Thermodynamic and Acoustical Study of 1-(4-Fluorophenyl)-3-(napthalen-1-yl)-2-propen-1-one
Department of Chemistry, Sri Dharmasthala Manjunatheshwara College (Autonomous), Ujire- 574240, India.
Corresponding Author E-mail: sowmyakalai@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380123
Article Received on : 23-Aug-2021
Article Accepted on : 10-Jan-2022
Article Published : 14 Jan 2022
Reviewed by: Dr. Jamshidkhan Chamani
Second Review by: Dr. Munther Abdul-Jaleel Mohammed-Ali
Final Approval by: Dr. S. A. Iqbal
The various concentrations (ranging from 0.01M to 0.1 M) of synthesized 1-(4-fluorophenyl)-3-(napthalen-1-yl)-2-propen-1-one1 (4FNP) solutions in DMF were prepared. Density (ρ), viscosity (η) and ultrasonic velocity (U) measurements of pure solvent and solutions were carried out at 288.15 K, 293.15 K, 298.15 K and 303.15 K. The observed values of ρ, η and U were found at 298.15K in the range of 944.50-954.25 kg.m-3, 0.8440 x10-3-1.4195 x10-3 Nm-2s and 1454-1513 ms-1 respectively. The values of η and U decreased with increase of temperature, reveals the weakening of intermolecular forces between the molecules at higher temparature. Calculated thermodynamic parameters such as free volume, Gibbs free energy and internal pressures were interpreted in terms solute-solvent interaction. Increase of Gibbs free energy with increase of concentration clearly indicates the strong interaction between solvent and solute molecules. Decrease of free volume with increase in mole fraction gives evidence of strong intermolecular interaction in solution. Positive excess values of internal pressure represent the presence of dispersive forces between molecules.
KEYWORDS:Acoustical Parameters; Chalcone; Gibb’s free Energy; Ultrasonic Velocity; Viscosity
Download this article as:| Copy the following to cite this article: Sowmya B. P. Thermodynamic and Acoustical Study of 1-(4-Fluorophenyl)-3-(napthalen-1-yl)-2-propen-1-one. Orient J Chem 2022;38(1). |
| Copy the following to cite this URL: Sowmya B. P. Thermodynamic and Acoustical Study of 1-(4-Fluorophenyl)-3-(napthalen-1-yl)-2-propen-1-one. Orient J Chem 2022;38(1).Available from: https://bit.ly/33yKpBQ |
Introduction
Chalcones are α, β unsaturated ketones that exhibits different activities includes antimalaria 2, antitumor 3, antiviral 4, antidiabetic 5, antituberculosis 6, anticancer 7, anti-urease inhibitory 8, and others. It is found that the chalcones derived from alpha-naphthyl generally exhibit more potent anticancer activity than those of beta-naphthyl. Assessment of series of naphthalene derivatives exhibit antidepressant, anti- inflammatory and analgesic effects1. The studies showed that naphthalene derivatives used as a potent antimicrobial agent against different human pathogens 9. Further, many studies revealed that fluorine substituted drug leads alter the physical properties and binding characteristics 10. Also, fluorine substituted chalcones exhibited maximum antifungal, antibacterial and antitubercular activities 11.
The study of solute-solvent interaction plays important role in medicinal chemistry and ultrasonic interferometric measurement helps to calculate the parameters related to solute- solvent interaction 12. Also, it gives information about complex formation and molecular association of solute in solution 13–14. Ultrasonic parameters are directly linked with different thermodynamic quantities which help to study the interaction of solute in solution. The values of acoustical parameters as a function of concentration will be of much help in providing about the types of molecular interactions. In order to understand the kind of interaction between solute and solvent, it is of interest to calculate the acoustical parameters.
In this study density, viscosity and ultrasonic velocity measurement of 1-(fluoro)-3-(napthalen-1-yl)-2-propen-1-one (4FNP) in dimethylformamide (DMF) were carried out at 288.15 K, 293.15 K, 298.15 K and 303.15 K. The aim of carrying out this research is to study the drug suitability by studying the solute solvent interaction by viscometry and interferometric methods. The calculation of acoustical parameters in terms of concentration give information about molecular interactions in solution. Intermolecular forces between the molecules can be predicted by measuring ultrasound velocity, density and viscosity of solution of 4FNP in DMF at different temperatures.
Materials and Methods
Synthesis of 1-(4-fluorophenyl)-3-(napthalen-1-yl)-2-propen-1-one
To a mixture of 4-fluoroacetophenone (0.01 mol) and 1-naphthaldehyde (0.01 mol) in ethanol (50 mL), 15 mL of 10% sodium hydroxide solution was added dropwise. The mixture was allowed to stand at 60oC with stirring for 6 to 8 hrs. The reaction mixture was kept for overnight and acidified with glacial acetic acid. The precipitated yellow solids were collected by filtration, washed with distilled water and recrystallized from DMF. The completion of reaction was checked by TLC analysis and the products were visualized by UV detection. (Yellow solid, yield: 60% and mp: 84-85oC)1,15 . The reaction scheme of 4FNP is shown in Fig.1
 |
Figure 1: Reaction scheme of synthesis of 4FNP. |
Spectral Characterisation
1H NMR spectrum of 4FNP was measured in CDCl3 on an Agilent 400 MHz NMR spectrometer is shown in Fig.2.
 |
Figure 2: 1H NMR spectrum of 4FNP |
1H NMR (400 MHz, CDCl3, delta, ppm)
6.997-7.019 (2H, d, H-1, J=8.8 Hz), 7.506-7.594 (4H, m, H-3, H-6, H-9 & H-10), 7.887-7.906 (3H, d, H-8 & H-5, J=7.6 Hz), 8.089-8.111 (2H, d, H-2, J=8.8 Hz), 8.266-8.286 (1H, d, H-7, J=8 Hz), 8.638-8.677 (2H, d, H-4, J=15.6Hz)
Study of Acoustical Parameters16
Single crystal interferometer has been used for the measurement of ultrasonic velocities. Pycnometer and Ubbelohde viscometer have been used for the measurement of density and viscosity respectively. Using the data obtained from the experiment, various acoustical parameters are calculated in different solutions. The purified and AR grade DMF used as a solvent in present work. The calculations of acoustical properties require the measurements of ultrasonic velocity (U), viscosity (η) and density (ρ).
Density Measurements
The weight of distilled water, DMF and different concentrated solutions of 4FNP in DMF were measured at 288.15 K, 293.15 K, 298.15 K and 303.15 K by using pycnometer. The density (ρ) of different concentrated solutions were determined by using following equation (1).

where w & w0 are weight of solution & water respectively and ρo is the density of water17.
Viscosity Measurements
10 mL of the test solution was pipetted and introduced to the Ubbelohde viscometer. The Ubbelohde viscometer was suspended in a viscometer bath at specific temperature. The time of flow for DMF and different concentrated solution of 4FNP were measured at 288.15 K, 293.15 K, 298.15 K and 303.15 K. The viscosity of solutions was determined using equation (2).

where t, to and ηo are the flow time of solution, time of solvent and viscosity of DMF18 respectively.
Sound Velocity Measurement
Sound velocity of test solution was determined using Ultrasonic interferometer. Mittal Ultrasonic interferometer of Model No. F-81 was used for the study. The measuring cell consists of quartz crystal and micrometre was filled with 2mL of solvent / solution. The temperature of DMF / different concentrated solution of 4FNP in the cell was maintained by water circulation from the thermostat. Maximum/minimum of anode current (n) was obtained by slow rotation of micrometre. The wave length (λ) and sound velocity (U) were calculated using the equations (3) & (4).
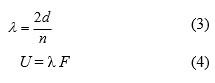
where, F= 3 x 106 Hertz.
