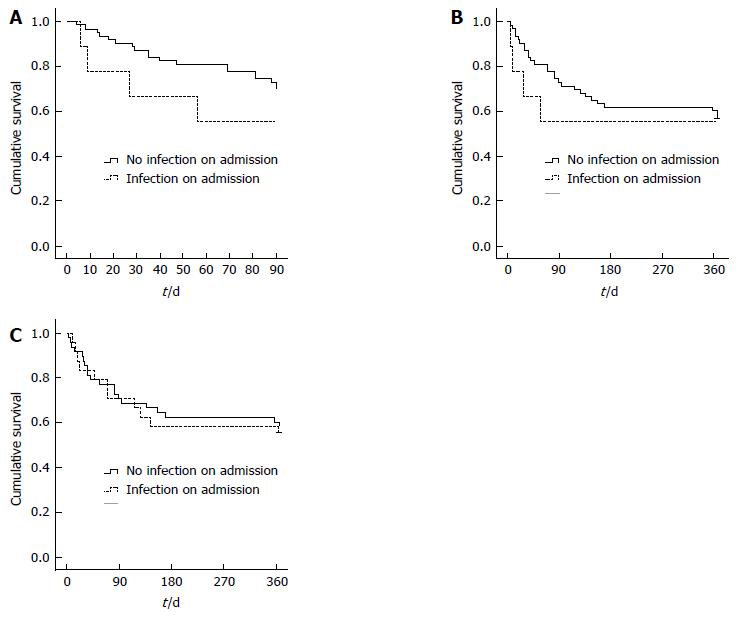
In total 11 and 15 out of 36 patients who developed infection died at 90 d or 12 mo respectively compared to 12 and 16 out of 36 of the patients without infection. Kaplan-Meier survival analysis from the time of steroid commencement did not demonstrate a significant survival difference between groups at any time. The same was true in patients with clinically relevant infection at the time of hospital admission (P = 0.26 at 90 d and P = 0.67 at 1 year; no censored data; Figure 2A and B). There was neither a 90 d nor 1 year survival difference in patients with and without an incident infection after commencement of steroids (P = 0.78 at 90 d and P = 0.98 at 1 year; no censored data; Figure 2C).
In this cohort of patients with AAH in which clinically relevant infections were clearly defined and documented prospectively, 50% developed an infection during the study period which was not associated with increased mortality at 90 d or 1 year. 11% of patients presented with clinically relevant infection, 10% developed infection after admission but prior to steroid initiation and 32% developed an incident infection during steroid treatment but the timing of infection did not influence survival. In agreement with recently published studies, Escherichia and Enterococcus genii were the commonest bacterial pathogens isolated[12,13]. The commonest site of infection was ascites (31%) and 31% had bacterial septicaemia. In only 3 patients a site of infection could not be identified.
Although this is a single centre study, the patient cohort is representative of United Kingdom patients with AAH with study selection criteria similar to the recent STOPAH clinical trial[3]. Mean age in this study was 47.9 years (in comparison to 48.7 years in STOPAH), with 74% male (68% male in STOPAH) and mean discriminant function 53.0 (62.6 in STOPAH). Mortality is also comparable with the STOPAH study and other recent clinical trials with overall mortality of 21%, 32% and 43% at day 28, day 90 and 1 year respectively[3,6,14,15]. This study has the added advantage over multicentre studies of having standardised patient management by a small team of clinicians which reduces variability in patient outcome.
Data regarding infection in AAH are sparse in the literature and of varying quality. Uniquely, this study has robustly prospectively recorded clinically relevant infections. This is reflected in the higher rate of infections noted here compared to only 13% recorded in the prednisolone treated patients in the STOPAH trial, which was likely an underestimate of the true rate of infection since it relied on clinician judgement to report it as a serious adverse event[3].
A meta-analysis of 12 RCTs with sufficient infection data (including the STOPAH study) described incident infections occurring in 20% of patients without a higher rate in steroid treated patients[10]. The commonest infection was sepsis of unknown source followed by respiratory tract infections. It concluded that infection after steroid commencement was not associated with increased mortality at 28 d. However, the effect on longer term mortality was not assessed and most of the 12 studies were historic from more than 20 years ago highlighting the fact that many recent studies have not collected high quality data on infections.
A French single centre prospective study of 246 patients reported infection in 26% at presentation and 24% developed an incident infection after commencement of steroid treatment[7]. In agreement with the current study SBP was once again the commonest infection on admission but respiratory tract infections were more common in those with incident infection. Patients who had an infection at admission had a similar outcome to other patients but those who developed infection on steroid treatment had a reduced 60-d survival. However, they demonstrated that the biochemical response to steroids (using the Lille score) and not infection itself was the most important determinant of survival. A more recent RCT also concluded that treatment non-responders rather than responders were at higher risk of death from infection (14% vs 4%[6]). In the present study, clinically relevant infection at any time during hospital admission was associated with a significantly higher Lille score. Conversely, Lille non-responders (Lille score > 0.45) had a trend to increased rates of clinically relevant incident infection (48% vs 24%, P = 0.07) but the Lille score itself was not identified as an independent predictor of outcome. These data suggest there is an interaction between biochemical response to treatment and infection which influences survival. However, it cannot be determined whether infection itself has an effect on liver biochemistry or whether treatment non-response increases susceptibility to infection.
