Congo Red and Nigrosine Dye Degradation using Dimethyl Dioxirane as an Oxidising Agent
Department of Chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Trichy-2.
Corresponding Author E-mail: vinoyoga61805@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370529
Article Received on : 20-Mar-2021
Article Accepted on :
Article Published : 28 Oct 2021
Reviewed by: Dr. Mayank M Dalal

Second Review by: Dr. Khalid Siraj

Final Approval by: Dr. Ioana Stanciu
The advanced oxidation of Congo red (CR) and Nigrosine (NI) using the combined action of dimethyl dioxirane as an oxidising agent is described in this study. The effects of several parameters, such as the concentration of the oxidising agent, the initial dye concentration, and the pH, have been investigated. At room temperature, the oxidising agent dimethyl dioxirane was employed to test the degradation of CR and NI dyes. On the degradation efficiency of CR and NI, pH’s effects, oxidising agent, and initial dye concentration were examined. The absorbance of CR and NI dyes before and after degradation was measured using UV-visible spectroscopy. The functional group existing in the dyes before and after degradation was determined using FT-IR spectroscopy.
KEYWORDS:Advance Oxidation Process; Congo Red; Dye concentration; Nigrosine; Oxidizing agent; pH
Download this article as:| Copy the following to cite this article: Vinotha S, Rose A. L. Congo Red and Nigrosine Dye Degradation using Dimethyl Dioxirane as an Oxidising Agent. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Vinotha S, Rose A. L. Congo Red and Nigrosine Dye Degradation using Dimethyl Dioxirane as an Oxidising Agent. Orient J Chem 2021;37(5). Available from: https://bit.ly/3GxqH8A |
Introduction
New, sophisticated, and low-cost ways for dealing with commercial wastewater are needed. On a variety of polluting chemical agents that are mostly found in wastewater originating from the industrial region, various separation, degradation, and eradication strategies are applied. To mention a few, there are aromatic and halogenated substances 1,6, thiols2, acids3, alcohols4, dyes1,5 and insecticides. Some physical7,8, biological9-11, and chemical12-14 approaches have been successful in the treatment and control of specific types of pollutants. Some pollutants, on the other hand, cannot be reduced using the methods outlined above but they’re well or have been largely degraded to less harmful molecules. Along with its efficacy and cost-effectiveness, and also the fact that it allows for the complete breakdown of organic contaminants into CO2 and inorganic acids, it is becoming increasingly popular, an advanced oxidation method1-6,15,16 is frequently utilised to treat such contaminants.
Nigrosine dye is a mixture of fake dark colours (CI 50415, Solvent dark 5) made by warming a mixture of aniline, nitrobenzene, and aniline hydrochloride in a copper or iron-like atmosphere. The benefit of sulfonating nigrosine dye is that it retains its ability to dissolve in water as an anionic dye. The primary mechanism for nigrosine dye production is marker-pen ink and stain colourant The nigrosine dye, like the container holding parasite and Cryptococcus neoformans 8,11, is used in science for negative staining microorganisms. In Figure 1a, the chemical structure of nigrosine is depicted.
 |
Figure 1: a. Chemical structure of nigrosine. |
An azo dye (congo red) produced from the sodium salt of 3,3′- ([1,1′- biphenyl]-4,4′- diyl) bis ([1,1′- biphenyl]-4,4′- diyl) bis ([1,1′- biphenyl]-4,4′- diyl) bis ([1,1′- biphen (4-aminonaphthalene-1-sulfonic destructive). Although azo dye dissolves in water and generates a red colloidal arrangement, its dissolvability is more apparent in natural solvents. Regardless, for its cancer-causing qualities, azo dye has been banned for a elongated time. The chemical structure of azo dye is shown in Figure 1b.
 |
Figure 1: b. Chemical structure of congo red |
The current paper focuses on advanced oxidation of azo dye and nigrosine dyes to improve the effectiveness of this AOP by adding low dimethyl dioxirane concentrations and producing a nontoxic colourless effluent in a short amount of time. The substrate was a brightly coloured solution of azo dye and nigrosine dyes with optical absorbance at 498 and 570 nm. Various parameters have been explored, including oxidant concentration, starting dye concentration, and pH. This method is both inexpensive and environmentally beneficial. These dyes are widely used in the textile industry, and we can reduce pollution by employing this oxidation approach.
