Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.716
Revised: May 29, 2005
Accepted: July 15, 2005
Published online: February 7, 2006
AIM: To examine the pathway related to the IL-1β-induced activation of mitogen-activated protein (MAP) kinases in cat esophageal smooth muscle cells.
METHODS: Culture of the esophageal smooth muscle cells from cat was prepared. Specific inhibitors were treated before applying the IL-1β. Western blot analysis was performed to detect the expressions of COX, iNOS and MAP kinases.
RESULTS: In the primary cultured cells, although IL-1β failed to upregulate the COX and iNOS levels, the levels of the phosphorylated forms of p44/42 MAP kinase and p38 MAP kinase increased in both concentration- and time-dependent manner, of which the level of activation reached a maximum within 3 and 18 h, respectively. The pertussis toxin reduced the level of p44/42 MAP kinase phosphorylation. Tyrphostin 51 and genistein also inhibited this activation. Neomycin decreased the density of the p44/42 MAP kinase band to the basal level. Phosphokinase C (PKC) was found to play a mediating role in the IL-1β-induced p44/42 MAP kinase activity. In contrast, the activation of p38 MAP kinase was inhibited only by a pretreatment with forskolin, and was unaffected by the other compounds.
CONCLUSION: Based on these results, IL-1β-induced p44/42 MAP kinase activation is mediated by the Gi protein, tyrosine kinase, phospholipase C (PLC) and PKC. The pathway for p38 MAP kinase phosphorylation is different from that of p44/42 MAP kinase, suggesting that it plays a different role in the cellular response to IL-1β.
- Citation: Lee TS, Song HJ, Jeong JH, Min YS, Shin CY, Sohn UD. IL-1β activates p44/42 and p38 mitogen-activated protein kinases via different pathways in cat esophageal smooth muscle cells. World J Gastroenterol 2006; 12(5): 716-722
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.716
Esophageal reflux is a common condition that affects both children and adults. If left untreated, it can result in chronic esophagitis, aspiration pneumonia, esophageal strictures and Barrett’s esophagus, which is a premalignant condition[1]. Reflux esophagitis (RE) is a multifactorial disease that may depend on the relaxation of the transient lower esophageal sphincter (LES), the speed of esophageal clearance, the mucosal resistance and other factors, and is often associated with the LES pressure[2]. Cytokines exhibit potent chemotactic activity toward different populations of leukocytes, and are essentially involved in the induction of acute and chronic inflammatory reactions[3,4]. The selective infiltration of various leukocyte subsets into the esophagus indicates the participation of chemokines in the immune and inflammatory processes of RE.
Interleukin-1 (IL-1) has several biological effects on many cell types, and plays a key role in regulating the inflammatory reaction[5]. The IL-1 family is an important part of the innate immune system, which regulates functions of the adaptive immune system. In the presence of excess IL-1, inflammatory and autoimmune diseases can develop in many organs, including the joints, lungs, gastrointestinal track, central nervous system, or blood vessels. Stimulation of the cells by IL-1 initiates the transcription of many pro-inflammatory genes, including IL-6 and IL-8. The levels of the inflammatory genes are higher in the esophageal mucosa of those patients with RE[6]. IL-1 has its own identical biological functions and it is induced by gastric ulceration in rats[7,8]. Increased mucosal levels of IL-1 are constantly observed during acute and chronic intestinal inflammation in human beings[9,10] and animals[11,12]. The immunoneutralization of the IL-1 activity has been reported to greatly reduce the severity of colitis in a murine model of the disease, suggesting that this cytokine plays an important role in initiating inflammation[12]. The IL-1 mRNA and protein were strongly expressed in the gastric mucosa of gastritis and ulcer patients[13,14]. Therefore, it is suggested that the pro-inflammatory effects of IL-1 are due to the induction of COX-2 gene expression in many tissues, including mucous[14]. However, there are few reports about the effect on the smooth muscle.
In mammalian cells, there have been at least three different subfamilies of mitogen-activated protein (MAP) kinases reported. They include the extracellular signal-regulated kinases (ERKs, p44/42 MAP kinase), c-Jun N-terminal kinase (JNK), and p38. These kinases are activated by distinct upstream MAP kinases (MEKs), which phosphorylate the residues within a tripeptide motif (Thr-X-Tyr) on the MAP. Once activated, MAP kinases, in turn, phosphorylate a variety of intracellular substrates, including certain transcription factors[15]. IL-1β is known to activate all three MAP kinase subfamilies in human airway smooth muscle cells. IL-1β stimulation has been associated with several MAP kinases cascades involved in the transmission of a signal from the cell surface to the nucleus. Although these pathways have not been demonstrated to be involved in the IL-1β-mediated effects on the esophageal smooth muscle, p38 and ERK have been shown to contribute to such effects in human[16] or canine airway[17] or myometrial[18] smooth muscle. Therefore, in this study, we examined the mechanism associated with IL-1β-induced MAP kinases activation in cat esophageal smooth muscle cells.
Fetal bovine serum (FBS) was purchased from Biofluids (Rockville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM), antibiotic-antimycotic (penicillin, streptomycin, amphotericin B), and trypsin-EDTA were obtained from Invitrogen (Grand Island, NY, USA). Phospho-p44/p42 monoclonal MAP kinase antibody, phospho-SAPK/JNK monoclonal antibody, phospho-p38 monoclonal MAP kinase antibody, p44/42 MAP kinase antibody, SAPK/JNK antibody and p38 MAP kinase antibody were acquired from Cell Signaling (Beverly, MA, USA). Goat anti-mouse IgG-HRP was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The rainbow prestained molecular weight marker was obtained from Amersham (Arlington Heights, IL, USA). The enhanced chemiluminescence agents were purchased from PerkinElmer Life Sciences (Boston, MA, USA). Sodium dodecyl sulfate (SDS) sample buffer was acquired from Owl Scientific, Inc. (Woburn, MA, USA). Nitrocellulose membrane, Tris/glycine/SDS buffer and Tris/glycine buffer were purchased from BioRad (Richmond, CA, USA). Phosphate-buffered saline (PBS) was acquired from Boehringer Mannheim (Indianapolis, IN, USA). The PD 98059 and SB202190 were obtained from Calbiochem (La Jolla, CA, USA). The GF 109203X was purchased from Tocris (Ellisville, MO, USA). RestoreTM Western Blot Stripping Buffer was obtained from Pierce (Rockford, IL, USA). Horseradish peroxidase-conjugated goat anti-rabbit antibody, phorbol-12-myristate-13-acetate (PMA), genistein, tyrphostin 51 and other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Adult cats of either gender, weighing between 2.5 and 3.5 kg, were used in this study. The cats were anesthetized with ketamine (50 mg/mL per kg) and the abdomen was then opened with a midline incision. The esophagus and stomach were excised together, cleaned and maintained in Krebs buffer with the following composition (mmol/L): NaCl 116.6, NaHCO3 21.9, NaH2PO4 1.2, KCl 3.4, CaCl2 2.5, glucose 5.4 and MgCl2 1.2. The esophagus and stomach were opened along the lesser curvature. The location of the squamocolumnar junction was identified and the mucosa was peeled off. The high-pressure zone was identified by a visible thickening of the circular muscle layer in conjunction with the squamocolumnar junction and immediately proximal to the sling fibers of the stomach. It has been shown that a 5- to 8-mm band of tissue coinciding with the thickened area constitutes the LES, and has distinct in vivo characteristics in an organ bath or as single cells after enzymatic digestion[19,20].
After opening the esophagus and stomach and identifying the LES, the mucosa and submucosal connective tissues were removed via a sharp dissection. The LES was excised and a 3- to 5-mm wide strip at the junction of the LES and esophagus was discarded in order to avoid potential overlap. The circular muscle layer from the esophagus was cut into 0.5-mm-thick slices using a Stadie Riggs tissue slicer (Tomas Scientific Apparatus, Philadelphia, PA, USA). The last slices containing the myenteric plexus, the longitudinal muscle and the serosa were discarded, and the remaining slices were then cut into 2 mm × 2 mm tissue squares by hand.
The sliced tissue was then placed into DMEM supplemented with 500 mL/L FBS containing 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 0.25 μg/mL amphotericin B and incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2 and 950 mL/L air. On the following day, fresh DMEM containing 1 0 0 mL/L FBS was added. Ten days later, the tissue explants were removed and the medium was exchanged with fresh DMEM containing 100 mL/L FBS. After the cells reached confluence, the cells were detached with 10 g/L trypsin-EDTA in HBSS with sodium bicarbonate. The cells were then counted, seeded at 1×106 cells/mL on 100-mm culture dishes, and maintained in DMEM containing 100 mL/L FBS. The medium was changed every 48 h until the cells reached confluence. The experiments were performed on the cells of passage 2.
In order to characterize the isolated and cultured esophageal smooth muscle cells as well as to exclude contamination by epithelial cells and fibroblasts, the cells were identified using an indirect immunofluorescent staining method with a monoclonal antibody of a light chain myosin[21]. More than 95% of the cell preparation was found to be composed of smooth muscle cells.
For the animal esophagitis model, esophageal smooth muscle tissue squares were homogenized in an homogenizing buffer containing 20 mmol/L Tris (hydroxymethyl) aminomethane (Tris), 160 mmol/L HCl (pH 7.5), 0.5 mmol/L EGTA, 0.5 mmol/L EDTA, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 mmol/L β-mercaptoethanol and centrifuged at 14 000 g for 15 min at 4 °C. The supernatants were used as the whole cell extract.
For the IL-1β treatment experiments, the cells were plated in six-well culture plates and made quiescent at confluence by incubating them in serum-free DMEM for 24 h. The growth-arrested esophageal smooth muscle cells were incubated with or without IL-1β at 37 °C at various times. When the inhibitors were used, they were added 1 h before applying the IL-1β according to the inhibitors, with the exception that the cells were pretreated with the pertussis toxin 24 h prior to IL-1β. After incubation, the cells were rapidly washed with ice-cold PBS and lysed for 5 min in an ice-cold lysis buffer containing 25 mmol/L Tris-HCl (pH 7.4), 25 mmol/L NaCl, 25 mmol/L NaF, 25 mmol/L sodium pyrophosphate, 1 mmol/L sodium vanadate, 2.5 mmol/L EDTA, 2.5 mmol/L EGTA, 0.5 g/L Triton X-100, 5 g/L SDS, 0.5 g/L deoxycholate, 0.5 g/L NP-40, 5 μg/mL leupeptin, 5 μg/mL aprotinin, and 1 mmol/L PMSF. After incubation, the lysates were scrapped and collected by centrifugation at 45 000 g for 1 h at 4 °C to yield the whole cell extract.
Equal amounts of the protein from each sample were resolved on a SDS-polyacrylamide gel by electrophoresis. Prestained molecular mass markers were also run in an adjacent lane to determine the molecular mass. The separated proteins were transferred to a 0.45-μm nitrocellulose membrane in 25 mmol/L Tris (pH 8.3), 192 mmol/L glycine, and 100 mL/L methanol using a power supply (Power Pac 1000, BioRad, Melville, NY, USA). The membranes were incubated in a PBS buffer containing 50 g/L non-fat dry milk for 1 h at room temperature in order to block the nonspecific binding. After washing thrice with PBS, the blots were incubated with the primary antibodies in a PBS solution containing 1 g/L BSA at 4 °C overnight. The membranes were washed using PBS containing 0.5 g/L Tween 20 and then incubated with the horseradish peroxidase-conjugated secondary antibody (1:1 000 dilution) for 1 h. The immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham). The total MAP kinase expression level was determined by subsequently stripping the same blot with a stripping buffer and reproving the blot with p44/42 MAP kinase antibodies. Developed films from ECL were scanned and analyzed densitometrically using Scion Image.
The protein concentration of the supernatant in each reaction vial was measured spectrophotometrically at a wavelength of 595 nm using a BioRad assay (BioRad Chemical Division, Richmond, CA, USA).
The data were expressed as mean ± SE of three separate experiments, and the statistical differences between means were determined by using Student’s t test. A P < 0.05 was considered statistically significant.
The treatment of the esophageal smooth muscle cells (ESMC) with IL-1β did not increase the COX-2 expression level (Figure 1). In other cell types such as canine tracheal smooth muscle cells[22], human gastric cancer cells[23], and human myometrial smooth muscle cells[18], it has been reported that IL-1β exerts its biological effects partly by inducing COX-2 expression. However, the cells treated with IL-1β at a concentration of 0.5-50 ng/mL for 18 h did not show an increased COX-1 and COX-2 expression levels (Figure 1A). In addition, when treated with 25 ng/mL IL-1β for 15 min to 24 h, the COX expression level in the cat ESMC was not altered (Figure 1B). These results corresponded to previous results showing that an experimentally induced rat esophagitis did not cause an increase in the COX-2 level, suggesting that COX-2 might not mediate esophagitis (unpublished data).
It is also known that IL-1β mediates inflammation, at least in part, by inducing the expression of inducible nitric oxide synthase (iNOS)[7], with the concomitant enhanced synthesis of NO, which is a critical effector molecule, in rat pulmonary artery smooth muscle cells. The cat ESMCs were treated with IL-1β (25 ng/mL) for 18 h and the iNOS level was determined by using Western blotting analysis (Figures 1C and 1D). This treatment did not increase the iNOS expression level, suggesting that iNOS, like COX-2, might not be a mediator of the cellular response to IL-1β in ESMC.
Although there was no change in the COX and iNOS levels, which is similar to other reports, IL-1β activated p44/42 MAP kinase with increasing concentration (Figure 2). After a treatment with 0.5-50 ng/mL IL-1β for 3 h, p44/42 MAP kinase was phosphorylated at concentrations above 10 ng/mL. Densitometric assessments demonstrated that the exposure of ESMCs to 25 ng/mL IL-1β for 3 h significantly increased the level of activation of p44/42 MAP kinase by 2.9 ± 0.1 times over the level of control samples (Figure 2A). Furthermore, IL-1β (25 ng/mL) produced a time-dependent increase in p44/42 MAP kinase phosphorylation (Figure 2B). The effect of IL-1β on p44/42 MAP kinase was observed within 30 min. The maximum increase (2.6 ± 0.1-fold increase over the control) in this response was observed within 3 h, and a gradual decrease to the basal level within 18 h was observed. Based on the concentration-response and time-course data, the ESMCs were exposed to IL-1β at a final concentration of 25 ng/mL for 3 h in later experiments, aiming at identifying the signaling pathway of p44/42 MAP kinase activation. Similarly, p38 MAP kinases were activated by IL-1β in the cat ESMCs. As shown in Figure 2A, an 18-h IL-1β treatment caused phosphorylation of p38 MAP kinase in a concentration-dependent manner. A clear increase (1.9 ± 0.1-fold) was observed at concentrations above 10 ng/mL IL-1β. The time course of p38 MAP kinase phosphorylation indicated that the IL-1β increased the activity within 12 h, which was maintained up to 24 h (1.5 ± 0.04-fold increase over the control). Therefore, even though IL-1β activated p38 MAP kinase as well as p44/42 MAP kinase, a difference in the activated time of the two MAP kinases was also observed. This suggests that IL-1β can evoke these cellular responses via at least two pathways, involving p38 MAP kinase and p44/42 MAP kinase, in the cat ESMCs.
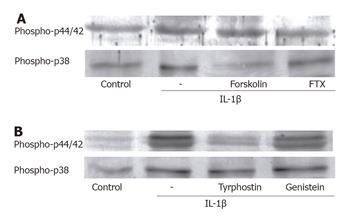
In order to determine if the effects of IL-1β on p44/42 and p38 MAP kinases were mediated through the activation of a receptor coupled to the pertussis toxin (PTX)-sensitive G protein, the ESMCs were pretreated with 100 ng/mL PTX for 24 h, followed by stimulation with IL-1β for 3 and 18 h, respectively (Figure 3A). Previous studies showed that various types of G proteins, such as Gs, Gq, Go and Gi1-3, could be detected in the cat ESMCs[24]. As shown in Figure 3A, pretreatment of these cells with PTX caused a slight attenuation of p44/42 phosphorylation, indicating that IL-1β exerts its effects through the receptor-coupled Gi protein. However, p38 MAP kinase phosphorylation was maintained without being mediated by the Gi protein. In order to determine the involvement of adenylate cyclase (AC) in the activation of MAP kinases, the cells were pretreated with forskolin 24 h before IL-1β in order to activate the AC (Figure 2A). AC had little effect on p44/42 MAP kinase activation, but was found to decrease the phosphorylated forms of p38 MAP kinase. Therefore, it is believed that IL-1β-induced p38 MAP kinase activation is negatively regulated by cAMP.