The following thermodynamic parameters are calculated by using following standard expressions19
Isentropic compressibility20 (β):

Intermolecular free path length20 (Lf):

where KJ =2.0965 X 10-6is known as Jacobson constant
Acoustic impedance (Z):
Z= U x ρ (7)
Relaxation Strength (r):

where U∞ = 1.6 x 105 cm.s-1
Molar compressibility (W):

The apparent molecular weight (M) of the solution can be calculated as16:
M = M1 X1 +M2 X2 (10)
Apparent molar volume (ɸV)
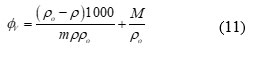
Apparent molar compressibility (ɸβ)
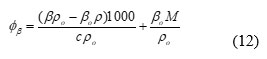
where c is the molar concentration of solute and M is the molar mass of the solute.
Solvation number (Sn)

Free Volume (Vf)

Internal Pressure (π)
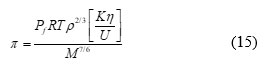
where Pf is the packing factor (=2), η is viscosity of solution and K is a constant (K =4.28 x 109)
Gibb’s Free Energy (∆G)
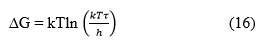
where τ is the viscous relaxation time
Results and Discussion
The density (ρ), viscosity (η) and sound velocity (U) of pure DMF and solutions of 4FNP in DMF were determined experimentally at 288.15 K, 293.15 K, 298.15 K and 303.15 K and are given in Table 1. It is observed that ultrasound velocity, density and viscosity were decreases when the temperature increased and also ultrasound velocity, density and viscosity were increases with increase of concentrations of solution and are shown in Fig 3 & 4. Decrease of Ultrasound velocity and viscosity with increase of temperature reveals the weakening of intermolecular forces between the molecules. So molecular interaction between the molecules decreases.
Table 1: The density (ρ), ultrasonic velocity (U) and viscosity (η) of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K.
|
Conc. (M) |
ρ |
U |
η x 10-3 |
Conc. (M) |
ρ (kg.m-3) |
U (m.s-1) |
η x 10-3 (Nm-2s) |
|
T= 288.15 K |
T= 293.15 K |
||||||
|
0.00 |
954.20 |
1478 |
0.940 |
0.00 |
949.40 |
1460 |
0.8920 |
|
0.01 |
955.53 |
1491 |
1.0087 |
0.01 |
950.42 |
1473 |
0.9564 |
|
0.02 |
957.85 |
1504 |
1.0941 |
0.02 |
952.74 |
1489 |
1.0331 |
|
0.04 |
959.36 |
1517 |
1.2288 |
0.04 |
954.25 |
1503 |
1.1501 |
|
0.06 |
960.91 |
1529 |
1.3652 |
0.06 |
955.82 |
1515 |
1.2592 |
|
0.08 |
962.21 |
1539 |
1.4992 |
0.08 |
957.21 |
1527 |
1.3816 |
|
0.10 |
964.12 |
1551 |
1.6311 |
0.10 |
959.23 |
1539 |
1.5186 |
|
T= 298.15 K |
T= 303.15 K |
||||||
|
0.00 |
944.50 |
1454 |
0.8440 |
0.00 |
939.80 |
1447 |
0.8090 |
|
0.01 |
946.32 |
1462 |
0.8811 |
0.01 |
940.19 |
1454 |
0.8409 |
|
0.02 |
947.62 |
1471 |
0.9475 |
0.02 |
942.49 |
1461 |
0.9220 |
|
0.04 |
949.16 |
1482 |
1.0676 |
0.04 |
944.01 |
1470 |
1.0161 |
|
0.06 |
950.71 |
1493 |
1.1822 |
0.06 |
945.56 |
1482 |
1.1044 |
|
0.08 |
952.25 |
1505 |
1.3032 |
0.08 |
947.08 |
1495 |
1.1954 |
|
0.10 |
954.25 |
1513 |
1.4192 |
0.10 |
949.14 |
1507 |
1.2911 |
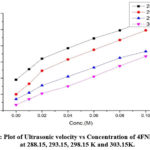 |
Figure 3: Plot of Ultrasonic velocity vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
 |
Figure 4: Plot of Viscosity vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
Different acoustical parameters were calculated from these experimental data using equations (1) to (16) at 288.15 K, 293.15 K, 298.15 K and 303.15 K. The calculated acoustical parameters are given in Table 2 and shown in Fig. 5- Fig.10
The variation of isentropic compressibility (β) and the intermolecular free length (Lf) with concentration in DMF at different temperatures are shown in Fig. 5 and Fig. 6. It depicts that both isentropic compressibility (β) and the intermolecular free length (Lf) decreases continuously with increase of concentration of solutions and the same increases with rise in temperature. The decrease of Lf indicates that there is strong interaction between solvent and solute molecules. Fig. 7 shows variation of molar compressibility (W) with concentration at different temperatures. The increase of W with concentration indicates that there is an increase of molecular interaction. Free volume (Vf) decreases with increase in mole fraction indicates intermolecular interaction seems to be stronger than the intramolecular interaction shown in Fig. 8. Variation of Internal Pressure (π) and Gibbs free energy (∆G) with increase of concentration at different temperatures are shown in Fig. 9 and Fig. 10. Gibbs free energy increases with increase in concentration of solution. This gives evidence for the strong interaction between solvent and solute molecules. The increase in Gibbs free energy also suggests shorter time for rearrangement of the solute molecules in the solution. Positive excess values of internal pressure represent the presence of dispersive forces between molecules.
Table 2: Various Acoustical parameters of 4FNP at 298.15K.
|
Conc. (M) |
β x 10-10 m2N-1 |
Lf x10-11 (m) |
Z x 10-6 (Nm-2) |
r |
Wx10-3 |
Vf x10-3 (m3mol-1) |
π x 103 (atm) |
∆Gx10-21 (kJmol-1) |
ɸV (m3mol-1) |
ɸβ (m2N-1) |
Sn |
|
T= 288.15 K |
|||||||||||
|
0.00 |
4.7975 |
4.5920 |
1.4103 |
0.1467 |
7.4239 |
4.3918 |
1.6799 |
0.5420 |
– |
– |
– |
|
0.01 |
4.7076 |
4.5488 |
1.4247 |
0.1316 |
7.4506 |
4.0198 |
1.7285 |
0.5633 |
3.3886 |
-64.3037 |
-37.5194 |
|
0.02 |
4.6154 |
4.5040 |
1.4406 |
0.1164 |
7.4700 |
3.6200 |
1.7896 |
0.5886 |
3.3722 |
-23.2423 |
-13.5612 |
|
0.04 |
4.5295 |
4.4619 |
1.4553 |
0.1011 |
7.4751 |
3.1065 |
1.8782 |
0.6286 |
3.3616 |
-7.8314 |
-4.5694 |
|
0.06 |
4.4515 |
4.4233 |
1.4692 |
0.0868 |
7.4815 |
2.7065 |
1.9615 |
0.6648 |
3.3507 |
-0.8141 |
-0.4750 |
|
0.08 |
4.3879 |
4.3916 |
1.4808 |
0.0748 |
7.4818 |
2.3943 |
2.0378 |
0.6974 |
3.3417 |
0.8880 |
0.5181 |
|
0.10 |
4.3117 |
4.3533 |
1.4954 |
0.0603 |
7.4907 |
2.1519 |
2.1068 |
0.7249 |
3.3285 |
1.9411 |
1.1326 |
|
T= 293.15 K |
|||||||||||
|
0.00 |
4.9413 |
4.6603 |
0.1673 |
1.3861 |
7.3926 |
4.6645 |
1.6409 |
0.5326 |
– |
– |
– |
|
0.01 |
4.8493 |
4.6167 |
0.1524 |
1.4000 |
7.4191 |
4.2755 |
1.6873 |
0.5536 |
3.4251 |
-63.8108 |
