Ytterbium(III) Chloride: A superior Reagent for the Synthesis of 4-Chloro-5, 6-Dihydro-2H-Pyran Derivatives via Cyclization of Epoxides and Homopropynyl Alcohols
Tirukovela Manjula1,2 and Jeyanthi Arasan1*
1Department of Chemistry, Satavahana University, Karimnagar-505002, (Telangana) India.
2Department of Chemistry, Government Degree College, Luxettipet-504231, (Telangana) India.
Corresponding Author E-mail: manjulatirukovela.chem@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390433
Article Received on : 09 Jun 2023
Article Accepted on : 15 Jul 2023
Article Published : 25 Jul 2023
Reviewed by: Dr. Jatin Mehta
Second Review by: Dr. Rajasekhar Koorella
Final Approval by: Dr. S. A. Iqbal
Prins type cyclization reaction of epoxides and homopropargyl alcohols in the presence of Ytterbium trichloride results in high yields of the corresponding dihydropyran derivatives under mild conditions.
KEYWORDS:Dihydropyran, Epoxide; Homopropargyl Alcohol; Ytterbium trihloride
Download this article as:| Copy the following to cite this article: Manjula T, Arasan J. Ytterbium(III) Chloride: A superior Reagent for the Synthesis of 4-Chloro-5, 6-Dihydro-2H-Pyran Derivatives via Cyclization of Epoxides and Homopropynyl Alcohols. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Manjula T, Arasan J. Ytterbium(III) Chloride: A superior Reagent for the Synthesis of 4-Chloro-5, 6-Dihydro-2H-Pyran Derivatives via Cyclization of Epoxides and Homopropynyl Alcohols. Orient J Chem 2023;39(4). Available from: https://bit.ly/44ZJ15W |
Introduction
Dihydropyrans are six-membered oxygenated ring heterocycles that are important intermediates for the synthesis of various natural products and pharmaceutical agents.1,2 Among them, (+)-cacospongionolide B, a marine natural product has been shown to have potent anti-inflammatory activity in diseases such as asthma, psoriasis and rheumatoid arthritis (Figute 1).3 In addition, dihydropyrans are important intermediates for the synthesis of tetrahy- dropyrans and glycals, which in turn are important intermediates for the synthesis of carbohydrate compounds.4-12 Since dihydropyrans contain olefin functionality, they are also suitable for further functionalization such as epoxidation, dihydroxylation, and halogenation leading to the formation of multi-substituted ring systems.13-16 Prins cyclization between aldehydes and homopropargyl alcohols is a common method for the synthesis of dihydropyrans. However, the dihydropyrans can also be synthesized by the cyclization reaction between epoxides and homopropargyl alcohols and are the emerging method for dihydropyran synthesis.17-21 Since there are few methods for the cyclization of epoxides and homopropargyl alcohols in the literature, there is an opportunity to develop a better method with improved yield and reduced reaction time for dihydropyran synthesis. In particular, epoxides are more favorable starting materials compared to aldehydes because aldehydes undergo aerobic oxidation to form carboxylic acids.22 In recent years, Ytterbium trichloride has been shown to be a good Lewis acid for many organic reactions.23-25 In this study, we describe the improvement in terms of yield and reaction time reduction for the synthesis of dihydropyran derivatives from the cyclization reaction of epoxides and homopropargyl alcohol with ytterbium trichloride.
 |
Figure 1 Click here to View Figure |
Materials and Methods
The chemicals used for these reactions were purchased from Sigma-Aldrich and used as received. All epoxides were prepared from the relevant olefins according to the methods described in the literature. For TLC, silica gel 60 F254 coated on aluminium sheets was used and purchased from Merck. Silica gel with 60-120 meshes was used for column chromatography. 1HNMR and 13CNMR spectra were recorded on a Bruker 300-MHz NMR spectrometer using trimethylsilane as an internal standard on a δ-scale of parts per million. Mass spectra were recorded using a Waters G2- XS QTof mass spectrometer. IR Spectra were recorded using the Nexus 670 Thermo Nicolet Fourier transform infrared spectrometer
Results and Discussion
The reaction was studied by treating a mixture of styrene oxide and 3-butyn-1-ol with ytterbium trichloride in anhydrous dichloromethane at room temperature under a nitrogen atmosphere. The mixture is stirred at room temperature for 30 min. and the complete transformation of the products is found. After post-treatment, the crude product was separated by silica gel column chromatography, which separated the major product in 80% yield. The major product was determined as 3a from the spectral data and compared with the literature data.11 (Scheme 1).
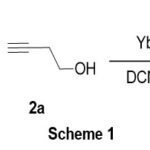 |
Scheme 1 |
The above result was encouraged us to explore the method on different combination of epoxides and homopropargyl alcohols. The reaction with all combinations gave corresponding 4-chloro dihydropyran derivatives in good yield in shorter reaction time. This demonstrates the wide applicability of this method for the synthesis of a variety of 4-chloro dihydropyrans using epoxides as starting materials (Table 1). However, slight variation in reaction time is observed with different combinations. It is observed that, reaction of homopropargylic alcohols with 1,2-dihydronaphthalene oxide, stilbene oxide and methylenecyclohexane oxide took longer reaction time with low yield compared to styrene oxide. The variation in reaction time may possibly depending on steric effects and stability of carbonium ion after epoxide ring opening.
 |
Table 1: YbCl3 mediated Prins_type reaction epoxide and homopropargylic alcohols |
The expected reaction mechanism for this cyclization may possibly Ytterbium trichloride mediated ring opening of epoxide to form alkoxy carbonuim ion, which is after rearrangement nucleophilic addition with alcohol produces dihydropyran carbonium species. The dihydropyran carbonium species is further undergoes nucleophilic addition with chloride nucleophile originated from Ytterbium trichloride to give 4-Chloro-dihydropyran derivative (Scheme 2).
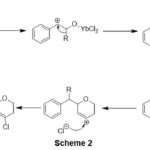 |
Scheme 2 |
Conclusion
In conclusion, we investigated a simple method for the synthesis of dihydropyran derivatives using Ytterbium trichloride. The method has the characteristics of short reaction time, clean reaction and high yield, and is a good alternative to the existing dihydropyrans synthesis method.
General Procedure
To a magnetically stirred mixture of styrene oxide (200 mg, 1.66 mmol) and 3-butyn-1-ol (117 mg, 1.66 mmol) in anhydrous dichloromethane (15 mL) was added Ytterbium trichloride (232 mg, 0.83 mmol) under a nitrogen atmosphere and stirred for 15 minutes at room temperature (25-30 oC). After 30 minutes, TLC analysis showed that the reaction was complete. The reaction mixture is then poured onto ice, extracted with dichloromethane and concentrated. The reaction mixture was purified by silica gel column chromatography and the product was eluted with ethyl acetate-hexane mixture (3:7) as mobile phase to obtain 275 mg of compound 3a (80% yield).
Acknowledgement
The authors thank the Department of Chemistry, Satavahana University for the cooperation and support.
Conflict of Interest
The authors declare no conflict of interest.
References
- Oishi, T.; Ohtsuka, Y. “Studies in Natural Products Synthesis”, ed., Elsevier, 1989, 3, Atta-ur-Rahman, Amsterdam, 73.
- Yet, L. Chem. Rev. 2000, 100, 2963-3008. https://doi.org/10.1021/ cr990407q
CrossRef - Atwood, K.C.; Marc L.S. J. Am. Chem. Soc. 2002, 124, 11584-11585. https://doi.org/10.1021/ja026899x.
CrossRef - Clarke, P. A. Tetrahedron 2011, 67, 4949. https://doi.org/10.1016/j. tet.2011.04.069.