Materials and Methods
All the chemicals used in this experiment were of analytical grade. The potassium peroxy monosulfate was purchased from TCI Chemicals (purity 99%). The acetone and sodium bicarbonate was purchased from Pure Chems and Merck. Without additional purification, all compounds are used in their natural state. The pH of the solution was measured using Elico Digital pH meter. The Dye used for representing organic dyes is Congo red dye (molecular formula C32H22N6Na2O6S2, MW 696.665=498nm) and nigrosine (molecular formula C22H14N6Na2O9S2, MW 616.49=570nm), as shown in Fig 2a & 2b, because they are readily soluble in water. Thus, they are prepared using distilled water and used for all the experiments. These dyes are purchased from S.D. fine chemicals as a result of their high water solubility. The instrument UV-Visible spectrophotometer (Hitachi U2910) was used to monitor the decolourization of Congo red and Nigrosine dye for all the parameters optimized.
 |
Figure 2: a. UV-visible spectra for Congo red |
 |
Figure 2: b. UV-visible spectra for nigrosine. |
Preparation of an Oxidizing Agent
Acetone and distilled water (1:2) are combined in a 1-L round bottom flask and refrigerated in an ice bath with magnetic stirring for 20 minutes. After 20 minutes, the stirring is stopped, and a single part of Oxone and sodium bicarbonate is added. While still submerged in the ice bath, the flask is loosely covered and the slurry is vigorously agitated for 15 minutes. The stir bar is removed from the reaction flask after 15 minutes and washed with distilled water.
The reaction slurry-containing flask is then attached to a rotating evaporator with a room-temperature bath. During this step, the flask is vigorously rotated (210 rpm) to prevent the slurry from bouncing into the bump trap. After 15 minutes, the bath temperature is raised to 40 °C for 10 minutes. When the bath reaches 40 °C, the distillation is immediately stopped by releasing the vacuum and lifting the flask away from the heated water bath. The pale yellow DMDO acetone solution is obtained and transferred immediately from the bump bulb into a graduated cylinder to assay the whole volume of the solution20,21. The degradation process continues with the freshly prepared DMDO.
 |
Equation 1: Preparation of dimethyl dioxirane. |
Degradation Experiments
The dye stock solutions (10mg to 100mg/L) for Congo red and nigrosine were produced, and 50 mL was taken for each analysis. All of the experiments were conducted in a dark environment at room temperature. Prior to execution, the dye solution’s initial absorption peak was recorded. After 30 minutes of experimentation, 1 mL of dye solution was removed from the reaction mixture and diluted, and dye absorption was assessed. The effect of pH was studied by changing the solution pH with 0.1 N NaOH and 0.1 N HCl. As previously stated, the blank trials were directed in the dark at ambient temperature. The formula was utilised to compute the percentage of decolorization.
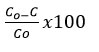
Where C0 is the initial concentration of the dye solution and C is the dye solution concentration at time t.
Results and Discussion
Visual Observation of Oxidizing Agent Formation
Visual observation also revealed the presence of DMDO. The synthesis of dimethyl dioxirane is confirmed by the change in colour of the reaction mixture. The colour of the aqueous solution of acetone and water will change from colourless to pale yellow when sodium bicarbonate and potassium peroxy monosulfate are added 20,21. As illustrated in figs. 3a and 3b, this is related to the production of dimethyl dioxirane.
 |
Figure 3: (a) Acetone and water mixture solution and sodium bicarbonate (b) After addition of potassium peroxy monosulfate, formation of dimethyl dioxirane. |
Uv-Visible Spectroscopy
Without an oxidising agent, congo red and nigrosine were stable at room temperature. When CR and NG dye solutions were destroyed when there is not DMDO oxidant, there was no noticeable change in absorbance. In the presence of dimethyl dioxirane, however, CR and NG solutions dropped considerably. The absorbance spectra of Congo red (CR) dye and Nigrosine (NG) dye after degradation in the presence of dimethyl dioxirane are shown in Fig. 4(a&b). The oxidation performance of DMDO, which was assessed in terms of variation in the intensity of the absorption maximum of CR and NG dye over reaction time, was a term used to denote the rate at which decolorization of CR and NG dye because of the influence of DMDO. Within 30 minutes, higher degradation performances of 99.2% and 100% were reached. The level of intensity absorbance peak has completely decreased and there aren’t no longer any peaks visible. It proves that contaminates are degraded by indicating that pollutants are removed throughout the degradation process.
 |
Figure 4: (a) Before and after degradation of Uv-Visible absorption spectra for Congo red. (b) Before and after degradation of Uv-Visible absorption spectra for Nigrosine. |
FT-IR Spectroscopy
The sharpest, best-defined, and most frequently recurring peaks were selected because they occurred regularly and had previously been detected in investigations spectra. Other minor peaks were so small that background noise in the spectra may have hidden them. Infrared spectroscopy is one of the most effective methods for characterising organic molecules.
 |
Figure 5: (a) and (b) Before and after degradation of FT-IR spectra for Congo red. |
 |
Figure 6: (a) and (b) Before and after degradation of FT-IR spectra for Nigrosine. |
The varied absorption bands for congo red dye before and after the advanced oxidation process are shown in FT-IR spectral analysis. The findings show that dye molecules may have acquired structural modifications throughout the AOP process. Figure 5 (a) shows the FTIR analysis of the congo red dye molecule before the AOP operation (b) shows the FT-IR analysis of the congo red after DMDO use. Congo red’s FT-IR spectra shows peaks at 3,466 cm-1 for primary amine N-H stretching vibration, 1,584 cm-1 for aromatic -C=C- stretching vibration, 1,446 cm-1 for -N=N- stretching vibration, 1,351 cm-1 for -C-N bending vibration, and 1,062 cm-1 for -S=O stretching vibration of sulfonic acid. After decolorization, the IR spectra of Congo red exhibits eliminated peaks at 1,446 cm-1 for the chromophoric group’s -N=N- stretching vibration. Only peak at 3,435 cm-1 for –O-H- stretching, 2,074 cm-1 for –C-C- stretching, 1,633 cm-1 for –C-C- stretching, and 1,112 cm-1 for –C-O- stretching remain after degradation. The distinct absorption bands for congo red dye before and after the advanced oxidation process are shown in FT-IR spectral analysis. The findings revealed that there is a significant structural change, which can be verified by comparing before and after degradation. Similarly, Figure 6 (a) shows the FTIR analysis of the nigrosine dye molecule before the AOP method (b) shows FT-IR analysis of the nigrosine after DMDO use. Nigrosine’s FT-IR spectra shows peaks at 3,422 cm-1 for primary amine N-H stretching vibration, 1,588 cm-1 for aromatic -C=C- stretching vibration, 1,004 cm-1 for –C-H- bend, 1,490 cm-1 and 1,461 cm-1 for -N-O- asymmetry, and 2,850 cm-1 for -C-H- stretching. Only peak at 3,435 cm-1 for –O-H- stretching, 2,074 cm-1 for –C-C- stretching, 1,633 cm-1 for –C-C- stretching, and 1,112 cm-1 for –C-O- stretching remain after degradation. The distinct absorption bands for nigrosine dye before and after the advanced oxidation process are shown in FT-IR spectral analysis. The findings revealed that there is a significant structural change, which can be verified by comparing before and after degradation.