Recently, it has been proposed that IL-1β causes the activation of tyrosine kinase and regulates protein tyrosine phosphorylation[22]. There are two classic tyrosine kinases, namely, receptor and nonreceptor tyrosine kinase. The receptor tyrosine kinases are actually the growth factor receptors located in the inner side of the cytoplasmic membrane and undergo dimerization and autophosphorylation upon activation. In this study, we examined the possibility that the observed increase in the level of MAP kinases phosphorylation after an IL-1β treatment might be mediated by tyrosine kinase (Figure 3B). Therefore, the cells were pretreated with genistein for 1 h prior to adding IL-1β. This resulted in a decrease in IL-1β-induced p44/42 MAP kinase activation. In addition, tyrphostin 51, which is a member of the genistein family of tyrosine kinase inhibitors and is specific to the receptor tyrosine kinase, significantly decreased the level of p44/42 MAP kinase phosphorylation, suggesting that tyrosine kinase might participate in these responses to IL-1β.
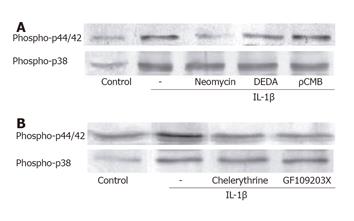
The involvement of phospholipase in the IL-1β-induced p44/42 MAP kinase activation was examined using a phospholipase C (PLC) inhibitor (neomycin), a PLA2 inhibitor (DEDA), and a PLD inhibitor (ρCMB). As shown in Figure 4A, pretreatment of the ESMCs with neomycin attenuated the p44/42 MAP kinase activation. DEDA also slightly decreased the band density, but could not reach statistical significance. In addition, pretreatment of the ESMCs with ρCMB did not decrease the level of p44/42 MAP kinase activation induced by IL-1β, suggesting that IL-1β-stimulated p44/42 activation in ESMC might be mediated via the activation of PLC. In this study, we therefore investigated whether or not phosphokinase C (PKC) activation enhanced the p44/42 MAP kinase levels. The ESMCs were pretreated with the PKC inhibitor, GF109203X and chelerythrine (Figure 4B), which reduced the level of p44/42 MAP kinase phosphorylation induced by IL-1β. It was assumed that there was no relationship between p38 MAP kinase phosphorylation and the phospholipases (Figure 4A). As the pretreatment with the phospholipase inhibitors had no effect on phospho-p38 MAP kinase band density, the PKC inhibitors did not decrease the level of activation by IL-1β (Figure 4B). Overall, IL-1β might activate p38 MAP kinase in cat ESMCs via a pathway other than the phospholipase- and PKC-associated pathway.
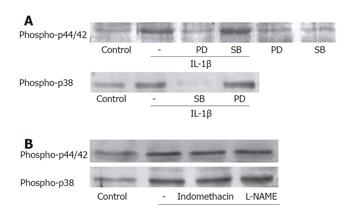
The relationship between the activation of the two MAP kinases was identified after treating the ESMCs with either 10 μmol/L PD98059 (a synthetic inhibitor of MEK1/2 activation) or 30 μmol/L SB202190 (a p38 MAP kinase inhibitor) for 1 h (Figure 5A). PD98059 resulted in inhibition of the IL-1β-induced p44/42 MAP kinase activation. However, SB202190 had little effect, indicating that p44/42 MAP kinase activation in ESMCs was mediated by the upstream kinase MEK, which was not affected by p38 MAP kinase. SB202190 markedly decreased the level of p38 MAP kinase phosphorylation to almost the basal control level, whereas PD98059 had little effects on this activation. This means that the IL-1β-induced p38 MAP kinase and p44/42 MAP kinase activation might be accomplished by independent pathways.
Although IL-1β did not enhance the COX-2 and iNOS expression levels, the participation of COX and NOS in the activation of p44/42 MAP kinase by IL-1β is still unclear. Therefore, the cells were treated with a COX inhibitor, indomethacin, and a NOS inhibitor, L-NAME, for 1 h before IL-1β stimulation in order to determine whether COX and NOS play a role in the activation (Figure 5B). The increase in the phosphorylated forms of MAP kinases was maintained even with the pretreatment of inhibitors. This suggested that COX and NOS played no role in IL-1β-induced MAP kinases activation.