An increased infection rate is seen in patients with AAH due to generalised immune dysfunction. Recent studies have investigated the immune defect in more detail and describe impaired monocyte oxidative burst, phagocytic capacity as well as increased T cell exhaustion[12,16]. The current data support these findings since the majority of bacterial isolates were able to produce catalase which is protective against phagocytic oxidative burst. Interestingly these effects were independent of steroid treatment[12] suggesting that steroids do not increase susceptibility to infection through this mechanism.
Local clinical protocols in our liver unit mean all patients with clinically relevant infection are treated rapidly (within 12 h) after first identification of infection. Additionally, in those presenting with infection, steroids were withheld until at least 48 h of intravenous broad spectrum antibiotics had been received and they were free from clinical signs of infection for this period. This protocol was adhered to in all patients in this study. This proactive antibiotic treatment policy may partly explain why no increased risk of mortality is associated with infection in these patients with AAH.
This prospective observational cohort study demonstrates that clinically relevant infections that are sought and treated early with broad spectrum antibiotics do not influence short- or long-term mortality in patients with severe AAH treated with steroids. However, long-term mortality of patients with AAH remains high primarily through alcohol recidivism and progressive liver disease[3,4]. Continuing efforts to improve long-term outcome from severe AAH should be directed to modify behaviour after hospital discharge.
We thank all the clinicians involved in the management of these patients in University Hospitals Bristol NHS Foundation Trust, in particular Dr Anne McCune, Dr Fiona Gordon and Dr Jim Portal. We thank all the patients for participating in this study.
Acute severe alcoholic hepatitis (AAH) is a serious complication of chronic heavy alcohol misuse resulting in progressive liver failure with a high mortality. Corticosteroids are the only treatment with a proven survival benefit but may be associated with higher rates of infection. The current study describes clinically relevant infections in a prospective cohort of patients at a large single National Health Service hospital in the United Kingdom.
The existing literature reports increased rates of infection associated with corticosteroid treatment but there are conflicting data regarding whether this is linked to higher mortality. This information is particularly relevant since it will help guide clinician treatment of AAH.
The current study collected infection data prospectively using a clear definition of clinically relevant infection. This is an improvement on previous studies which have less robust definitions of infections, collected infection data retrospectively or relied on clinician discretion to report relevant infections.
This study demonstrates that although clinically relevant infections are common in AAH patients they are not associated with higher mortality if actively sought and treated. These data will provide clinicians with more confidence to select appropriate patients with AAH for treatment with corticosteroids.
AAH is defined as new onset jaundice (within the previous 3 mo) with serum bilirubin > 80 μmol/L and coagulopathy in a heavy drinker [more than 10 units alcohol (80 g ethanol) daily in males and 7.5 units (60 g ethanol) in females within the previous 4 wk]. Clinically relevant infections were defined as a body temperature > 38 °C or < 36 °C for more than 4 h, ascitic neutrophil count > 0.25 × 109 L, consolidation on chest radiograph or clinically relevant positive microbiological culture of bodily fluid.
Alcoholic hepatitis is still a clinical challenge, despite improved nutrition management. The study carries important information also regarding the aetiology of infectious complications, which would provide help on proper antibacterial choice. The predictive value of urea is also very important in this setting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Malnick S, Petrova M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 639] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 2. | Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193-199. [PubMed] [Cited in This Article: ] |
| 3. | Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 461] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 4. | Orntoft NW, Sandahl TD, Jepsen P, Vilstrup H. Short-term and long-term causes of death in patients with alcoholic hepatitis in Denmark. Clin Gastroenterol Hepatol. 2014;12:1739-1744.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, Thursz MR, Loomba R, Shah VH. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology. 2015;149:958-970.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Park SH, Kim DJ, Kim YS, Yim HJ, Tak WY, Lee HJ, Sohn JH, Yoon KT, Kim IH, Kim HS. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. 2014;61:792-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, Deltenre P, Mathurin P. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Potts JR, Goubet S, Heneghan MA, Verma S. Determinants of long-term outcome in severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;38:584-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Atkinson S, Vergis N, Thursz M, Investigators ST. Infection in severe alcoholic hepatitis: results from the STOPAH trial. Journal of hepatology. 2016;64:S174-175. [Cited in This Article: ] |
| 10. | Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int. 2016;36:721-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 451] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 12. | Vergis N, Khamri W, Beale K, Sadiq F, Aletrari MO, Moore C, Atkinson SR, Bernsmeier C, Possamai LA, Petts G. Defective monocyte oxidative burst predicts infection in alcoholic hepatitis and is associated with reduced expression of NADPH oxidase. Gut. 2017;66:519-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Beisel C, Blessin U, Schulze Zur Wiesch J, Wehmeyer MH, Lohse AW, Benten D, Kluwe J. Infections complicating severe alcoholic hepatitis: Enterococcus species represent the most frequently identified pathogen. Scand J Gastroenterol. 2016;51:807-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Mathurin P, Louvet A, Duhamel A, Nahon P, Carbonell N, Boursier J, Anty R, Diaz E, Thabut D, Moirand R. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310:1033-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Sidhu SS, Goyal O, Singla P, Gupta D, Sood A, Chhina RS, Soni RK. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Dig Dis Sci. 2012;57:1664-1671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590-602.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |