Non-metastatic Ewing’s sarcoma family of tumors arising from head and neck: a single institution experience
Introduction
Peripheral primitive neuroectodermal tumors (pPNETs) are small round blue cell tumors of neural crest origin. Tumor cells are uniformly vimentin positive and often cytokeratin positive, indicating origin from neuronal and epithelial elements. Together with Ewing’s sarcoma (both osseous and extraosseous) and Askin’s tumors of chest wall, they are referred to as Ewing’s sarcoma family of tumors (ESFT) which are characterised by 11:22 chromosomal translocation and expression of MIC2 gene product on the tumor cell membrane (1). They need to be distinguished from the central nervous system (CNS) primitive neuroectodermal tumors which lack the characteristic translocation and have divergent clinical behavior. Over half the cases are located in extremities. Management is multimodal, consisting of multiagent systemic therapy and local therapy in the form of surgery, radiation therapy, or a combination of both.
Head and neck pPNETs are uncommon, constituting 1–9% of all pPNETs (2,3). Management is challenging due to the unique anatomy of head and neck region, limiting extensive surgery due to functional and cosmetic issues. Since most patients belong to pediatric or adolescent age group, long-term sequelae of various treatment modalities, such as retardation of musculoskeletal development, hearing and vision problems etc., also need to be considered when planning therapy. Outcome of this group of patients has been reported only in isolated case reports or small series, thereby highlighting the lack of understanding of the clinical behavior of this group of patients compared with extremity ESFT (2-9).
We attempted to study the disease characteristics, treatment and outcome of head and neck ESFT patients treated at our department over a 5-year period. To our knowledge, this is the second largest single institution study till date evaluating the clinical outcome of such patients.
Methods
We reviewed the records of patients with extracranial ESFT of head and neck region, treated with curative intent at our institution in the period May 2004 to June 2009. Demographic and disease-related information including age, gender, tumor location, initial symptoms, diagnosis, treatment, follow-up and outcome, was obtained by chart review. Histopathological diagnosis was available for all patients. Tumors with metastases to any sites other than cervical lymph nodes, and patients with any prior treatment were excluded. All patients underwent a diagnostic and staging workup including biopsy from primary site, imaging of head and neck region, either computed tomography (CT) or magnetic resonance imaging (MRI) scan, complete blood counts, renal and liver function tests, bone scan, bone marrow biopsy, high resolution CT chest, and serum lactate dehydrogenase.
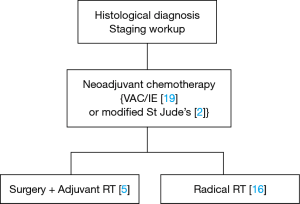
Combined modality treatment was the standard of care. All patients were planned for neoadjuvant chemotherapy (NACT) with VAC/IE regimen, followed by local therapy (surgery /radiotherapy) and adjuvant chemotherapy (Figure 1). Chemotherapy protocol was as follows: inj vincristine 1.4 mg/m2 IV D1, inj adriamycin 60 mg/m2 IV D1, inj cyclophosphamide 1 g/m2 IV D1 (VAC) alternating with inj ifosfamide 1.8 g/m2 IV D1-5 (with mesna uroprotection), inj etoposide 100 mg/m2 IVD1-5 (IE), q 3 weeks for 5 cycles, followed by surgery or radiotherapy. In patients treated with radical radiotherapy, clinical target volume (CTV) consisted of gross disease as seen on CT or MRI with 1–2 cm margin for microscopic disease. For postoperative cases, CTV included tumor bed with appropriate margin. Regional nodes were included in CTV whenever involved at baseline. Radiation therapy was planned using 3D conformal technique and delivered with 6 MV X-rays using a Linear Accelerator. Radiotherapy dose was tailored to the radiotherapy indication (postoperative vs radical) as well as the presence of microscopic or gross residual disease. Neck nodes were treated only when involvement was confirmed on imaging or operative histopathology. Radiotherapy dose was reported in gray (Gy) and treatment was delivered at 1.8–2.0 Gy per fraction, 1 fraction per day and 5 fractions per week, for a total of 5–6 weeks. Local therapy was followed by adjuvant chemotherapy for a total of 12 cycles.
Follow-up evaluation consisted of a clinical examination every 3 months and CT scan of primary site and chest X-ray every 6 months or as warranted by symptoms. Other investigations were directed by any additional symptoms.
Statistical analysis was done using SPSS software version 16.0.
Results
A total of 21 patients were identified and included in the analysis. There were 13 males and 8 females. Median age at diagnosis was 13 years (range, 3–31 years). Median duration of symptoms at presentation was three months (range, 1 month to 2 years). Most common presenting feature was a swelling (100%) followed by pain (57%). Most common tumor location was mandible (N=7) followed by maxilla (N=5). Three patients had cervical lymph nodes at presentation, the primary sites being maxilla, nasal cavity and parotid. Seventeen patients had bone involvement while the remaining four involved only soft tissues. Five patients had intracranial extension from primary tumor. Figure 2 shows a few representative clinical, pathology and imaging features. No patients had vascular involvement, joint space involvement or skip metastases on CT scan (Table 1).
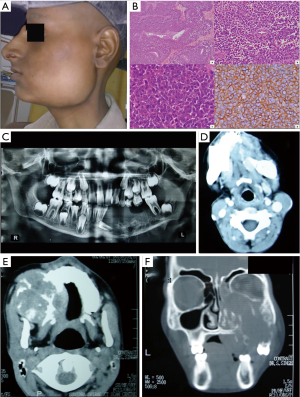
Table 1
| Patient/tumor features | No. of patients (n=21) |
|---|---|
| Age (in years) | |
| Median (range) | 13 [3–31] |
| Gender | |
| Male | 13 |
| Female | 8 |
| Primary site | |
| Mandible | 7 |
| Maxilla | 5 |
| Orbit | 4 |
| Parotid | 2 |
| Soft tissues of neck | 2 |
| Nasal cavity | 1 |
| Symptoms | |
| Swelling | 21 |
| Pain | 12 |
| Vision loss | 7 |
| Size on imaging (cm) | |
| Median (range) | 6 [3–15] |
| Size >10 cm | 5 |
| Cervical nodes at presentation | 3 |
| Performance status (Eastern Cooperative Oncology Group) | |
| 1 | 15 |
| 2 | 6 |
| Systemic symptoms (fever, cough, fatiguability) | 2 |
| Serum lactate dehydrogenase >250 | 7/14 |
| Serum alkaline phosphatase >150 | 12/15 |
Treatment regimen details are given in Table 2. Treatment essentially consisted of a combination of chemotherapy, radiotherapy and surgery. All patients received chemotherapy as per planned schedule. Two patients treated in early 2004 received chemotherapy as per modified St. Jude’s protocol. Median duration of chemotherapy was 33 weeks (range, 12 to 60 weeks). Only one patient required chemotherapy dose reduction to 75% of original dose. Chemotherapy induced morbidity consisted of 9 episodes of febrile neutropenia in 6 patients, 3 episodes of mucositis in 2 patients, and grade 2 anemia in 1 patient. One patient developed scalp & submandibular abscesses followed by respiratory arrest at 21 weeks of chemotherapy.
Table 2
| Treatment | No. of patients (n=21) |
|---|---|
| Surgery | |
| Biopsy only | 16 |
| Complete excision | 5 |
| Chemotherapy regime | |
| VAC/IE | 18 |
| VAC/ICE | 1 |
| Modified St. Jude’s protocol | 2 |
| Chemotherapy duration (weeks) | |
| Range | 12–60 |
| Median | 33 |
| Radiation therapy | |
| Dose range | 30–60 Gy |
| Median dose | 55 Gy |
| Dose >/=50 Gy | 16/21 |
Surgery was undertaken in 5 patients, all of which had complete tumor excision and one of these was an excision biopsy at diagnosis. Surgical margins were negative in all but one case. Primary tumor sites in patients managed with surgery included mandible (N=2), orbit (N=2) and maxilla (N=1). Out of 4 patients who had surgery following NACT, one had complete pathologic response and three had partial responses.
All patients received local or locoregional radiation therapy (radical or postoperative). Radiotherapy was delivered to gross disease (pre-operative or -chemotherapy extent, with margin for microscopic extension or set-up errors), using 3D conformal technique. Three patients with neck node involvement received radiotherapy to neck as well. Radiotherapy was delivered at 1.8–2 Gy per fraction, 5 fractions per week. Median radiotherapy dose was 55 Gy (range, 30 to 60 Gy). There were no grade 3 or 4 skin reactions or mucositis.
Following therapy, 13 patients had complete response, 5 patients had partial response, 1 patient had stable disease and 2 patients had progressive disease. One patient developed local recurrence following complete response, 24.2 months following diagnosis. Mean follow-up duration was 26.7 months (range, 7.4 to 77.5 months). At last follow-up, 12 patients were disease-free, 6 were alive with disease and 1 patient had succumbed to his disease. The only observed death was unrelated to disease or therapy, and was due to sepsis consequent to a submandibular abscess. The child had a mandibular primary and had achieved complete remission after successfully completing treatment. Status of 2 patients was unknown.
Discussion
ESFTs are aggressive tumors of bone or soft tissue, diagnosed in the second decade of life with a male predominance, with a common genetic origin and clinical behavior. Of these, Ewing’s sarcomas are the second most common group of pediatric bone tumors after osteosarcoma. pPNETs may appear similar to neuroblastoma on morphology or neuron specific enolase (NSE) expression but can be distinguished by CD99 (MIC2) antigen expression and different biology and clinical behavior. Common osseous sites of origin include lower extremities (40–45%), pelvis (20–25%), chest wall (15–20%), and upper extremities (10%). Non-osseous tissues of origin may include gastrointestinal tract, kidneys and skeletal muscle. Symptoms usually depend on the site of involvement and in 25% cases, may be attributable to metastases to lungs, bones or bone marrow. Nodal or liver involvement is rare (10).
Involvement of sites in the head and neck is rare and most commonly involves mandible, vertebrae and skull bones. This could possibly be due to the paucity of hematopoietic marrow in these regions (11).
Only five reports have reported results on four or more patients with either Ewing’s sarcoma of bones or pPNETs in head and neck region (2-6). Apart from these, there are nearly 100 single case reports in English literature after 1985. Head and neck Ewing’s sarcomas constitute 1–7% among all sites. Frequency is higher in males, similar to the overall distribution of ESFT. The largest single institution series on head and neck PNETs by Allam et al. reported on 24 cases of Ewing’s sarcomas, all but one of which had non-metastatic disease. Their reported a male:female ratio of 2.4, higher than 1.6 observed in our series, or 1.2 observed in another large series by Siegal et al. which included 29 patients included in four Intergroup Ewing’s Sarcoma Study (IESS) protocols (7299, 7450, 7895 and 7896) (2,3). Presenting features include pain and soft tissue or bony swelling. Lesions originating in the orbit or periorbital tissues may produce visual symptoms such as nerve palsies and loss of vision (6). Unlike Ewing’s sarcoma of bone in other sites where nearly 90% complain of pain, pain is less common in head and neck pPNET (57% in our series).
The most common site in Siegal’s series was flat bones of skull (11/29); in others, mandible appears to be a site which is more common and has a better prognosis as well. Nearly one-third of our patients had mandibular primaries, though their survival did not differ with respect to site of origin. We did not have any tumor originating in flat bones of skull or cervical vertebrae which are believed to have a poorer prognosis (2,12,13). Median age at presentation in our series was the second decade, which agreed with the observations of previous series. Interestingly, of the 5 patients reported by Nikitakis et al., three were over the age of 30 years (5). Only one of our patients presented in fourth decade, at 31 years of age, with a supraclavicular soft tissue mass.
On laboratory tests, ESFTs may demonstrate increase in leucocyte counts, serum alkaline phosphatise levels and lactate dehydrogenase levels, which may have prognostic significance (14-16). Typical radiologic features of Ewing’s sarcoma are permeative destructive bone lesions with origin in metaphysis or diaphysis of long bones. Periosteal elevation by the tumor producing the Codman’s triangle sign, and bone expansion are common though new bone formation beyond the periosteal margin is rare. More than 50% of long bone neoplasms have associated soft tissue masses. Both CT and MRI have complementary roles in delineating the disease extent (17,18). Classical signs described for Ewing’s sarcomas such as periosteal reaction, cortical thickening, permeative changes, sclerotic change or soft tissue mass are less often noted in head and neck Ewing’s sarcomas. Pure lytic change, honeycombing, bone expansion and cortical violation are more frequently observed (2). Soft tissue pPNETs typically appear as heterogeneous soft tissue density masses. Tumors larger than 5 cm may show some low attenuating cystic or necrotic areas. Calcification may be present, but is uncommon. Skull base invasion may occasionally be seen (19). Bone scans show increased radiotracer uptake in the primary site in bony primaries. Positron emission tomography CT (PET-CT) is appearing as a new tool for determining the initial tumor bulk and extent as well as prognostication (20).
Treatment of ESFT is typically multimodal. Combination chemotherapy has helped improve survival rates from less than 10% to over 60%. Induction chemotherapy (3–6 cycles) is followed by local therapy and 8–10 cycles of consolidation chemotherapy. Local treatment may include surgery, radiation therapy or combination thereof. For most sites, surgery with adequate margins is the preferred mode of treatment, with radiation given for marginal or intralesional surgery or inoperable tumors (21). In head and neck ESFTs, surgery is less frequently employed due to the cosmetic and functional implications in the age group which typically consists of children or young adults. In our study, only 5 of 21 underwent complete excision, the rest only had a biopsy from the primary lesion. Similar findings were reported by Allam (8/24 underwent surgery, 2/8 had complete excision), Siegal (14/29 surgery, 7/14 complete excision), Jones (5/11 surgery, 2/5 complete excision), Vaccani (2/4 surgery), the only exception being the report by Nikitakis et al. (5/5 surgery, 2/5 had microscopic positive margin).
Chemotherapy is an integral part of therapy since the disease has a propensity for systemic dissemination. The most common drugs used are vincristine, adriamycin, cyclophosphamide, actinomycin, ifosfamide and etoposide. Over the years, many IESS protocols have evolved defining treatment regimes. Nineteen of our patients were treatment according to IESS protocols and two as per modified St. Jude’s protocol (22,23). Radiation therapy has been used frequently in these cases, since complete ablative surgery is usually not possible due to the difficult location of these tumors (2,3,24). All our patients received radical or adjuvant radiation therapy, only one received a dose under 40 Gy. Median dose was 55 Gy. Other series have also reported similar radiation doses (mean dose of 53 Gy by Siegal and 50.4 Gy by Allam). Nikitakis et al. also reported on a dose range of 45–60 Gy to local disease.
Siegal et al., have reported on a mean follow-up of 56 months (range, 7–123 months). Ten of their patients had a follow-up over 5 years. Of the five deaths reported in their series, three had vertebral primaries and four were teenagers; both these factors denoted a poorer prognosis. When compared to all other Ewing’s sarcoma sites, head and neck primaries had a better survival, which improved further when cervical vertebral primaries were excluded. The authors have also reported on several earlier studies reported between 1950 and 1975, which reiterated the authors’ observation that patient’s with mandibular primaries survive the longest (25,26). Metastases at presentation, quite predictably, drastically reduce survival rates, e.g., 65% vs. 38% at 2 years for non-metastatic vs. metastatic presentation (4). Mean follow-up reported by Allam et al. was 3.4 years, 5-year overall and disease free survival rates noted at 53% and 30%, respectively. Response to chemotherapy was the only significant prognostic factor. Our study has a relatively shorter mean follow-up of 26.7 months. Eleven patients had achieved complete remission; one of these with a maxillary primary developed a local recurrence at 24.3 months for which salvage chemotherapy was attempted. At last follow-up, 11 patients had no evidence of recurrence while 6 were alive with disease. Only one of our patients had died during the study period, the reason was sepsis following a submandibular abscess, after having achieved a complete response.
Conclusions
Earlier series have reported a high failure rate in ESFT due to systemic dissemination. However, modern imaging and treatment techniques including conformal radiation and intensive chemotherapy regimens have drastically improved survival. The few small series of head and neck primary ESFTs so far have focused either on bone Ewing’s sarcomas alone or soft tissue pPNETs. However, current understanding merges these two as a single entity due to similar histomorphologic appearance and clinical behavior. The follow-up in our series is smaller compared to the other two larger series. In the observed follow-up period, we have encountered good local and systemic control rates. Head and neck ESFT appear to be a good prognosis entity, with the likelihood of durable disease control and survival even with limited or no surgery, provided adequate radiation doses are delivered along with intensive systemic chemotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tro.2019.05.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the local Ethics Committee. The treatment informed consent states upfront that anonymised data may be used for compilation and future research. Care was taken not to disclose identity of patients in any manner.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marcus KJ, Tarbell NJ. Ewing’s sarcoma. In: Halperin EC, Constine LS, Tarbell NJ, et al. editors. Pediatric Radiation Oncology. 4th edition. Philadelphia: Lippincott Williams & Wilkins, 2005:272-91.
- Siegal GP, Oliver WR, Reinus WR, et al. Primary Ewing's sarcoma involving the bones of the head and neck. Cancer 1987;60:2829-40. [Crossref] [PubMed]
- Allam A, El-Husseiny G, Khafaga Y, et al. Ewing's Sarcoma of the Head and Neck: A Retrospective Analysis of 24 Cases. Sarcoma 1999;3:11-5. [Crossref] [PubMed]
- Jones JE, McGill T. Peripheral primitive neuroectodermal tumors of the head and neck. Arch Otolaryngol Head Neck Surg 1995;121:1392-5. [Crossref] [PubMed]
- Nikitakis NG, Salama AR, O'Malley BW Jr, et al. Malignant peripheral primitive neuroectodermal tumor-peripheral neuroepithelioma of the head and neck: a clinicopathologic study of five cases and review of the literature. Head Neck 2003;25:488-98. [Crossref] [PubMed]
- Vaccani JP, Forte V, de Jong AL, et al. Ewing's sarcoma of the head and neck in children. Int J Pediatr Otorhinolaryngol 1999;48:209-16. [Crossref] [PubMed]
- Bakhshi S, Meel R, Naqvi SG, et al. Therapy and outcome of orbital primitive neuroectodermal tumor. Pediatr Blood Cancer 2009;52:544-7. [Crossref] [PubMed]
- Fiorillo A, Tranfa F, Canale G, et al. Primary Ewing's sarcoma of the maxilla, a rare and curable localization: report of two new cases, successfully treated by radiotherapy and systemic chemotherapy. Cancer Lett 1996;103:177-82. [Crossref] [PubMed]
- Deb RA, Desai SB, Amonkar PP, et al. Primary primitive neuroectodermal tumour of the parotid gland. Histopathology 1998;33:375-8. [Crossref] [PubMed]
- Heidi V. Russell HV, Pappo AS, et al. Childhood cancers. Solid tumors of childhood. In: DeVita VT, Lawrence TS, Rosenberg SA. editors. Devita, Hellman & Rosenberg's Cancer: Principles & Practice of Oncology. 8th edition. Philadelphia: Lippincott Williams & Wilkins, 2008;2034-85.
- Berk R, Heller A, Heller D, et al. Ewing's sarcoma of the mandible: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;79:159-62. [Crossref] [PubMed]
- Kuzeyli K, Aktürk F, Reis A, et al. Primary Ewing's sarcoma of the temporal bone with intracranial, extracranial and intraorbital extension. Case report. Neurosurg Rev 1997;20:132-4. [Crossref] [PubMed]
- Schuck A, Ahrens S, von Schorlemer I, et al. Radiotherapy in Ewing tumors of the vertebrae: treatment results and local relapse analysis of the CESS 81/86 and EICESS 92 trials. Int J Radiat Oncol Biol Phys 2005;63:1562-7. [Crossref] [PubMed]
- Fletcher JA. Ewing's sarcoma oncogene structure: a novel prognostic marker? J Clin Oncol 1998;16:1241-3. [Crossref] [PubMed]
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol 1990;8:1664-74. [Crossref] [PubMed]
- Farley FA, Healey JH, Caparros-Sison B, et al. Lactase dehydrogenase as a tumor marker for recurrent disease in Ewing's sarcoma. Cancer 1987;59:1245-8. [Crossref] [PubMed]
- Murphy WA Jr. Imaging bone tumors in the 1990s. Cancer 1991;67:1169-76. [Crossref] [PubMed]
- Peersman B, Vanhoenacker FM, Heyman S, et al. Ewing's sarcoma: imaging features. JBR-BTR 2007;90:368-76. [PubMed]
- Dick EA, McHugh K, Kimber C, et al. Imaging of non-central nervous system primitive neuroectodermal tumours: diagnostic features and correlation with outcome. Clin Radiol 2001;56:206-15. [Crossref] [PubMed]
- Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828-34. [Crossref] [PubMed]
- Saeter G, Oliveira J, Bergh J, et al. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of Ewing's sarcoma of bone. Ann Oncol 2005;16:i73-4. [Crossref] [PubMed]
- Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol 2009;27:2536-41. [Crossref] [PubMed]
- Marina NM, Pappo AS, Parham DM, et al. Chemotherapy dose-intensification for pediatric patients with Ewing's family of tumors and desmoplastic small round-cell tumors: a feasibility study at St. Jude Children's Research Hospital. J Clin Oncol 1999;17:180-90. [Crossref] [PubMed]
- La TH, Meyers PA, Wexler LH, et al. Radiation therapy for Ewing's sarcoma: results from Memorial Sloan-Kettering in the modern era. Int J Radiat Oncol Biol Phys 2006;64:544-50. [Crossref] [PubMed]
- Falk S, Alpert M. Five-year survival of patients with Ewing's sarcoma. Surg Gynecol Obstet 1967;124:319-24. [PubMed]
- Pritchard DJ, Dahlin DC, Dauphine RT, et al. Ewing's sarcoma. A clinicopathological and statistical analysis of patients surviving five years or longer. J Bone Joint Surg Am 1975;57:10-6. [Crossref] [PubMed]
Cite this article as: Goyal S, Biswas A, Mohanti BK, Bakhshi S. Non-metastatic Ewing’s sarcoma family of tumors arising from head and neck: a single institution experience. Ther Radiol Oncol 2019;3:22.