It is evident that the proinflammatory cytokines and chemokines play an important role in the pathogenesis of various inflammatory conditions[3,4,25]. Recently, it was reported that there was a significantly higher expression level of IL-8 mRNA in biopsy specimens obtained from RE patients than that from non-inflamed samples, which was determined by competitive reverse transcriptase polymerase chain reaction[26]. Furthermore, in addition to IL-8, the MCP-1 and RANTES levels were also significantly higher in esophagitis patients. In that report, although there were no significant differences in the mucosal IL-1β levels between the RE samples and the control group, the tissue IL-1β levels correlated significantly with the level of IL-8 production[6]. Cytokines, such as IL-1β and TNF-α, act locally and systemically to recruit and activate the target cells that can produce additional cytokines. IL-1 induces the gene expression of neutrophils and monocyte chemotactic cytokines, such as IL-8, IL-9, and macrophage inflammatory proteins that in turn stimulate migration and degranulation of neutrophils in vivo[27]. The surgically induced esophagitis rats resulted in an approximately two-fold increase in the level of TNF-α and IL-1β production[28].
Previous studies demonstrated that COX-2 and iNOS were the effectors of the IL-1β-induced cellular responses, products of which were PGE2 and NO, respectively. In our previous study, although iNOS expression and nitrite levels in the tissue were higher after inducing esophagitis in rats (unpublished data), the COX-2 level was unaffected by each IL-1β concentration ranged from 0.5 to 50 ng/mL. In addition, the IL-1β treatment by 24 h failed to increase the iNOS concentration in the cultured cat ESMCs. Therefore, IL-1β did not play a direct role in the inflammatory process in the esophagus, suggesting that IL-1β might be related to the induction of other cytokines, which would be an immediate cause of the inflammatory cellular response, such as COX-2 and iNOS, in cat ESMC.
IL-1β plays its own role in the cellular signaling components in the cat ESMC. The MAP kinase pathway has been suggested to be a mechanism by which various signals are transduced from the cell surface to the nucleus in response to a variety of different stimuli. These proteins participate in several intracellular processes by further inducing the level of phosphorylation of various intracellular substrates, such as other protein kinases and transcription factors. This signaling mechanism is believed to control a wide spectrum of cellular physiological and pathophysiological processes including cell growth, differentiation, and the stress response[29]. The treatment of the ESMC with > 10 ng/mL IL-1β resulted in the activation of p44/42 MAP kinase, which was generated by 30 min and reached a maximum within 3 h, with a subsequent decrease. A similar pattern was observed with p38 phosphorylation, which was also concentration-dependent above 10 ng/mL. This increase was observed after 12 h, suggesting that p44/42 MAP kinase might participate in the early phase response to IL-1β, and p38 MAP kinase might be a mediator of the late phase IL-1β effects.
In order to determine whether IL-1β interacts with the G proteins, the requirement of the G protein was evaluated using ESMC pretreated with the pertussis toxin (PTX). PTX has been shown to inhibit the intrinsic GTPase activity of the Gα subunit by the ADP-ribosylation of specific residues. A previous report demonstrated that Gq, G11, Gi1, Gi2, and Gi3 were present in the esophagus and LES[24]. In this study, the IL-1β-induced activation of p44/42 MAP kinase was affected by the PTX pretreatment, suggesting the involvement of a PTX-sensitive G protein in these processes. However, PTX had little effect on the IL-1β-induced activation of p38 MAP kinase. This inhibitory effect of the toxin might result from the increases in the cyclic AMP (cAMP) level via the abrogation of the Gi protein in ESMC. A previous report demonstrated that IL-1β activated AC in vascular smooth muscle via COX-2[30]. Therefore, a cAMP elevating agent, forskolin, was used to activate adenylate cyclase in order to evaluate its role in IL-1β-induced responses. Forskolin failed to attenuate the IL-1β-induced p44/42 MAP kinase activation, excluding the involvement of elevated cAMP. In contrast, the cAMP concentration affected the level of p38 MAP kinase phosphorylation, suggesting that the delayed activation of p38 MAP kinase is due to the elevation of cAMP by IL-1β.