Effect of pH
The pH has an impact on the oxidant’s ability to degrade organic contaminants. The dye solution’s acidic composition provides more reactive sites on the oxidant’s surface, causing organic pollutants to breakdown. However, a solution with a relatively acidic pH (pH 3) produces rapid oxidant activation, which could impair pollutant degradation efficiency22. The bulk of advanced oxidation processes, according to many experts, are dependent on pH and acidic conditions, which are favourable to the reaction 23,24. The effects of several pH values (pH 2, 3, 4, and 5) on CR elimination were studied. The following were the circumstances of the experiment: starting concentration of CR=10, oxidant dose=400µl, initial concentration of CR=10 mg/L at temperature. At a pH of 3, CR degrading proficiency was attained by roughly 84.2 percent in 30 minutes of reaction period. With rising pH from 2 to 5 during a reaction period of 30 minutes, the degradation efficiency of CR reduced from 84.2 to 52.4 percent; this happens as a result of a reduction in the activity of the oxidant25. Figure 7a shows the % decolorization vs pH plot for the elimination of CR at various pH values. At pH 3 of the CR solution, a rapid breakdown of CR was noticed when the pH value was amplified from 2 to 5. Further hydroxyl radicals were produced at pH 3 of the solution, resultant in the rapid breakdown of CR26. The elimination of CR has decreased at pH 4 and 5, owing to the low concentration of H+ in the solution and, as a result, the low production of hydroxyl radicals. Congo red dye was subjected to more experiment at an acidic pH of 3.
Similarly, pH ranges of 2-8 were tested for nigrosine dye using 600µl of oxidant and 10 mg of 50 mL dye solution at room temperature. At alkaline pH (pH=8), a pH range of 2-8 results in extreme removing rate of nigrosine dye indication. While pH removal efficiency increased, the rate of productivity achieved a whole maximum of 99.4%.at pH 8 for nigrosine as revealed in Fig 7b. At higher pH values, on the other hand, there is an increase in many hydroxyl ions; As a result, the decolorization process accelerates. However, when the pH level is very high, there are too many hydroxyl ions around the oxidant, which compete with dye molecules for adsorption on the surface of the oxidant, resulting in a drop in decolorization efficiency27. For the nigrosine dye, another test was performed at an alkaline pH of 8.
 |
Figure 7: a. 10mg/L Congo red dye Solution, 400µl of DMDO, pH varied from 2 to 5 under room temperature. b. 10mg/L nigrosine dye solution, 600 µl of DMDO, pH varied from 2 to 8 under room temperature. |
Effect of Dimethyl Dioxirane
By changing the oxidising agent concentration from 100µl to 1000µl for 10mg/L of the congo red dye solution shown in Fig.8a, the optimum quantity of dimethyl dioxirane oxidant for successful congo red and nigrosine dye decolurization was determined. When the oxidising agent concentration is increased to 400µl, the dye degradation continues to the point where a drop in the decolorization rate is noticed. This is due to the fact that active sites are required for dye decolorization to rise as the oxidising agent concentration increases, and beyond the addition of 400µl of dimethyl dioxirane to the congo red dye solution, the decolorization rate decreased. This is evident by the fact that active sites are required for dye decolorization, which rises as the oxidising agent concentration increases and beyond the addition of 400µl of dimethyl dioxirane to the congo red dye solution, a decrease in the decolorization process was observed. Similarly, for 10mg/L of the dye solution illustrated in Fig.8b, the concentration of nigrosine dimethyl dioxirane ranges from 100µl to 1000µl. Decolorization efficiency increases to 600µl when the oxidising agent concentration is increased. After 600l, there are no changes in decolorization efficiency. As a result, it was determined that 400µl and 600µl are sufficient to decolorize the entire dye solution. For congo red, an acidic pH 3, 400l of oxidising agent was used, while for nigrosine, an alkaline pH 8, 600µl of oxidising agent was used.
 |
Figure 8: a. 10mg/L Congo red dye solution, DMDO dosage varied from 100 to 400µl, pH =3, under room temperature. b. 10mg/L nigrosine dye solution, DMDO dosage varied from 100 to 600µl, pH =8, under room temperature. |
Effect of Initial Dye Concentration
The effect of varying initial dye concentrations was examined by choosing a concentration range of 10 mg to 100 mg/L for both congo red and nigrosine dyes under the experimental conditions of dimethyl dioxirane concentrations 400µl and 600µl, acidic pH range 3 for congo red dye and basic pH range 8 for nigrosine dye at room temperature, as shown in Figures 9a and 9b. The results show that for congo red, there is a 99.2% increase in dye decolorization up to 10 mg of dye solution, and for nigrosine dye solution, there is a complete 100% decolorization within 30 minutes, after which there is a decrease in the rate of degradation. At increased dye concentrations, the probability of dye molecules interacting with •OH radicals decreases, and hence a decrease in the dye degradation rate28,29,30. Therefore, congo red and nigrosine, the ideal beginning dye concentration is 10 mg/L, as well as the rate of degradation is 99.2% and 100%, respectively.
 |
Figure 9: a. DMDO=400 µl, pH = 3, Initial congo red dye concentration varied from 10mg/L to 100mg/L, under room temperature. b. DMDO=600 µl, pH = 8, Initial nigrosine dye concentration varied from 10mg/L to 100mg/L, under room temperature. |
Conclusion
The study focusses; the Dimethyl Dioxirane was prepared successfully by using acetone, deionized water; potassium peroxy monosulfate and sodium bicarbonate as a base. High production yield was achieved by this method. The method used is simple and inexpensive.
The optical absorption peak intensity for congo red and nigrosine were established to be at 498 and 570nm respectively.
Absorbance peak for before and after degradation of CR and NG dyes were observed by UV-Visible spectroscopy.
The functional group of congo red and nigrosine, before and after degradation band were determined by FT-IR spectroscopy.
Dimethyl dioxirane was employed as an oxidizing agent for the decolourization of congo red and nigrosine dye.
The removal efficiency of Dimethyl dioxirane was decreased by increasing the pH and initial dye concentration.
The decolourization proficiency was increased when the concentration of oxidant greater, 500μl is sufficient for the decolourization of congo red and nigrosine dye.
The decolourization efficiency was falling as the concentration of the dye solution increases.
As a whole, dimethyl dioxirane used as an oxidizing agent for the decolourization of Congo red and nigrosine dye was nontoxic, economically feasible and environmentally benign under the present reaction conditions. Compare to others, the time required is less and normal room temperature is more sufficient to do this work.
Further more, this work may be extended for the decolourization of industrial effluents containing dyes as major pollutants.
Acknowledgement
The authors would like to express their gratitude to Dr. S. Thennarasu, Sr. Principal Scientist, Department of Organic and Bio-Organic Chemistry, Central Leather Research Institute, Chennai, for his insightful comments that helped them complete the task efficiently. Dr. S. Vidhya and Dr. F. Janeeta Priya, Assistant professors in the Department of Chemistry at Holy Cross College, were also quite helpful during the research. We also appreciate the laboratory facilities provided by the PG and Research Department of Chemistry at Holy Cross College in Trichy.
Conflict of interest
There is no conflict of interest.
References
- Mrowetz.M.; Selli.E.; Pirola.C.; Ultrason Sonochem., 2003, 10, 247–254.
CrossRef - Matzusawa.S.; Tanaka.J.; Sato.S.; Ibusuki. T.; J. Photochem Photobiol A: Chem., 2002, 149, 183–189.
CrossRef - Davydov. L.; Reddy.E.; France.P.; Smirniotis.P.; Appl Catal B:Environ. 2001, 32–95.
CrossRef - Kado.Y.; Atobe. M.; Nonaka.T.; Ultrason Sonochem. 2001, 8, 69–74.
CrossRef - Selli.E.; Chem Phys. Chem. Phys. 2002, 4 ,6123.
CrossRef - Shirgaonkar. I.Z.; Pandit.A.; Ultrason Sonochem. 1998, 5, 53.
CrossRef - Acemiocglu.B.; J. Colloids Interf Sci. 2004, 274, 371–379.
CrossRef - Namasivayam.C.; Kavitha.D.; Dyes Pigm. 2002, 54, 47–58.
CrossRef - Lo´pez Cisneros.R.C., Revista de la facultad de ciencias UNI.
CrossRef - Bali.U.; Dyes Pigm. 2004, 60, 187–195.
CrossRef - Caplan Can.H.; Ul Kirci.B.; Kavlak.S.; Radiat Phys Chem. 2003, 68, 811–818.
CrossRef - Boer. C.G.; Obici.L.; Marques de Souza. C.G.; Biores Technol. 2004, 94, 107–112.
CrossRef - Iy´k .M.; Sponza.D.T.; Biores Technol. 2004, 94, 208–212.
- Molinari.R.; Pirillo.F.;. Falco.M.; Loddo.V.; Palmisano. L.; Chem. Eng Process. 2004, 43, 1103–1114.
CrossRef - Guillard.C.; Lachheb.H.; Houas.A.; Ksibi.M.; Elaloui.E.; Herrmann.J.M.; J Photochem Photobiol A: Chem. 2003, 158, 27–36.
CrossRef - Lachheb.H.; Puzenat.E.; A. Houas, M. Ksibi, E. Elaloui, C. Guillard, J.M. Herrmann, Appl Catal Environ. 2002, 39, 75–90.
CrossRef - Green, F.J. The Sigma-Aldrich Handbook of Dyes, Stains and Indicators. Aldrich, Milwaukee, 1990, 513-515.
- Clark, G. Staining Procedures Used by the Biological Stain Commission. 4th Edition, Williams & Wilkins, Baltimore, London, 1981, 412.
- Klaus Hunger, Peter Mischke, Wolfgang Rieper, Roderich Raue, Klaus Kunde, Aloys Engel: “Azo Dyes” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005.
- Robert W.; Murray and Ramasubbu jeyaraman.; J. Org. Chem. 1985, 50, 2847-2853.
CrossRef - Adam, W.; Chan, Y. Y.; Cremer, D.; Gauss, J.; Scheutzow.D.; Schindler, M.; Org.Chem, 1987, 52,2800-2803.
CrossRef - Tian.H.; Li.JJ.; Mu.Z.; Li. L.D.; Hao. ZP.; Sep Purif Technol, 2009, 66(1):84–89.
CrossRef - Bokare.AD.; Choi.W.; Environ Sci Technol, 2009, 43:7130–7135.
CrossRef - Liu.WP.; Zhang.HH.; Cao.BP.; Lin.K.; Gan.J.; Water Res ., 2011, 45:1872–1878.
CrossRef - Babuponnusami.A.; Muthukumar.K.; Sep Purif Technol., 2012, 98:130–135.
crossRef - Xu.L.; Wang.J.; J Hazard Mater, 2011, 186:256–264.
CrossRef - Thennarasu.G and Sivasamy.A, Ecotoxic Environ Safety, 2016, 134 (2), 412.
CrossRef - El-bahy.Z.M.; Ismail. A. A.; and Mohamed. R. M.; J. Hazard. Mater. 2009, vol.166, no.1, pp. 138–143.
CrossRef - Byrappa. K.; Subramani. A. K.; Ananda. S.; Lokanatha rai. K.M.; Dinesh. R.; and Yoshimura.M.; Bulletin Mater Sci, 2006, vol. 29, no. 5, pp. 433–438.
CrossRef - Behnajady. M.A.; Modirshahla. N.; and Hamzavi. R.; J Hazard Mater, 2006, 133(1-3).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









