Effect of Li Electrolyte on Structural, Morphological and Thermal Properties of PVDF and PEG Polymer Blend Thin Films
K. Venkata Ramana1* , M. Chandra Shekar2
, M. Chandra Shekar2 , V. Madhusudhana Reddy3
, V. Madhusudhana Reddy3 , A.R. Subrahmanyam1, M. Ravindar Reddy1
, A.R. Subrahmanyam1, M. Ravindar Reddy1 and T. Ramesh Reddy1
and T. Ramesh Reddy1
1Department of Applied Sciences, Maturi Venkata Subba Rao Engineering College, Hyderabad, Telangana-501510.
2Department of Physics, JNT University, Hyderabad, Telangana-500085.
3Department of S and H, Malla Reddy college of Engineering and Technology, Hyderabad-500043.
Corresponding Author E-mail: kotharamana@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380216
Article Received on : 10-Feb-2022
Article Accepted on : 16-Mar-2022
Article Published : 23 Mar 2022
Reviewed by: Dr. Ioana Stanciu
Second Review by: Dr. Yodthong Baimark
Final Approval by: Dr. Haresh Thakellapalli
PVDF polymer, LiClO4 salt doped PVDF polymer electrolyte, PVDF/PEG polymer blend and LiClO4 salt doped PVDF/PEG polymer blend electrolyte thin films prepared via solution cast method further these prepared thin films undergone with different characterization techniques i.e. XRD, FTIR, SEM and DSC. XRD and SEM results confirmed that reduction in crystalline nature of PVDF polymer in the presence of PEG polymer and LiClO4 salt. FTIR spectra results revealed that complexation/interaction of LiClO4 salt with polymers. Shifting of glass transition temperature (Tg) and disappearance of melting temperature(Tm) on the DSC curves of PEG and LiClO4 salt doped thin films was observed, which indicated that thermal stability and reduction in crystalline nature of thin films. These results confirmed that LiClO4 salt doped PVDF/PEG thin films may offer higher ionic conductivity for the fabrication of electrochemical cell.
KEYWORDS:Amorphous Nature; Conductivity; LiClO4; PVDF; PEG
Download this article as:| Copy the following to cite this article: Ramana K. V, Sekhar A. M C, Reddy V. M, Subrahmanyam A. R, Reddy M. R, Reddy T. R. Effect of Li Electrolyte on Structural, Morphological and Thermal Properties of PVDF and PEG Polymer Blend Thin Films. Orient J Chem 2022;38(2). |
| Copy the following to cite this URL: Ramana K. V, Sekhar A. M. C, Reddy V. M, Subrahmanyam A. R, Reddy M. R, Reddy T. R. Effect of Li Electrolyte on Structural, Morphological and Thermal Properties of PVDF and PEG Polymer Blend Thin Films.Orient J Chem 2022;38(2). Available from: https://bit.ly/3quQFmP |
Introduction
Polymers and their composite materials are creating high demand in the electronic market i.e. batteries, computers, portable electronic televisions, telephones and etc 1. For this purpose some polymeric materials and their blends were used by many researchers such as PEO, PAN, PMMA and PVDF polymers 2-7, these polymers may offer strength, stability, conductivity, etc for electronic related applications. To modify the properties of polymers, blending of two or more polymers is one of the methods which also assist to modify conductivity 8-9 because enhancement in conductivity was important to use polymers and their blends in the said applications. The addition of electrolytes i.e. Li+, Na+, Mg+, etc to the polymers also enhance ionic conductivity. In the present study, PVDF and PEG polymers and LiClO4 salt were used for the electrolyte purpose. PVDF polymer itself crystalline in nature which not suitable for electrical applications but provides more strength to the electrochemical cell; hence addition of PEG and LiClO4 salt leads modification of crystalline nature to amorphous nature as a result increment in ionic conductivity may be achieved. To study the properties of polymers and salt doped polymers without difficulty which convert powder form to thin films by using some methods. PVDF, LiClO4 salt added PVDF, PVDF+PEG blend and LiClO4 salt added PVDF+PEG polymer blend thin films were prepared with the help of solution cast method which is the easiest and best method to prepare thin films, further modifications in properties of all prepared thin films were investigated by different characterization techniques.
Experimental
The two polymers i.e. PVDF (32000 MW), PEG (6000 MW) and LiClO4 (99% of purity) salt were purchased from Merck company. To dissolve polymers and salt THF solvent were used which purchased from local made. The solution cast method was obtained to prepare pure PVDF polymer, PVDF+PEG blend and LiClO4 salt doped polymer blend thin films 10-12 in which different compositions of PVDF, PEG polymers and LiClO4 salt were mixed together shown in Table 1.
Table 1: Compositions of PVDF, PEG polymers and LiClO4salt
|
Sample code |
wt.% |
||
|
PVDF |
PEG |
LiClO4 salt |
|
|
(a) |
100 |
0 |
0 |
|
(b) |
70 |
0 |
30 |
|
(c) |
70 |
30 |
0 |
|
(c) |
70 |
30 |
10 |
These polymers and salt were dissolved in DMF solvent and further stirred using magnetic stirrer until to get homogeneous solution. To evaporate excess solvent in homogeneous solution which was shifted to glass Petri dish and kept inside a heat Owen at temperature of 800C for 4 hours and further observed that a uniform thin film of thickness 1mm was produced. The prepared thin films were examined using different characterization techniques such as XRD, FTIR, SEM and DSC and the results were reported.
Results and Discussion
XRD
The results of XRD spectra of prepared thin films have been revealed that structural modification from one phase to another phase. The XRD spectra of all the prepared thin films were recorded at room temperature and shown in Fig.1. The sharp and high intense peak of pure PVDF was recorded at 2θ = 20.20 and other two small and sharp peaks also were recorded at 2θ = 18.50 and 2θ = 26.20 (Fig.1 (a)), these peaks related to crystalline phase of PVDF polymer 13-14. Even though characteristic peak of 2θ = 20.20 of PVDF thin film still present and remaining peaks were some disappeared and some were shifted with addition of LiClO4 salt and PEG polymer (Fig.1 (c-d)), which exposed that slight phase modification of PVDF thin film from crystalline to amorphous in the presence of LiClO4 salt and PEG polymer. The decrease in crystalline nature of PEG and LiClO4 salt presented PVDF leads towards better thermal stability and higher ionic conductivity.
 |
Figure 1: XRD spectra of (a) pure PVDF polymer, (b) PVDF+LiClO4 (70:30) salt doped polymer, (c) PVDF+PEG (70:30) polymer blend, (d) PVDF+PEG+LiClO4 (70:30:10) electrolyte thin films |
FTIR
FTIR spectroscopy is a useful technique to identify chemical groups and phase modification regions in the polymer materials 15-16. FTIR spectra results of prepared thin films from wave number range of 500cm-1 to 4000cm-1 as shown in Fig.2. The occurrence of vibrational bands corresponds to pure PVDF polymer 820cm-1 (β phase crystalline) i.e. CH2 rocking and 420cm-1, 790cm-1 and 1080cm-1, 1110cm-1 (α phase crystalline)17. The symmetric and asymmetric vibrational bands of CH2 bonds of PVDF polymer thin film were observed at 2995cm-1 and 3020cm-1 18. It was observed that some vibrational bands were shifted, some known peaks were disappeared and some new peaks were appeared by the addition of LiClO4 salt and PEG polymer to PVDF polymer, which indicated that interaction/complexation of polymers and salt and also evident that existence of amorphous nature in the presence of salt.
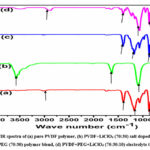 |
Figure 2: FTIR spectra of (a) pure PVDF polymer, (b) PVDF+LiClO4 (70:30) salt doped polymer, (c) PVDF+PEG (70:30) polymer blend, (d) PVDF+PEG+LiClO4 (70:30:10) electrolyte thin films |
SEM
The top surface SEM images of pure PVDF, PEG and LiClO4 salt doped polymer thin films shown in Fig.3. The surface in the SEM image of PVDF thin film appeared as rough and dominated with spherulitic crystalline structure 19 which also confirmed with XRD results(Fig.3(a)). With the addition of PEG and LiClO4 salt to PVDF polymer, appearance of pores and their sizes increases which established reduction in crystalline nature or increase in amorphous content of PVDF (Fig.3(b-d))20. This amorphous nature of polymer blend thin films create path for mobility of ions throughout the polymer chains which leads better ionic conductivity side 21.
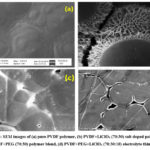 |
Figure 3: SEM images of (a) pure PVDF polymer, (b) PVDF+LiClO4 (70:30) salt doped polymer, (c) PVDF+PEG (70:30) polymer blend, (d) PVDF+PEG+LiClO4 (70:30:10) electrolyte thin films |
DSC
The variation in melting temperature(Tm) and glass transition temperature (Tg) of PVDF polymer thin film in the presence of PEG and LiClO4 salt has been investigated via DSC technique and DSC thermo grams of all prepared thin films shown in Fig.4. The glass transition temperature (Tg) of pure PVDF thin film observed at 1480C but inability of finding the melting temperature(Tm) on the curve which may be evident to the crystalline nature. It was observed that slight shifting of glass transition temperature (Tg) of PVDF towards lower temperature side with the addition of PEG and LiClO4 salt, which indicated that compatibility of polymers and interaction of salt with polymers 22. The disappearance of melting temperature(Tm) also, depicted that existence of amorphous nature by the presence of PEG and LiClO4 salt with PVDF polymer.
 |
Figure 4: DSC thermo grams of (a) pure PVDF polymer, (b) PVDF+LiClO4 (70:30) salt doped polymer, (c) PVDF+PEG (70:30) polymer blend, (d) PVDF+PEG+LiClO4 (70:30:10) electrolyte thin films |
Conclusion
Solution cast method was used to prepare pure PVDF polymer, LiClO4 salt doped PVDF polymer electrolyte, PVDF+PEG polymer blend and PEG, LiClO4 salt doped PVDF polymer blend electrolyte thin films. Modifications in different properties of prepared thin films were examined with the techniques of XRD, FTIR, SEM and DSC. XRD, SEM and DSC results confirmed that reduction in crystalline nature of PVDF polymer in the presence of PEG polymer and LiClO4 salt. FTIR spectra results revealed that complexation/interaction of LiClO4 salt with polymers. The reduction in crystalline nature or increase in amorphous nature of PVDF polymer in the presence of PEG polymer and LiClO4 salt may be provide better ionic conductivity for the Li ion batteries and further work is in progress will be reported.
Acknowledgement
The authors are expressing gratitude to Maturi Venkata Subba Rao Engineering College and JNTU, Hyderabad for their support to carry out this research work.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Sources
There is no funding source.
References
- Jian-hua, Cao; Bao-ku, Zhu; Dan-ying, Zuo; You-yi, Xu; Ji-ding, Li. Chinese Journal of Polymer Science. 2008, 26, 13−21.
CrossRef - Hayamizu, K.; Aihara, Y.; Arai, S.; Price, W.S. Solid State Ionics. 1998, 107: 1.
CrossRef - Huang, B.Y.; Wang, Z.X.; Li, G.B.; Huang, H.; Xue, R.J.; Chen, L.Q.; Wang, F.S. Solid State Ionics. 1996, 85, 79.
CrossRef - Appetecchi, G.B.; Croce, F.; Scrosati, B. Electrochim. Acta. 1995, 40, 991.
CrossRef - Wang, H.P.; Huang, H.T.; Stephanie, L.W. J. Electrochem. Soc. 2000, 147, 2853.
CrossRef - Prosini, P.P.; Villano, P.; Carewska, M. Electrochim. Acta. 2002, 48, 227.
CrossRef - Magistris, A.; Quartarone, E.; Ustarelli, P.; Saito, Y.; Kataoka, H. Solid State Ionics. 2002, 347, 152-153.
CrossRef - Abdelrazek, E.M.; Elashmawi, I. S.; Labeeb, S. Physica B, 2010, 405, 2021-2027.
CrossRef - Lee, L.; Park, S.J.; Kim, S. Solid State Ionics. 2013, 234, 19-24.
CrossRef - Venkata Ramana, K.; Chandra Shekar, M.; Subrahmanyam, A.R.; Ravindar Reddy, M.; Madhusudhana Reddy, V. Int. J. Emer. Tech. Adv. Engg. 2017, 7, 484.
- Venkata Ramana, K.; Subrahmanyam, A.R.; Bhanu Prasad, B.; Ravinder Reddy, M.; Madhusudana Reddy, V.; Chandra Sekhar, M. Int. J. Sci. & Tech. Res. 2020, 9, 2607.
- Ravindar Reddy, M.; Anna Mallikarjun.; Jaipal Reddy, M.; Subrahmanyam, A.R.; Vikranth Reddy, M. J Polym Eng. 2021, 41, 654–659.
CrossRef - Sabah Salman, A.; Farah Noori, T.M.; AwS Mohammed, K. International Journal of Applied Engineering Research, Volume. 2018, 13, 5008-5013.
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Progress in polymer science. 2014, 39, 683-706.
CrossRef - Bauman, R.P. absorption Spectroscopy, New York, John Wiley and Sons, Inc. 1962.
- Brugel, W. An introduction to Infrared spectroscopy, London: Methuen and Co Ltd. 1962.
- Gregorio, R.; Borges, D.S. Polymer. 2008, 49, 1–8.
CrossRef - Ramazanov, M.A.; Maharramov, A.M.; Shirinova, H.A.; Luca Di Palma.Journal of thermoplastic composite materials. 2020, 33, 138-149.
CrossRef - Gaurav Mago.; Dilhan Kalyon, M.; Frank Fisher, T. Nanomechanics and Nanostructured Multifunctional Materials: Experiments, Theories, and Simulations. Special issue, 2008.
CrossRef - Sangeetha Mahendrakar.; Mallikarjun Anna.; Siva Kumar, J.; Jaipal Reddy, M. International Journal of Applied Chemistry. 2017, 13, 477-490.
- Ulaganatham, M.; Sunder Pethaiah, S.; Rajendran, S. Mater. Chem. Phys. 2011, 129, 471-476.
CrossRef - Blonsky, P.M.; Shriver, D.F.; Austin, P.; Allock, H.R. Solid State Ionics. 1986, 258, 18-19
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