It is well known that IL-1β triggers the activation of PI-PLC and PC-PLC in U937 cells and tracheal smooth muscle cells to generate DAG and IP3[31,32]. DAG and IP3 activate PKC and release Ca2+ from the intracellular stores, respectively, and may play an important role in regulating the cellular functions in several cell types[33]. PKC is a major component in the kinase cascade that is initiated by ligand attachment to both the G protein-coupled receptors and the receptors possessing intrinsic tyrosine kinase activity. Different PKC isozymes appeared to mediate the LES tone as well as the phasic contraction of the ESMC[34]. In order to confirm the participation of phospholipase in the IL-1β-induced activation of MAP kinases, the cells were pretreated with inhibitors of each phospholipase. Neomycin markedly decreased the activity of p44/42 MAP kinase, but failed to reduce the activity of p38 MAP kinase. Therefore, p44/42 MAP kinase and p38 MAP kinase are on a different IL-1β signaling pathway at least downstream from the receptors. The regulatory mechanisms involved in IL-1β-induced MAP kinase activation by PKC were further investigated. These results showed that pretreatment with the PKC inhibitors attenuated the IL-1β-induced p44/42 MAP kinase activation in the ESMC. These results were consistent with those showing that cytokine activated the PLC-coupling signaling pathways in the human histiocytic lymphoma cell line, U937[31]. Despite the relationship between p44/42 MAP kinase activation by IL-1β and PKC, the PKC inhibitors did not exert any inhibitory effects on p38 MAP kinase activation.
The implication of a tyrosine kinase in the activation MAP kinases by IL-1β was investigated using tyrosine kinase inhibitors, genistein and tyrphostin 51. Genistein and tyrphostin 51 inhibited p44/42 MAP kinase phosphorylation, indicating the involvement of tyrosine kinase in this response. In contrast, these inhibitors did not block the phosphorylation of p38 MAP kinase by IL-1β.
The activation of p44/42 MAP kinase requires both tyrosine and threonine phosphorylation as a result of the dual specificity MEK1/2. PD98059, a synthetic and highly specific MEK1/2 inhibitor, has been shown to inhibit p44/42 MAP kinase activation by several stimuli[35]. In this study, a PD98059 pretreatment attenuated the p44/42 MAP kinase activation, leaving p38 MAP kinase phosphorylated. In addition, IL-1β has been shown to phosphorylate p38 MAP kinase, which appears to be distinct from p44/42 MAP kinase in several cell types[36,37]. SB202190 inhibited the IL-1β-induced p38 MAP kinase activation, but did not show any inhibitory effect on p44/42 MAP kinase activation. Overall, the activation of p44/42 MAP kinase and p38 MAP kinase appears to involve independent pathways.
The absence of a role of COX and iNOS in these pathways was confirmed by their respective inhibitors, indomethacin and L-NAME. Indomethacin plays a role in preventing COX-2 from producing prostaglandin, and L-NAME blocks NO synthesis by NOS. The IL-1β-induced activity of p44/42 MAP kinase and p38 MAP kinase remained unchanged even after the indomethacin and L-NAME treatment, thereby excluding the role of prostaglandin and NO in this responses.
In conclusion, IL-1β, which is believed to act as a pro-inflammatory mediator by evoking other cytokines in the esophagitis, can induce p44/42 MAP kinase and p38 MAP kinase activation. The pathway related to p44/42 MAP kinase is composed of a PTX-sensitive G protein, tyrosine kinase, PLC and PKC. These components are believed to play a role in the early cellular responses via IL-1β-induced p44/42 activation, but have little effect on p38 MAP kinase phosphorylation, which is generated later and is regulated negatively by cAMP.
S- Editor Kumar M and Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Biancani P, Sohn UD, Rich HG, Harnett KM, Behar J. Signal transduction pathways in esophageal and lower esophageal sphincter circular muscle. Am J Med. 1997;103:23S-28S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51 Suppl 1:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 365] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 288] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 289] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095-2147. [PubMed] [Cited in This Article: ] |
| 6. | Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. 2003;98:551-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Kinoshita Y, Nakata H, Hassan S, Asahara M, Kawanami C, Matsushima Y, Naribayashi-Inomoto Y, Ping CY, Min D, Nakamura A. Gene expression of keratinocyte and hepatocyte growth factors during the healing of rat gastric mucosal lesions. Gastroenterology. 1995;109:1068-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Takahashi S, Shigeta J, Inoue H, Tanabe T, Okabe S. Localization of cyclooxygenase-2 and regulation of its mRNA expression in gastric ulcers in rats. Am J Physiol. 1998;275:G1137-G1145. [PubMed] [Cited in This Article: ] |
| 9. | Youngman KR, Simon PL, West GA, Cominelli F, Rachmilewitz D, Klein JS, Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993;104:749-758. [PubMed] [Cited in This Article: ] |