CrossRef - Smoot, J. T.; Demchenko, A. V. Adv Carbohydr Chem Biochem. 2009, 62, 161-250. https://doi.org/10.1016/s0065-2318(09)00005-5.
CrossRef - Boltje, T. J.; Buskas, T.; Boons, G.-J. Nat. Chem. 2009, 1, 611-622. https://doi.org/10.1038/nchem.399.
CrossRef - Schmidt, R. R.; Vankar, Y. D. Acc. Chem. Res. 2008, 41, 1059-1073. https://doi.org/10.1021/ar7002495.
CrossRef - Ramesh, N. G.; Balasubramanian, K. K. Eur. J. Org. Chem. 2003, 4477-4487. https://doi.org/10.1002/ejoc.200300383.
CrossRef - Seeberger, P. H.; Bilodeau, M. T.; Danishefsky, S. J. Aldrichimica Acta. 1997, 30, 75-92.
- Yu, B.; Wang, L.-X.; Danishefsky, S.; Crich, D. Carbohydrate Synthesis towards Glycobiology. Ding, K.; Dai, L.-X., Eds.; Wiley-VCH: Weinheim, 2012.
CrossRef - Nicolaou, K. C.; Mitchell, H. J. Angew.Chem. Int. Ed. 2001, 40, 1576-1624. https://doi.org/10.1002/1521-3773(20010504)40:9%3C1576::AID-ANIE15760%3E3.0.CO;2-G.
CrossRef - Boivin, T. L. B. Tetrahedron. 1987, 43, 3309-3362. https://doi.org/ 10.1016/S0040-4020(01) 81626-4.
CrossRef - Coppi, L.; Ricci, A.; Tadei,M. J. Org. Chem. 1988, 53, 911-913. https://doi.org/10.1021/jo00239a053.
CrossRef - Zhang, W.-C.; Li, C.-J. Tetrahedron. 2000, 56, 2403-2411. https://doi.org/10.1016/S0040-4020(00)00152-6.
CrossRef - Schmidt, B.; Westhus, M. Tetrahedron. 2000, 56, 2421-2426. https://doi.org/10.1016/S0040-4020(00)00150-2.
CrossRef - Dobbs, A. P.; Martinovic, S. Tetrahedron Lett. 2002,43, 7055-7057. https://doi.org/10.1016/S0040-4039(02)01558-7.
CrossRef - Miranda, P.O.; Diaz, D.D.; Padron, J.I.; Bermejo, J.; Martin, V. S. Org. Lett. 2003, 5, 1979-1982. https://doi.org/10.1021/ol034568z.
CrossRef - Yu, C. M.; Shin, M.S.; Cho, E.Y. Bull. Korean Chem. Soc. 2004,25, 1625-1626. https://doi.org/10.5012/bkcs.2004.25.11.1625.
CrossRef - P. O. Miranda, R. M. Carballo, V. S. Martin and J. I. Padron, Organic Lett. 2009, 11, 357-360. https://doi.org/10.1021/ol802593u.
CrossRef - Yadav, J. S.; Rajasekhar, K.; Murty, M. S. R. Tetrahedron Lett. 2006, 47, 6149-6151. https://doi.org/10.1016/j.tetlet.2006.06.063.
CrossRef - Laurent, V.; Alain F.R.; Asma, A.; Philippe, R.; Bellefon, C. RSC Adv. 2013, 3, 18931-18937. https://doi.org/10.1039/C3RA42385A
CrossRef - Rao, G. B. D.; Kaushik, M. P. Tetrahedron Lett. 2011, 52, 5104-5106. https://doi.org/10.1016/J.TETLET.2011.07.108
CrossRef - Huang, J.; Yu, Y.; Hua, L. Chin. Sci. Bull. 2013, 58, 717-723. https://doi.org/10.1007/s11434-012-5631-z
CrossRef - Masamichi, Y.; Atsushi, N.; Masako, N. Org. Lett. 2000, 2, 159-161. https://doi.org/10.1021/ol991260s
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