-37.0446 |
|
0.02 |
4.7341 |
4.5616 |
0.1339 |
1.4186 |
7.4430 |
3.8865 |
1.7415 |
0.5754 |
3.4084 |
-23.0394 |
-13.3752 |
|
0.04 |
4.6390 |
4.5155 |
0.1176 |
1.4342 |
7.4496 |
3.3834 |
1.8190 |
0.6112 |
3.3977 |
-7.7457 |
-4.4967 |
|
0.06 |
4.5583 |
4.4760 |
0.1034 |
1.4481 |
7.4562 |
3.0136 |
1.8858 |
0.6413 |
3.3865 |
-0.7786 |
-0.4520 |
|
0.08 |
4.4804 |
4.4376 |
0.0892 |
1.4617 |
7.4595 |
2.6750 |
1.9570 |
0.6723 |
3.3767 |
0.9028 |
0.5241 |
|
0.10 |
4.4015 |
4.3984 |
0.0748 |
1.4763 |
7.4687 |
2.3677 |
2.0339 |
0.7039 |
3.3625 |
1.9422 |
1.1275 |
|
T= 298.15 K |
|||||||||||
|
0.00 |
5.0081 |
4.6917 |
0.1742 |
1.3733 |
7.3784 |
5.0368 |
1.5940 |
0.5040 |
– |
– |
– |
|
0.01 |
4.9439 |
4.6615 |
0.1651 |
1.3835 |
7.3987 |
4.7810 |
1.6209 |
0.5158 |
3.4549 |
-63.8965 |
-36.9028 |
|
0.02 |
4.8769 |
4.6298 |
0.1547 |
1.3940 |
7.4114 |
4.3454 |
1.6718 |
0.5487 |
3.4454 |
-22.8585 |
-13.2017 |
|
0.04 |
4.7969 |
4.5917 |
0.1421 |
1.4067 |
7.4141 |
3.7041 |
1.7587 |
0.5829 |
3.4342 |
-7.6756 |
-4.4330 |
|
0.06 |
4.7188 |
4.5542 |
0.1293 |
1.4194 |
7.4194 |
3.2406 |
1.8341 |
0.6099 |
3.4230 |
-0.7499 |
-0.4331 |
|
0.08 |
4.6363 |
4.5142 |
0.1152 |
1.4331 |
7.4232 |
2.8571 |
1.9079 |
0.6346 |
3.4119 |
0.9083 |
0.5246 |
|
0.10 |
4.5778 |
4.4857 |
0.1058 |
1.4438 |
7.4269 |
2.5545 |
1.9762 |
0.6588 |
3.3977 |
1.9428 |
1.1220 |
|
T= 303.15 K |
|||||||||||
|
0.00 |
5.0819 |
4.7262 |
0.1821 |
1.3599 |
7.3630 |
5.3284 |
1.5591 |
0.4865 |
– |
– |
– |
|
0.01 |
5.0310 |
4.7024 |
0.1742 |
1.3670 |
7.3802 |
5.0860 |
1.5810 |
0.4951 |
3.5000 |
-62.8200 |
-36.1005 |
|
0.02 |
4.9708 |
4.6742 |
0.1662 |
1.3770 |
7.3913 |
4.4804 |
1.6489 |
0.5248 |
3.4830 |
-22.6258 |
-13.0023 |
|
0.04 |
4.9022 |
4.6418 |
0.1559 |
1.3877 |
7.3911 |
3.9411 |
1.7164 |
0.5571 |
3.4718 |
-7.5716 |
-4.3512 |
|
0.06 |
4.8152 |
4.6005 |
0.1421 |
1.4013 |
7.3980 |
3.5496 |
1.7728 |
0.5842 |
3.4604 |
-0.7007 |
-0.4027 |
|
0.08 |
4.7242 |
4.5568 |
0.1269 |
1.4159 |
7.4033 |
3.2197 |
1.8268 |
0.6107 |
3.4493 |
0.9410 |
0.5408 |
|
0.10 |
4.6392 |
4.5156 |
0.1129 |
1.4304 |
7.4128 |
2.9265 |
1.8819 |
0.6351 |
3.4344 |
1.9607 |
1.1268 |
 |
Figure 5: Plot of Isentropic compressibility vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
 |
Figure 6: Plot of intermolecular free length (Lf) vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
 |
Figure 7: Plot of Molar compressibility (W) vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
 |
Figure 8: Plot of Free volume (Vf) vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
 |
Figure 9: Plot of Gibbs free energy (∆G) vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
 |
Figure 10: Plot of Internal Pressure (π) vs Concentration of 4FNP in DMF at 288.15, 293.15, 298.15 K and 303.15K. |
Acknowledgement
This research was supported by Vision Group of Science & Technology, Govt. of Karnataka and University Grant Commission, Government of India.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding source.
References
- Jin, Q.-H.; Chen, H.-H.; Chen, W.-B.; Fu, Z.-Y.; Guan; Jiang, H.-Y. Synthesis and Biological Effects of Naphthalene-Chalcone Derivatives. Med. Chem. Res. 2020, 29 (2), 1–10. https://doi.org/10.1007/s00044-020-02525-4.
CrossRef - Gutteridge, C. E.; Nichols, D. A.; Curtis, S. M.; Thota, D. S.; Vo, J. V.; Gerena, L.; Montip, G.; Gettayacamin, C. O.; Diaz, D. S.; DiTusa, C. A.; Smith, K. S.; Bhattacharjee, A. K. In Vitro and in Vivo Efficacy and in Vitro Metabolism of 1-Phenyl-3-Aryl-2-Propen-1-Ones against Plasmodium Falciparum. Bioorg. Med. Chem. Lett. 2016, 16, 5682–5686. https://doi.org/10.1016/j.bmcl.2006.08.009.
CrossRef - Yuan, J.-W.; Wang, S.-F.; Luo, Z.-L.; Qiu, H.-Y.; Wang, P.-F.; Zhang, X.; Yang, Y.-A.; Yin, Y.; Zhang, F.; Zhu, H.-L. Synthesis and Biological Evaluation of Compounds Which Contain Pyrazole, Thiazole and Naphthalene Ring as Antitumor Agents. Bioorg. Med. Chem. Lett. 2014, 24, 2324–2328. https://doi.org/10.1016/j.bmcl.2014.03.072.
CrossRef - Zhou, D.; Xie, D.; He, F.; Song, B.; Hu, D. Antiviral Properties and Interaction of Novel Chalcone Derivatives Containing a Purine and Benzenesulfonamide Moiety. Bioorg. Med. Chem. Lett. 2018, 28, 2091–2097. https://doi.org/doi.org/10.1016/j.bmcl.2018.04.042.
CrossRef - Rocha, S.; Ribeiro, D.; Fernandes, E.; Freitas, M. A Systematic Review on Anti-Diabetic Properties of Chalcones. Curr. Med. Chem. 2020, 27 (14), 2257–2321.
CrossRef - Ahmada, I.; Thakur, J. P.; Chanda, D.; Saikia, D.; Khan, F.; Dixit, S.; Kumar, A.; Konwar, R.; Negi, A. S.; Gupta, A. Syntheses of Lipophilic Chalcones and Their Conformationally Restricted Analogues as Antitubercular Agents. Bioorg. Med. Chem. Lett. 2013, 23, 1322–1325. https://doi.org/10.1016/j.bmcl.2012.12.096.
CrossRef - Yan, X.-Q.; Wang, Z.-C.; Qi, P.; Li, G. Design, Synthesis and Biological Evaluation of 2-H Pyrazole Derivatives Containing Morpholine Moieties as Highly Potent Small Molecule Inhibitors of APCeAsef Interaction. Eur. J. Med. Chem. 2019, 177, 425–447. https://doi.org/10.1016/j.ejmech.2019.05.056.
CrossRef - Mermer, A. Microwave- and Ultrasound-Promoted Greener Synthesis of Thiazolylpyrazoline Derivatives and Investigation of Their Biological Activities. JOTCSA 20AD, 7 (1), 25–36. https://doi.org/doi.org/10.18596/jotcsa.563286.
CrossRef - Huang, M.-H.; Wu, S.-N. W.; Wang, J.-P.; Lin, C.-H.; Lu, S.-I.; Liao, L.-F.; Shen, A.-Y. Biological Study of Naphthalene Derivatives with Antiinflammatory Activities. DRUG Dev. Res. 2003, 60, 261–269. https://doi.org/10.1002/ddr.10327.
CrossRef - Hagmann, W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51 (15), 4361–4369.
CrossRef - Burmaoglu, S.; Algul, O.; Gobek, A.; Anil, D. A.; Ulger, M.; Erturk, B. G.; Kaplan, E.; Dogen, A.; Aslan, G. Design of Potent Fluoro-Substituted Chalcones as Antimicrobial Agents. J. ENZYME Inhib. Med. Chem. 2017, 32 (1), 490–495. https://doi.org/10.1080/14756366.2016.1265517.
CrossRef - Abbot, V.; Sharma, P. Thermodynamic and Acoustic Studies of Quercetin with Sodium Dodecyl Sulfate in Hydro-Ethanolic Solvent Systems: A Flavonoid-Surfactant Interaction Study. Chem. Phys. 2020, 538, 110921. https://doi.org/10.1016/j.chemphys.2020.110921.
CrossRef - Baluja, S.; Shipra, P. Acoustical Studies of Some Chalcones in DMF and DMSO Solutions. Int. Lett. Chem. Phys. Astron. 2015, 44, 130–140.
CrossRef - Palani, R.; Geetha, A. Acoustical and Excess Thermodynamic Studies of Molecular Interaction in Aqueous Mixed Solvent Systems at 303, 308 and 313K. Phys. Chem. Liq. 2009, 47 (5), 542–552.
CrossRef - Holla, B. S.; Kalluraya, B.; Sridhar, K. R. Synthesis of Some 1-Aryl-3-(5- Nitro-2- Furyl)- 2-Propen- 1-Ones as Potential Antibacterial Agents. Curr. Sci. 1987, 56, 585–588.
- Nayan J, V. Studies of Some Bioactive Heterocyclic Entities, Saurashtra University, Rajkot, Gujarath, 2009.
- Narjes Mirheydari, S.; Barzegar-Jalali, M.; Golmohamadi, B.; Shekaari, H.; Martinez, F.; Jouyban, A. Density, Speed of Sound, and Viscosity of Diethylene Glycol Monoethyl Ether + N,N-Dimethylformamide (Ethanol, Water) at T = 288.15–318.15 K. J Chem Eng Data 2019, 64 (4), 1425–1436. https://doi.org/10.1021/acs.jced.8b01012.
CrossRef - Bernal-García, J. M.; Guzmán-López, A.; Cabrales-Torres, A.; Estrada-Baltazar, A.; Iglesias-Silva, G. A. Densities and Viscosities of (N,N-Dimethylformamide + Water) at Atmospheric Pressure from (283.15 to 353.15) K. J Chem Eng Data 2008, 53, 1024–1027. https://doi.org/10.1021/je700671t CCC: $40.75.
CrossRef - Vigoureux, P. Ultrasonics; Chapman and Hall: London, 1952.
CrossRef - Ana P.V., E.; Nieves M.C., T.-P.; Abel G.M., F.; Jaime B., S.; Mário J., S.; Zaida L., A.; Isabel M.A., F. Speed of Sound and Derived Thermodynamic Properties of Glycerol. J. Chem. Thermodyn. 2021, 156, 106367. https://doi.org/10.1016/j.jct.2020.106367.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