| 10. | Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434-2440. [PubMed] [Cited in This Article: ] |
| 11. | McCall RD, Haskill S, Zimmermann EM, Lund PK, Thompson RC, Sartor RB. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rats. Gastroenterology. 1994;106:960-972. [PubMed] [Cited in This Article: ] |
| 12. | Arai Y, Takanashi H, Kitagawa H, Okayasu I. Involvement of interleukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine. 1998;10:890-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 348] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 250] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Karin M. Signal transduction from cell surface to nucleus in development and disease. FASEB J. 1992;6:2581-2590. [PubMed] [Cited in This Article: ] |
| 16. | LaPointe MC, Isenović E. Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem. 1999;274:24211-24219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 333] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Bartlett SR, Sawdy R, Mann GE. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J Physiol. 1999;520 Pt 2:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Biancani P, Zabinski M, Kerstein M, Behar J. Lower esophageal sphincter mechanics: anatomic and physiologic relationships of the esophagogastric junction of cat. Gastroenterology. 1982;82:468-475. [PubMed] [Cited in This Article: ] |
| 20. | Biancani P, Hillemeier C, Bitar KN, Makhlouf GM. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am J Physiol. 1987;253:G760-G766. [PubMed] [Cited in This Article: ] |
| 21. | Gown AM, Vogel AM, Gordon D, Lu PL. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985;100:807-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 209] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Yang CM, Chien CS, Hsiao LD, Luo SF, Wang CC. Interleukin-1beta-induced cyclooxygenase-2 expression is mediated through activation of p42/44 and p38 MAPKS, and NF-kappaB pathways in canine tracheal smooth muscle cells. Cell Signal. 2002;14:899-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Fan XM, Wong BC, Lin MC, Cho CH, Wang WP, Kung HF, Lam SK. Interleukin-1beta induces cyclo-oxygenase-2 expression in gastric cancer cells by the p38 and p44/42 mitogen-activated protein kinase signaling pathways. J Gastroenterol Hepatol. 2001;16:1098-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Frantsuzova SB. [Level of nicotinamide coenzymes in the myocardium of rats during the effects of methylxanthines (theophylline, theobromine, caffeine) and catecholamines]. Biull Eksp Biol Med. 1975;79:68-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 25. | Isomoto H, Miyazaki M, Mizuta Y, Takeshima F, Murase K, Inoue K, Yamasaki K, Murata I, Koji T, Kohno S. Expression of nuclear factor-kappaB in Helicobacter pylori-infected gastric mucosa detected with southwestern histochemistry. Scand J Gastroenterol. 2000;35:247-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Oppenheim JJ, Matsushima K, Yoshimura T, Leonard EJ, Neta R. Relationship between interleukin 1 (IL1), tumor necrosis factor (TNF) and a neutrophil attracting peptide (NAP-1). Agents Actions. 1989;26:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Shin YK, Sohn UD, Choi MS, Kum C, Sim SS, Lee MY. Effects of rutin and harmaline on rat reflux oesophagitis. Auton Autacoid Pharmacol. 2002;22:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 593] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Beasley D. COX-2 and cytosolic PLA2 mediate IL-1beta-induced cAMP production in human vascular smooth muscle cells. Am J Physiol. 1999;276:H1369-H1378. [PubMed] [Cited in This Article: ] |
| 31. | Schütze S, Berkovic D, Tomsing O, Unger C, Krönke M. Tumor necrosis factor induces rapid production of 1'2'diacylglycerol by a phosphatidylcholine-specific phospholipase C. J Exp Med. 1991;174:975-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 161] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Yang CM, Luo SF, Wang CC, Chiu CT, Chien CS, Lin CC, Hsiao LD. Tumour necrosis factor-alpha- and interleukin-1beta-stimulated cell proliferation through activation of mitogen-activated protein kinase in canine tracheal smooth muscle cells. Br J Pharmacol. 2000;130:891-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484-496. [PubMed] [Cited in This Article: ] |
| 34. | Sohn UD, Zoukhri D, Dartt D, Sergheraert C, Harnett KM, Behar J, Biancani P. Different protein kinase C isozymes mediate lower esophageal sphincter tone and phasic contraction of esophageal circular smooth muscle. Mol Pharmacol. 1997;51:462-470. [PubMed] [Cited in This Article: ] |
| 35. | Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686-7689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2115] [Cited by in F6Publishing: 2275] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 36. | Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4113] [Cited by in F6Publishing: 4064] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 37. | Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 AND p38 mitogen-activated protein kinase pathways. J Biol Chem. 2000;275:14838-14845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |